Abstract
Background
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel, Abraxane®) is FDA approved for the treatment of several adult cancers. Antimitotic agents are essential components for curative therapy of pediatric solid tumors, although taxanes have shown limited activity. Because of the novel formulation, nab-paclitaxel was evaluated against a limited series of Pediatric Preclinical Testing Program (PPTP) solid tumors.
Procedures
Nab-paclitaxel was tested against a limited subset of PPTP solid tumor xenograft models at a dose of 50 mg/kg using a q4dx3 schedule intravenously.
Results
Nab-paclitaxel was well tolerated in vivo, producing maximum weight loss of approximately 10% with recovery to baseline weight in the week following the third dose. All 20 xenograft models tested were considered evaluable for efficacy. Nab-paclitaxel induced statistically significant differences in event-free survival (EFS) distribution compared to control in 19 of 20 (95%) of the solid tumors. Objective responses were observed in 12 of 20 (60%) solid tumor xenografts. Complete responses (CR) or maintained CR were observed in 5 of 8 Ewing sarcoma models and 6 of 8 rhabdomyosarcomas. There were no objective regressions in either neuroblastoma (n=2) or osteosarcoma (n=2) xenograft panels. At the dose tested, systemic exposures of nab-paclitaxel in mice were somewhat greater than those tolerated in humans.
Conclusions
The high level of activity observed against the rhabdomyosarcoma and Ewing sarcoma PPTP preclinical models makes nab-paclitaxel an interesting agent to consider for pediatric evaluation.
Keywords: Preclinical Testing, Developmental Therapeutics, Abraxane, nab-paclitaxel
INTRODUCTION
Paclitaxel is an antimitotic natural product extracted from the Pacific yew (Taxus brevifolia) [1], and it stabilizes tubulin by preventing depolymerization [2,3]. Stabilization of microtubules inhibits dynamic reorganization of microtubule networks that are essential for interphase and mitotic cellular functions [4], resulting in cell death. Paclitaxel has broad spectrum activity against human cancers including breast, ovarian and non-small cell lung cancer [5]. However, in addition to toxicities associated with the effect of antimitotic agents on proliferating tissues, the formulation of paclitaxel is also of concern. Because paclitaxel is a hydrophobic molecule, the original formulation required use of polyoxyethylated castor oil (Cremophor EL) and ethanol [6]. Cremophor EL leaches plasticizers from DEHP infusion sets and can cause severe or fatal hypersensitivity reactions [7–13]. As a consequence, patients have to be premedicated with corticosteroids and antihistamines to reduce the risk of hypersensitivity reactions. Additionally, Cremophor EL may cause neutropenia [13,14] and prolonged peripheral neuropathy associated with axonal swelling and degeneration, vesicular degeneration and demyelination [15–17]. In addition, the ethanol in the formulation, while not a significant problem in adults, can be a serious concern in children. Acute central nervous system toxicities following conventional paclitaxel administration to pediatric cancer patients could be correlated at least in part with the ethanol exposure [7].
Nanoparticle-albumin binding is a drug delivery technology that uses the biochemical properties of albumin to increase drug delivery to tumors through albumin initiated transcytosis. Albumin-bound drugs cross the endothelial cells into the interstitial space through binding to a cell surface glycoprotein (gp60) receptor that induces caveolin-1 and results in invagination of the endothelial cell membrane, trapping plasma constituents in structures known as caveolae. Accumulation of albumin in tumor tissue is thought to result from secretion of the albumin-binding protein SPARC (secreted protein, acidic and rich in cysteine). Hydrophobic molecules are non-covalently bound to albumin using a proprietary process that does not alter either component. The first commercial product using this technology, nab-paclitaxel (ABI-007, Abraxane®), is a solvent-free, 130 nM albumin particle form of paclitaxel. Consequently, high-level expression of caveolin-1 or SPARC have been proposed as potential biomarkers for tumor response of nab-paclitaxel [1,3].
A Phase 3 clinical trial directly compared the antitumour activity and tolerability of nab-paclitaxel with those of paclitaxel in women with measurable metastatic breast cancer [18]. Patients received 3-week cycles of either nab-paclitaxel 260 mg/m2 i.v. over 30 min without premedication with corticosteroids or antihistamines (n = 229), or paclitaxel 175 mg/m2 i.v. over 3 h (n = 225). Both the objective response rates (33% versus 19%, p = 0.001) and the median time to progression (23.0 versus 16.9 weeks, p = 0.006) significantly favored nab-paclitaxel over paclitaxel. Of importance, no severe hypersensitivity reactions occurred in the nab-paclitaxel group, despite the shorter administration time and the absence of premedication. Nab-paclitaxel also showed significantly higher objective response rates compared to paclitaxel for non-small cell lung cancer (NSCLC) [19], and the addition of nab-paclitaxel to gemcitabine resulted in significantly improved overall survival for patients with advanced pancreatic cancer [20]. Nab-paclitaxel also shows promise for treatment of metastatic melanoma [21]. A report also indicated that patients who previously had hypersensitivity reactions to Cremophor-formulated paclitaxel could be treated safely with nab-paclitaxel [2].
