Abstract
Background
Curative therapy for childhood sarcoma presents challenges when complete resection is not possible. Ionizing radiation (XRT) is used as a standard modality at diagnosis or recurrence for childhood sarcoma, however local recurrence is still problematic. Most childhood sarcomas are TP53 wild type at diagnosis, although approximately 5–10%have MDM2 amplification or overexpression.
Procedures
The MDM2 inhibitor, RG7388, was examined alone or in combination with XRT (20 Gy given in 2 Gy daily fractions) to immune-deficient mice bearing Rh18 (embryonal) or a total of 30 Gy in 2 Gy fractions to mice bearing Rh30 (alveolar) rhabdomyosarcoma xenografts. RG7388 was administered by oral gavage using two schedules (daily × 5; schedule 1 or once weekly; schedule 2). TP53, and TP53-responsive gene products (p21, PUMA, DDB2, MIC1) as well as markers of apoptosis, were analyzed.
Results
RG7388 showed no significant single agent antitumor activity. Twenty Gy XRT induced complete regressions (CR) of Rh18 with 100 percent tumor regrowth by week 7, but no tumor regrowth at 20 weeks when combined with RG7388. RG7388 enhanced time to recurrence combined with XRT in Rh30 xenografts compared to 30 Gy XRT alone. RG7388 did not enhance XRT-induced local skin toxicity. Combination treatments induced TP53 responsive genes more rapidly and to a greater magnitude than single agent treatments.
Conclusions
RG7388 enhanced the activity of XRT in both rhabdomyosarcoma models without increasing local XRT-induced skin toxicity. Changes in TP53-responsive genes were consistent with the synergistic activity of RG7388 and XRT in the Rh18 model.
Keywords: childhood sarcoma, MDM2 inhibitor, radiation treatment
Introduction
Rhabdomyosarcoma (RMS), a malignancy derived from primitive skeletal muscle precursor cells, is a rare cancer predominantly occurring in early childhood to young adulthood. There are approximately 350 new cases of pediatric rhabdomyosarcoma (RMS) diagnosed per year in the United States [1]. The 5-year overall survival (OS) rate for patients with RMS is 70% to 75% in the recent IRS and International Society of Pediatric Oncology (SIOP) study [2], a substantial improvement compared to the 55% 5-year survival in the first Intergroup study (IRS-I; [3]). However, control of the primary site is still a major source of treatment failure. In the Intergroup RMS Study-IV approximately two-thirds of relapses were local-regional [4], suggesting an inherent resistance to radiotherapy in this childhood malignancy.
The two predominant forms of RMS are embryonal and alveolar. Embryonal RMS is characterized by frequent loss of heterozygosity at 11p15 [5,6] with paternal disomy and overexpression of IGF-2 through loss of imprinting of the 11p15 locus [7]. The alveolar subtype is characterized by reciprocal translocations between chromosomes 2 and 13 [t(2;13)] [8] that encodes Pax3/FOXO1 or chromosomes 1 and 13 [t(1;13)] [9] that encodes Pax7/FOXO1 (termed fusion positive) [10]. With the exception of young patients with TP53 germline mutations [11], TP53 mutations in RMS are relatively rare, around 5% whereas amplification of MDM2 has a slightly greater incidence [12].
Reconstitution of a functional p53 pathway is an attractive anticancer strategy. The interactions between p53 and its two principal regulatory molecules (MDM2/MDM4) involve a large protein-protein interface hence it was for some time considered a difficult target for pharmacological intervention [13]. Recently drugs have been developed that effectively inhibit the MDM2-mediated degradation of p53 or inhibition of MDM2 transcription [14]. Among these, compounds with quite diverse structures have been investigated, including peptides, chalcones [15], spiro-oxindoles [16], benzodiazepinediones [17], the compound RITA [18], and cis-imidazolines (Nutlins) [14]. Most of these compounds exhibited in vitro activity, while the Nutlins have also demonstrated impressive activity in animal models with limited toxicity [14]. The cis-imidazoline, RG7112, is an orally available inhibitor of the MDM2-p53 interaction [19] that entered clinical trials. Dose limiting toxicity was neutropenia/thrombocytopenia in sarcoma patients [20] and thrombocytopenia in combination with cytarabine in AML patients [21]. RG7112 has been shown to promote apoptosis of megakaryocyte progenitor cells, and also affected mature megakaryocytes by blocking DNA synthesis during endomitosis and impairing platelet production, providing an explanation for RG7112-induced thrombocytopenia [22]. The finding of the p53-MDM2 auto-regulatory loop in normal megakaryocytopoiesis suggests that thrombocytopenia may be an on-target toxicity associate with targeting the MDM2-TP53 interaction, potentially making combination of these agents with hemato-toxic therapies a challenge.