Whereas paclitaxel is approved for use in several adult cancers, and nab-paclitaxel is approved for breast cancer, NSCLC and metastatic pancreatic cancer, use of taxanes in pediatric oncology has been limited, where paclitaxel has demonstrated very modest activity [4–6]. In addition, severe neurologic and allergic toxicity was encountered despite premedication [7]. Recently, the activity of nab-paclitaxel against pediatric models of rhabdomyosarcoma and neuroblastoma demonstrated marked in vivo activity superior to that of paclitaxel [8]. The activity of nab-paclitaxel was further tested against PPTP sarcoma (rhabdomyosarcoma, osteosarcoma and Ewing sarcoma) and neuroblastoma xenograft models.
MATERIALS AND METHODS
In vivo tumor growth inhibition studies
CB17SC scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma as previously described [9]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Ten mice were used in each control or treatment group. Tumor volumes (cm3) were determined and responses were determined using three activity measures as previously described [9]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Caveolin-1(CAV1) Immunohistochemistry
Slides from formalin fixed paraffin embedded (FFPE) tissue were deparaffinized in xylene (3 changes of 2 minutes each) and then rehydrated through graduated alcohols of 2 minutes each (100%, 95%, and 70%) and ended in distilled water. They were then microwaved for 10 minutes in pH 6.0 citrate buffer, cooled and washed in running water followed by a peroxidase block for 5 minutes. After rinsing in PBS, slides were incubated with a primary antibody for caveolin-1 (N-20, Santa Cruz) at 1:200 for 30 minutes in a humidified chamber, rinsed and incubated with a HRP Polymer conjugated rabbit secondary antibody (EnVision+, DAKO) for 30 minutes. Slides were rinsed again in PBS, incubated with DAB for 5 minutes, rinsed and counterstained with hematoxylin for a few seconds. Slides were then rinsed in water and dehydrated following the opposite order (70%, 95% and 100% alcohol) that ended in xylene, mounted and coverslipped. Slides were reviewed and scored for the intensity of staining in the tumor cells (0 = no staining to 3 = strong staining) and the percentage of stained tumor cells. An H score was calculated by multiplying the score for staining intensity times the percentage of tumor staining.
Expression of CAV1 and SPARC
The levels of CAV1 and the albumin-binding protein SPARC (secreted protein, acidic and rich in cysteine) were evaluated using Agilent gene expression arrays.
Nab-paclitaxel pharmacokinetics
Following intravenous nab-paclitaxel administration, plasma samples were collected from mice at 0.083, 0.25, 0.5, 1, 2, 4, 8 and 24 hours and analyzed for pharmacokinetics. For calibration curve, an aliquot of paclitaxel stock solution (0.2 mg/mL in acetonitrile) was spiked into plasma to make the initial plasma stock at 4,000 ng/mL, which was then diluted to make calibration standard samples at concentrations ranging from 2 to 4,000 ng/mL in plasma. Quality control (QC) samples were made similarly with concentrations of low QC at 6 ng/mL, medium QC at 100 ng/mL, and high QC at 3,200 ng/mL. Dilution QC at 20,000 ng/mL was made by spiking the stock solution into blank plasma and then diluted 10-fold with blank plasma. Plasma samples of 5 min to 4 hour were diluted 10-fold using blank plasma and plasma samples from other time points were crashed directly without dilution.
Aliquots (50 μL) of plasma samples (including standards, blanks, QCs) were transferred into a 96 well plate. To each well, 150 μL of a solvent mixture of acetonitrile:methanol (9:1) containing 100 ng/mL of d5-paclitaxel (as the internal standard) was added. The plate was capped, vortex mixed and centrifuged. A 150 μL aliquot of the supernatant was transferred into a clean 96-well plate for LC-MS/MS analysis.
Chromatographic separation was carried out using a Shimadzu LC20 system equipped with an Agilent Pursuit XRs 3 C18 column (100×2 mm). Mobile phases consisted of 5 mM ammonia acetate in water containing 0.1% formic acid (A) and 5 mM ammonia acetate in acetonitrol:water (9:1) mixture containing 0.1% formic acid (B), at a flow rate of 0.35 mL/min. The column was eluted with a gradient of 25% B for 0.9 minute, linearly increased to 100% B over 1.5 minute, and then maintained at 100% B for another 3.5 minutes. The LC elute was connected directly to a Sciex API4000 QTrap mass spectrometer equipped with an electrospray ionization (ESI) ion source. The negative multiple reaction monitoring (MRM) mode was used for the quantitation. Mass transitions of m/z 898.3 to 525.2 and 903.3 to 530.2 were optimized for paclitaxel and d5-paclitaxel, respectively. The mass spectrometer parameters were fixed as follows: capillary voltage, −4.5 kV; curtain gas, 35 psi; desolvation temperature, 500 °C; GS1, 50 psi; GS2, 60 psi; and CAD, medium.
Mean plasma concentration-time profile was used for pharmacokinetic (PK) calculation. PK parameters were calculated using Watson 7.4 Bioanalytical LIMS™ (Thermo Fisher Scientific, Philadelphia, PA). The peak plasma concentration (Cmax) and time to reach Cmax (tmax) were determined from the actual data. The area under the plasma concentration-time curve from 0 to 24 hr post-dose (AUC24h) of paclitaxel was determined by the non-compartmental model using the linear/log trapezoidal rule.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies.
Drugs and Formulation
Nab-paxlitaxel was provided to the Pediatric Preclinical Testing Program by Celgene Corporation, through the Cancer Therapy Evaluation Program (NCI). Nab-paxlitaxel was formulated as a 10 mg/ml stock in sterile saline and stored at −20°C until use. Nab-paxlitaxel was administered intravenously (IV) at 50 mg/kg to mice using a q 4 days x 3 schedule. Nab-paxlitaxel was provided to each consortium investigator in coded vials for blinded testing.
RESULTS
In vivo testing
Nab-paxlitaxel was tested against the PPTP solid tumor xenografts using a dose of 50 mg/kg administered by the IV route q4d x 3. The total planned treatment and observation period was 6 weeks. Nab-paxlitaxel was generally well tolerated, with a 0% toxicity rate in the treated groups (0 of 200), similar to that observed for control animals (0 of 200). All 20 tested xenograft models were considered evaluable for efficacy. A complete summary of results is provided in Supplemental Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
Nab-paxlitaxel induced significant differences in EFS distribution compared to control in 19 of 20 (95%) of the evaluable solid tumor xenografts (Table I). For those xenografts with a significant difference in EFS distribution between treated and control groups, the EFS T/C activity measure additionally requires an EFS T/C value of > 2.0 for intermediate activity and indicates a substantial agent effect in slowing tumor growth. High activity further requires a reduction in final tumor volume compared to the starting tumor volume. Sixteen of 20 (80%) solid tumor models evaluable for the EFS T/C activity metric demonstrated EFS T/C > 2.0, and 9 additionally met criteria for high EFS T/C activity. High activity was observed against 5 of 8 Ewing xenografts (Figure 1) and 4 of 8 rhabdomyosarcoma xenografts (Figure 2). Neither osteosarcoma (n=2) or neuroblastoma (n=2) models demonstrated objective regressions (Table I).
Table I.
In vivo activity of nab-paclitaxel
| Line Description | Tumor Type | Median Time to Event | P-value | EFS T/C | Median RTV at End of Study | Tumor Volume T/C | Median Group Response | EFS Activity |
|---|---|---|---|---|---|---|---|---|
| SK-NEP-1 | Ewing | >EP | <0.001 | >3.4 | 0.0 | 0.00 | MCR | High |
| EW5 | Ewing | >EP | <0.001 | >5.4 | 3.1 | 0.13 | CR | Int |
| EW8 | Ewing | >EP | <0.001 | 2.2 | 0.0 | 0.11 | MCR | High |
| TC-71 | Ewing | 20.1 | <0.001 | 4.6 | >4 | 0.19 | PD2 | Int |
| CHLA258 | Ewing | 31.7 | <0.001 | 4.8 | >4 | 0.10 | PR | High |
| ES1 | Ewing | >EP | <0.001 | >3.5 | 0.0 | 0.04 | MCR | High |
| ES4 | Ewing | 16.6 | <0.001 | 1.6 | >4 | 0.44 | PD2 | Int |
| ES6 | Ewing | >EP | <0.001 | >2.8 | 0.0 | 0.03 | MCR | High |
| Rh10 | Alveolar RMS | >EP | <0.001 | >3.4 | 0.0 | 0.05 | MCR | High |
| Rh28 | Alveolar RMS | >EP | 0.003 | > 1.6 | 0.0 | 0.08 | MCR | NE |
| Rh30 | Alveolar RMS | >EP | <0.001 | >2.7 | 2.9 | 0.05 | MCR | High |
| Rh30R | Alveolar RMS | >EP | <0.001 | >2.7 | 2.0 | 0.28 | CR | Int |
| Rh41 | Alveolar RMS | 28.3 | <0.001 | 2.7 | >4 | 0.59 | PD2 | Int |
| Rh65 | Alveolar RMS | >EP | <0.001 | >3.2 | 0.0 | 0.04 | MCR | High |
| Rh18 | Embryonal RMS | 31.0 | <0.001 | 1.8 | >4 | 0.55 | PD2 | Low |
| Rh36 | Embryonal RMS | >EP | <0.001 | >3.0 | 0.0 | 0.02 | MCR | High |
| NB-1691 | Neuroblastoma | 6.1 | 0.212 | 1.2 | >4 | 0.78 | PD1 | Low |
| NB-1643 | Neuroblastoma | 5.6 | 0.049 | 1.2 | >4 | 0.79 | PD1 | Low |
| OS-17 | Osteosarcoma | 31.2 | <0.001 | 1.4 | >4 | 0.64 | PD1 | Low |
| OS-9 | Osteosarcoma | 27.2 | 0.022 | 1.2 | >4 | 0.68 | PD1 | Low |
EP: time to event exceeds 42 days; EFS T/C: Ratio of median time to event for treated compared to control animals; PD1 (Progressive Disease 1, dark green): >25% ↑ in tumor volume, TGD value ≤1.5; PD2 (Progressive Disease 2, light green): >25% ↑ in tumor volume, TGD value >1.5; SD (Stable Disease): <25% ↑ in tumor volume, <50% regression; PR (Partial response): a tumor volume regression ≥50%; CR (Complete response): disappearance of measurable tumor mass (< 0.10 cm3); MCR (Maintained CR): absence of measurable tumor mass at day 42.