In preclinical studies, RG7112 showed excellent activity against childhood acute leukemia models, but rather disappointing single agent activity against a range of childhood solid tumor xenografts [23]. Because of its hemato-toxicity this agent may be a challenge to combine with current chemotherapy regimens used for treatment of childhood solid tumors. However, conceptually, activation of TP53 should enhance cell killing by agents such as ionizing radiation (XRT). Small molecule inhibitors of MDM2 have been shown to enhance radiation sensitivity in cell culture [24–26] whereas mixed backbone oligonucleotides were also shown to increase radiation-induced tumor inhibition in vivo [27]. RG7388, is a second generation agent based upon a pyrrolidine scaffold, that blocks the MDM2-TP53 interaction, leading to activation of TP53 and downstream genes. RG7388 is more potent and selective than RG7112, having good oral bioavailability and a superior pharmacokinetic profile compared to RG7112 [28]. Here we have evaluated RG7388 in combination with daily fractionated XRT against two models of childhood rhabdomyosarcoma.
Materials and Methods
In vivo studies
The patient derived rhabdomyosarcoma xenografts Rh18 (embryonal histology, fusion negative) and Rh30 (alveolar histology, fusion positive) have been described previously [29]. CB17 SC female mice bearing each xenograft line were dosed orally with RG7388 on two schedules. For Schedule 1 doses of 40 or 80 mg/kg were administered daily for 5 days. For Schedule 2, mice received 100 mg/kg or 100 mg/kg BID once per week for three consecutive weeks. Drug dosing was given 2–4 hr before XRT. Mice received fractionated flank-irradiation treatments in clinically-relevant 2-Gy daily doses as previously described [30,31]. Mice were monitored daily for skin reaction starting on day 15 from the first day of irradiation treatments through day 43. A numeric grade is given based on a rating system according to the severity of the reaction as described previously [32] Dose density, complete response (CR) rates, recurrence rates, 20-week failure rates, event-free survival, and treatment related events were assessed as previously described [30,32]. The experiment design was fully discussed and approved by the National Radiation Group (NRG) Oncology and Translational Research program (TRP) of the sarcoma working group.
Pharmacodynamic studies
The samples were ground under liquid nitrogen and stored at −80°C. 50 mg of samples was lysed with 400 μl of cell lysis buffer and spun through qiashredder to homogenize. For conventional western blotting 20 μg of lysate was run on a 4–12% Bis-Tris gel and transferred to PVDF membrane with an iBlot. The membranes were probed with appropriate antibodies and chemiluminescent substrate. p53 (2527), PUMA (12450), PARP (9532), Cleaved Caspase 3 (9661), and Mic1 (8479) were obtained from Cell Signaling Technologies (Danvers, MA). MDM2 (TA311996) was obtained from Origene.
For capillary electrophoresis separation (Protein Simple, Bio-Techne, Minneapolis, MN lysate was diluted to 2 mg/ml and electrophoresed as recommended by the manufacturer. Proteins were immobilized to the capillary and identified using a primary antibody and an HRP-conjugated secondary antibody and chemiluminescent substrate. The signal is detected and quantitated. Antibodies against DDB2 (5416), p21 (2947) and GAPDH (5174) were obtained from Cell Signaling Technologies.
Statistical Analysis
For in vivo testing xenograft models, criteria for defining an event (4 times the tumor volume at the start of treatment) were similar to that used by the Pediatric Preclinical Testing Program [29]. Log-rank test was used to compare the time-to-event curves between groups. The comparison of cumulative tumor volumes between treatment groups was conducted by using analysis of variance (ANOVA) and Holm’s method was used to adjust for multiplicity within each xenograft model. SAS 9.3 was used for this analysis (SAS, Inc.).