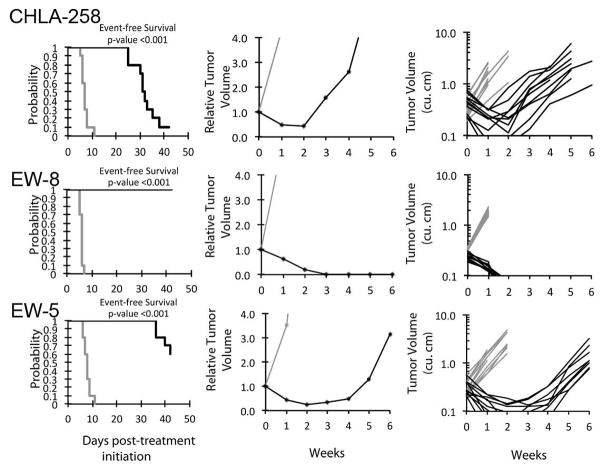
Figure 1.
Nab-paclitaxel in vivo objective response activity for Ewing sarcoma models having high Caveolin-1 expression. Ewing sarcomas (CHLA-258, EW-8, and EW-5): Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Controls (gray lines); Treated (black lines), statistical significance (p values) of the difference between treated and control groups are included.
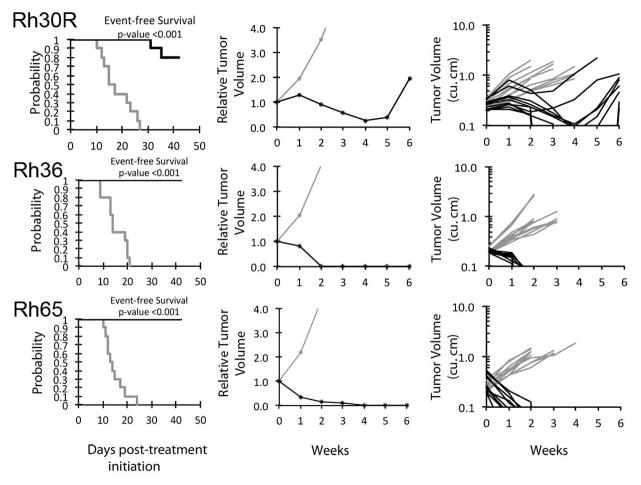
Figure 2.
Nab-paclitaxel in vivo objective response activity for rhabdomyosarcoma models having low Caveolin-1 expression. Rhabdomyosarcomas (Rh65, Rh36, and Rh30R): Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Controls (gray lines); Treated (black lines), statistical significance (p values) of the difference between treated and control groups are included.
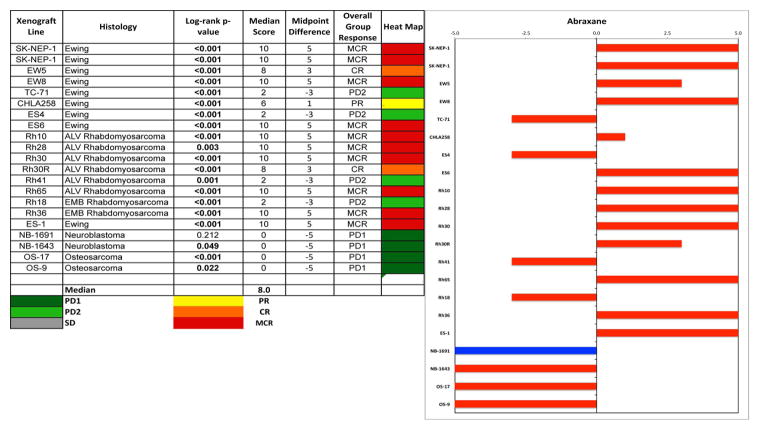
The in vivo testing results for the objective response measure of activity are presented in Figure 3 in a ‘heat-map’ format as well as a ‘COMPARE’-like format, based on the scoring criteria described in the Supplemental Response Definitions section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of 5 (stable disease). The figures illustrate the complete responses (CR) or maintained CRs for 6 of 8 rhabdomyosarcoma and 5 of 8 Ewing sarcoma xenografts.
Figure 3.
Left: The colored heat map depicts group response scores to nab-paclitaxel. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of >2 but <6, and low activity by a score of <2. Right: representation of tumor sensitivity to nab-paclitaxel based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
Caveolin-1 and SPARC Expression
Expression of CAV1 at the mRNA level (Agilent Sureprint 3 arrays) shows that expression was generally highest in Ewing sarcoma models (relative expression 10 to >70-fold the median for all tumors) whereas expression in alveolar rhabdomyosarcomas was at the median for the entire PPTP tumor panel (Supplemental Figure 1). Immunohistochemical staining of FFPE sarcoma tissues showed high-level caveolin-1 expression in EW-5, EW-8 and SKNEP-1 that was in good agreement with the mRNA expression data. In contrast, high-level expression detected by Agilent profiling did not correlate with IHC in Rh18 xenografts, and relatively high IHC H scores (200 and 100) were associated with low (<10) transcript expression for Rh65, Rh36 and Rh28 (Table II). Overall, there was no good correlation between caveolin-1 expression (IHC) and response to nab-paclitaxel. Although the mean level for the ‘responding’ tumors was greater than in the ‘non-responding’ group, there was considerable overlap. Because the ‘H score’ is not normally distributed, the Wilcoxon rank-sum test was used for comparison. Although there was a trend, the differences in H scores between responding and non-responding tumors was not significant (P = 0.069) (Figure 4A). Levels of CAV1 mRNA had a similar distribution in responding and non-responding xenografts (Figures 4B). Similarly, there was no obvious relationship between SPARC expression and response, as expression was low in non-reponding models (NB-1691, TC-71, NB-1643) and responding models (Rh28, Rh30R, Rh36, Rh41, Rh65), whereas relatively high-level SPARC (mRNA ~ 9-fold the median for all models) was associated with Rh18 tumors that were non-responsive to nab-paclitaxeltreatment (Figure 4B).
Table II.
Immunohistochemical staining for caveolin-1
| Tumor | Score Sensitivity | %Tumor cells Positive | H Score (intensity x % tumor cells stained) | Tumor Response | Cav-11 Expression | SPARC1 Expression |
|---|---|---|---|---|---|---|
| ES-6 | 3 | 95 | 285 | MCR | ND2 | ND |
| ES-4 | 1 | 70 | 70 | PD1 | ND | ND |
| SK-NEP-1 | 3 | 80 | 240 | MCR | 50.6 | 0.38 |
| CHLA-258 | 3 | 95 | 285 | PR | 11.9 | 2.28 |
| EW-8 | 3 | 100 | 300 | MCR | 47.2 | 1.01 |
| EW-5 | 3 | 100 | 300 | CR | 47.9 | 0.63 |
| Rh18 | 1 | 30 | 30 | PD1 | 47.9 | 6.36 |
| Rh65 | 2 | 100 | 200 | MCR | 0.84 | 2.10 |
| Rh10 | 0 | 0 | 0 | MCR | 0.83 | 1.43 |
| Rh36 | 1 | 100 | 100 | MCR | 0.74 | 4.62 |
| Rh28 | 1 | 100 | 100 | MCR | 1.3 | 2.93 |
| Rh30R | 2 | 100 | 200 | CR | 1.0 | 2.39 |
| Rh41 | 1 | 100 | 100 | PR | 0.89 | 2.45 |
| ES-1 | 3 | 100 | 300 | MCR | ND | ND |
| OS-9 | 1 | 100 | 100 | PD1 | 1.82 | 14.31 |
| NB-1691 | 1 | 100 | 100 | PD1 | 0.84 | 1.12 |
| TC-71 | 3 | 90 | 270 | PD2 | 32.9 | 0.6 |
| NB-1643 | 1 | 100 | 100 | PD1 | 0.84 | 0.69 |
Relative expression is the ratio of the expression value for each xenograft divided by the median expression value for the entire panel.
ND, not determined.
Figure 4.
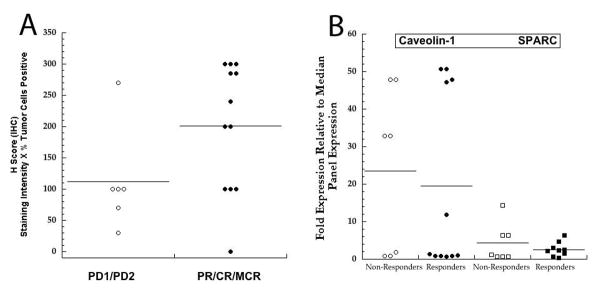
(A) Relationship between caveolin-1 immunohistochemical staining intensity (H Score) and response. PD1/PD2 (progressive disease, as defined in Supplemental Respose Definitions). PR partial response (>50% volume regression); CR (complete response with regrowth during 6 weeks; MCR, CR maintained at week 6. Bars indicate the mean H score for the group. (B) Relationship between caveolin-1 and SPARC gene expression (Agilent Sureprint 3 arrays) and response. Non-repoders (PD1/PD2); Responders (PR/CR/MCR). Progressive disease, as defined in Supplemental Respose Definitions). PR, partial response; CR (complete response with regrowth during 6 weeks); MCR, CR maintained at week 6.
Nab-paclitaxel pharmacokinetics
Total plasma levels of paclitaxel were determined in plasma after mice received 50 mg/kg nab-paclitaxel I.V. Results are summarized in Supplemental Table II. Similar to nab-paclitaxel pharmacokinetics reported for rat and human [22], there was rapid decline in plasma drug concentration and large volume of distribution. The AUC and extrapolated AUC∞ were similar at 36.54 and 36.65 μM*Hr, respectively.
DISCUSSION
Antimitotic agents such as vincristine have demonstrated single agent activity, and are used as standard of care for many curative regimens in pediatric patients with both solid and hematologic malignancies. Vinca alkaloids bind tubulin and cause destabilization of microtubules, leading to mitotic arrest and cell death. In contrast, taxanes induce microtubule stabilization, and have not shown significant activity against pediatric solid tumors [4–6]. Whether this represents an intrinsic difference in the mechanism of action of taxanes versus Vinca’s, or whether taxanes were tested in predominantly patients who had previously failed vincristine-based therapy, is unclear. For example, acquired or intrinsic resistance to both Vinca alkaloids and paclitaxel may be mediated through a common mechanism, by which drug is excluded from cells via drug efflux by ABC transporters such as P-glycoprotein (ABCB2) [23]. In addition to its lack of clinical activity in pediatric trials, paclitaxel demonstrated significant neurologic and allergic toxicities, hence enthusiasm for further development was low. Nab-paclitaxel, represents a novel nanoparticle bound formulation of paclitaxel that does not require formulation with surfactants such as Cremophor EL that is thought to contribute to neurologic and allergic toxicity.
Nab-paclitaxel was well tolerated at the dose and schedule used for testing. Of note, nab-paclitaxel is better tolerated in mice than in humans, with the 50 mg/kg q4d x 3 regimen evaluated being well below the mouse MTD (120–180 mg/kg, q4d x 3) [13]. The lower dose was selected as a more clinically relevant dose that produces drug levels that are reasonably comparable to those achieved in humans using weekly dosing (see below). Nab-paclitaxel showed very high level activity against Ewing sarcoma and rhabdomyosarcoma xenograft models. For the panel of 8 Ewing sarcomas there were 6 objective regressions (4 MCR, 1CR, 1PR), with a similar number observed for the rhabdomyosarcoma panel (5 MCR, 1CR). There were no objective regressions in neuroblastoma (n=2) or osteosarcoma (n=2) models. Of note, neither neuroblastoma or osteosarcoma models tested responded to the antimitotic agents vincristine [9] or eribulin [10], which may suggest a common mechanism of resistance to these agents, for example expression of ABC transporters that may reduce intracellular levels of abraxane (e.g. MDR1B). The PPTP results confirm and extend the data showing marked sensitivity for rhabdomyosarcoma xenografts [8], although the limited testing against neuroblastoma models did not demonstrate sensitivity to nab-paclitaxel. The PPTP results also extend data for the responsiveness of Ewing sarcoma xenografts to nab-paclitaxel to a large panel of tumors [24].
The PPTP xenograft models identified antimitotic agents as being very active against acute lymphoblastic leukemia and sarcoma xenografts [10,25], although ABT-751, an orally bioavailable sulphonamide that binds the colchicine binding site on β-tubulin, was not active [12]. A common reason for over-prediction of clinical activity by preclinical in vivo models is that mice may tolerate higher drug levels of the test compound compared to humans. However, incorporating modeling of mouse and human pharmacokinetic data with xenograft efficacy data enhances the predictive capability of in vivo testing [11]. In the case of nab-paclitaxel, the comparisons of species-specific differences in pharmacokinetics are more complex, as nanoparticle albumin-bound paclitaxel shows a greater volume of distribution, and greater tumor:plasma ratios [8,13]. The exposure to total paclitaxel following administration of nab-paclitaxel (50 mg/kg) was 36.54 μM*Hr in mice. In contrast the human exposure was 23.80 μM*Hr in patients receiving 260 mg/m2 [14]. Thus, the single dose exposure in mice is slightly higher than in man. However, two other factors also need to be considered. Protein binding is greater in mice than humans (98% vs 95%) so free drug exposure following a single administration of drug would be approximately 1.19 μM*Hr and 0.731 μM*Hr, in human and mouse, respectively. Secondly, in our study, mice were administered nab-paclitaxel on days 1, 4 and 8 whereas in humans nab-paclitaxel is administered at 260 mg/m2 on a 21-day cycle or at 125 mg/m2 days 1, 8 and 15 on a 28 day cycle. Administered at 50 mg/kg the total free drug exposure per cycle in mice would be 2.193 μM*Hr, or approximately two-fold greater than in patients receiving 260 mg/m2 every 21 days. Total exposure per 28 day cycle at a dose of 125 mg/m2 would result in a plasma exposure (assuming linear kinetics for free drug) of 1.716 μM*Hr, or 78 per cent of that in mice. Recognizing the multiple factors complicating comparisons of mouse to human exposure for nab-paclitaxel, the mouse exposures per cycle appear to be somewhat greater than exposures achieved in humans.
High expression of SPARC and caveolin-1 have been postulated to increase sensitivity to nab-paclitaxel, although their role in predicting sensitivity remains an open question [26]. Caveolin-1 expression was generally high in Ewing sarcoma xenografts, and some of the brain tumor models and several rhabdomyosarcoma models had levels above the median for the entire in vitro and in vivo tumor panels (Agilent profiling). In contrast, SPARC expression was highest in osteosarcoma xenografts, with some enhanced expression in brain tumors and rhabdomyosarcomas (Supplemental Figure 1). Ewing sarcoma models were uniformly low in expression of SPARC having median expression ratio’s (~1.0) relative to the panel median expression. There was no obvious relationship between caveolin-1 expression, IHC staining, and tumor response (Figure 4A). Similarly, neither caveolin-1 or SPARC mRNA expression levels correlated with tumor sensitivity (Figure 4B).
In conclusion, nab-paclitaxel showed high level in vivo activity against rhabdomyosarcoma and Ewing sarcoma xenograft models, but not in limited testing against neuroblastoma and osteosarcoma models resistant to vincristine. Comparisons of nab-paclitaxel preclinical activity to that of paclitaxel are difficult because of the limited published data for paclitaxel [27]. To some extent, the nab-paclitaxel results are similar to those previously observed for vincristine, with activity noted for such vincristine-responsive tumors as rhabdomyosarcoma. The in vivo activity for nab-paclitaxel against Ewing sarcoma xenografts exceeded that previously observed for vincristine. These results support evaluation of nab-paclitaxel in children with relapsed/refractory cancers, and if robust single agent activity is observed then proceeding with further development for the cancers identified as clinically responsive.
Supplementary Material
Agilent profiles for Caveolin-1 and SPARC for the in vitro and in vivo models used in the PPTP. The histograms show the relative expression is the ratio of the expression value for each xenograft divided by the median expression value for the entire panel.
Efficacy of Abraxane against PPTP Xenograft Models.
Summary of Pharmacokinetic Parameters.
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used nab-paclitaxel supplied by Celgene Corporation, Summit, NJ. In addition to the authors this paper represents work contributed by the following: Sherry Ansher, Catherine A. Billups, Joshua Courtright, Kathryn Evans, Edward Favours, Danuta Gasinski, Melissa Sammons, Joe Zeidner, Jianrong Wu, Ellen Zhang, and Jian Zhang.
Footnotes
Conflict of interest statement: Zeen Tong is an employee of Celgene Corporation.
References
- 1.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Translational oncology. 2009;2(2):59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leon MC, Bolla S, Greene B, Hutchinson L, Del Priore G. Successful treatment with nab-paclitaxel after hypersensitivity reaction to paclitaxel and docetaxel. Gynecologic oncology case reports. 2013;5:70–71. doi: 10.1016/j.gynor.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. International journal of nanomedicine. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi RJ, Blaney S, Sullivan J, Weitman S, Vietti T, Bernstein ML. Phase 1 study of Paclitaxel administered twice weekly to children with refractory solid tumors: a pediatric oncology group study. J Pediatr Hematol Oncol. 2003;25(7):539–542. doi: 10.1097/00043426-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz CA, Strauss LC, Kepner J, Kretschmar C, Harris MB, Friedman H, Kun L, Kadota R. Paclitaxel for the treatment of progressive or recurrent childhood brain tumors: a pediatric oncology phase II study. J Pediatr Hematol Oncol. 2001;23(5):277–281. doi: 10.1097/00043426-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kretschmar CS, Kletzel M, Murray K, Thorner P, Joshi V, Marcus R, Smith EI, London WB, Castleberry R. Response to paclitaxel, topotecan, and topotecan-cyclophosphamide in children with untreated disseminated neuroblastoma treated in an upfront phase II investigational window: a pediatric oncology group study. J Clin Oncol. 2004;22(20):4119–4126. doi: 10.1200/JCO.2004.08.174. [DOI] [PubMed] [Google Scholar]
- 7.Doz F, Gentet JC, Pein F, Frappaz D, Chastagner P, Moretti S, Vassal G, Arditti J, Tellingen OV, Iliadis A, Catalin J. Phase I trial and pharmacological study of a 3-hour paclitaxel infusion in children with refractory solid tumours: a SFOP study. British journal of cancer. 2001;84(5):604–610. doi: 10.1054/bjoc.2000.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Marrano P, Kumar S, Leadley M, Elias E, Thorner P, Baruchel S. Nab-paclitaxel is an active drug in preclinical model of pediatric solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(21):5972–5983. doi: 10.1158/1078-0432.CCR-13-1485. [DOI] [PubMed] [Google Scholar]
- 9.Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Tajbakhsh M, Reynolds CP, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Zeidner J, Wu J, et al. The Pediatric Preclinical Testing Program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 10.Kolb EA, Gorlick R, Reynolds CP, Kang MH, Carol H, Lock R, Keir ST, Maris JM, Billups CA, Desjardins C, Kurmasheva RT, Houghton PJ, Smith MA. Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr Blood Cancer. 2013;60(8):1325–1332. doi: 10.1002/pbc.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong H, Choo EF, Alicke B, Ding X, La H, McNamara E, Theil FP, Tibbitts J, Friedman LS, Hop CE, Gould SE. Antitumor activity of targeted and cytotoxic agents in murine subcutaneous tumor models correlates with clinical response. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(14):3846–3855. doi: 10.1158/1078-0432.CCR-12-0738. [DOI] [PubMed] [Google Scholar]
- 12.Morton CL, Favours EG, Mercer KS, Boltz CR, Crumpton JC, Tucker C, Billups CA, Houghton PJ. Evaluation of ABT-751 against childhood cancer models in vivo. Investigational new drugs. 2007;25(4):285–295. doi: 10.1007/s10637-007-9042-y. [DOI] [PubMed] [Google Scholar]
- 13.Desai NP, Trieu V, Hwang LY, Wu R, Soon-Shiong P, Gradishar WJ. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anti-cancer drugs. 2008;19(9):899–909. doi: 10.1097/CAD.0b013e32830f9046. [DOI] [PubMed] [Google Scholar]
- 14.Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, Hawkins MJ, Sparreboom A, Figg WD. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(13):4200–4205. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz W, Reimann HJ, Schmal A, Dormann P, Schwarz B, Neugebauer E, Doenicke A. Histamine release in dogs by Cremophor E1 and its derivatives: oxethylated oleic acid is the most effective constituent. Agents and actions. 1977;7(1):63–67. doi: 10.1007/BF01964882. [DOI] [PubMed] [Google Scholar]
- 17.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Jr, Van Echo DA, Von Hoff DD, Leyland-Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7):1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 18.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, Iglesias JL, Renschler MF. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 20.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kottschade LA, Suman VJ, Amatruda T, 3rd, McWilliams RR, Mattar BI, Nikcevich DA, Behrens R, Fitch TR, Jaslowski AJ, Markovic SN. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma : a North Central Cancer Treatment Group Study, N057E(1) Cancer. 2011;117(8):1704–1710. doi: 10.1002/cncr.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 23.Lage H. An overview of cancer multidrug resistance: a still unsolved problem. Cellular and molecular life sciences : CMLS. 2008;65(20):3145–3167. doi: 10.1007/s00018-008-8111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner LM, Yin H, Eaves D, Currier M, Cripe TP. Preclinical evaluation of nanoparticle albumin-bound paclitaxel for treatment of pediatric bone sarcoma. Pediatr Blood Cancer. 2014 doi: 10.1002/pbc.25062. [DOI] [PubMed] [Google Scholar]
- 25.Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Tajbakhsh M, Reynolds CP, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Zeidner J, Wu J, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 26.Viudez A, Ramirez N, Hernandez-Garcia I, Carvalho FL, Vera R, Hidalgo M. Nab-paclitaxel: A flattering facelift. Critical reviews in oncology/hematology. 2014 doi: 10.1016/j.critrevonc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Peterson JK, Tucker C, Favours E, Cheshire PJ, Creech J, Billups CA, Smykla R, Lee FY, Houghton PJ. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(19 Pt 1):6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Agilent profiles for Caveolin-1 and SPARC for the in vitro and in vivo models used in the PPTP. The histograms show the relative expression is the ratio of the expression value for each xenograft divided by the median expression value for the entire panel.
Efficacy of Abraxane against PPTP Xenograft Models.
Summary of Pharmacokinetic Parameters.