Results
Sensitivity to XRT
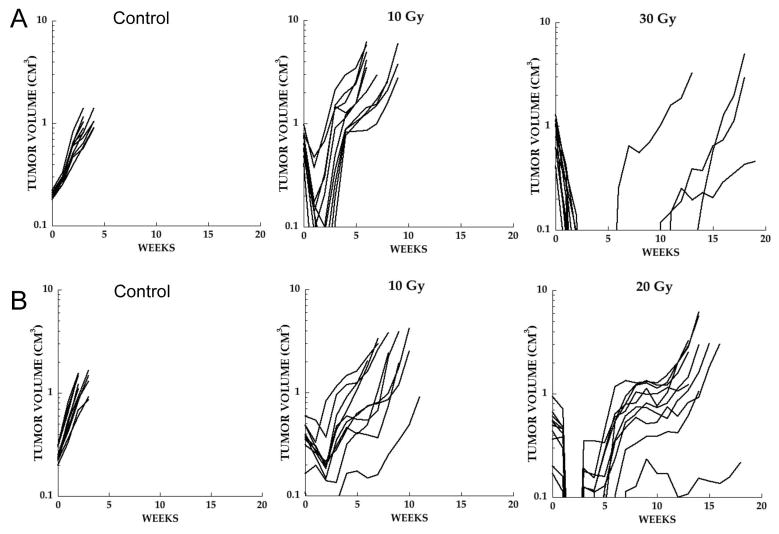
Ionizing radiation treatment was given as 2 Gy fractions 5-days per week. For Rh18 xenografts we assessed the response to 10 and 30 Gy, Figure 1A. With increasing dosage there was an increased number of tumors with complete regression (CR). Following 10 Gy there was transient regression of all tumors with rapid regrowth. In contrast, at 30 Gy only 4 of 10 tumors regrew with a median time to regrowth of 10 weeks. For Rh18 a dose of 20 Gy was selected for combination studies. Rh30 xenografts were more radio-resistant, Figure 1B. At 20 Gy 10 of 10 tumors recurred within 7 weeks. Because of the rapid relapse following 20 Gy in this model, 30 Gy was chosen as the dose to combine with XRT.
Figure 1.
Dose response to XRT given in daily 2 Gy fractions; tumor bearing mice received no treatment (Control), or received 2 Gy five-days per week until a cumulative dose of 10, 20 or 30 Gy was achieved. A. Rh18; B. Rh30.
Combination studies
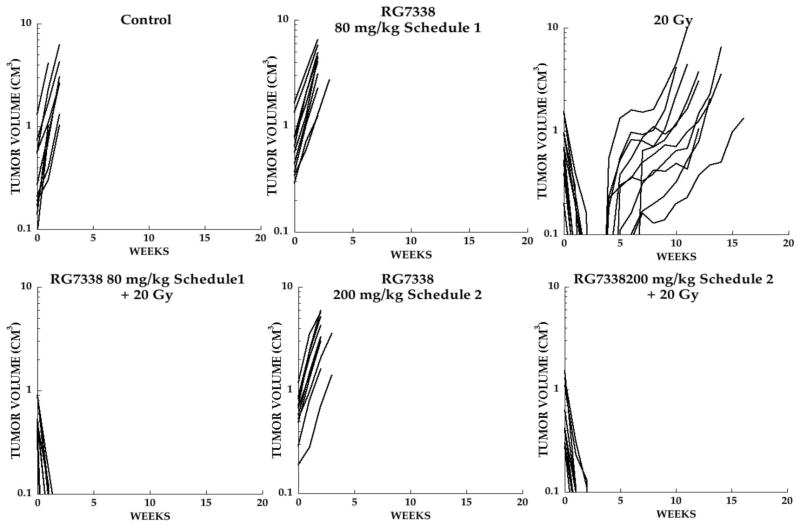
RG7388 was well tolerated at 80 mg/kg on the daily × 5 schedule (schedule 1) and once weekly × 2 at 200 mg/kg (given as split doses 12 hr apart; Schedule 2). RG7388 was administered 2 hr before XRT (2 Gy) daily for 5 days (Schedule 1) and a split dose given 12 hr and 2 hr pre-irradiation (Schedule 2). Consistent with our previous study of RG7112 [23], RG7388, as a single agent, had no significant effect on the growth of Rh18 xenografts administered on either schedule (P=0.8959 and P=0.5011 for Schedule 1 and 2, respectively), Figure 2. Radiation treatment (20 Gy) induced complete regressions followed by regrowth of all tumors with the median event time of 89.6 days compared to control tumors that evented at day 9.5 (P=0.0287). In contrast combination of XRT (20 Gy) with either 80 mg/kg RG7388 (Schedule 1) or 200 mg/kg (Schedule 2) resulted in complete tumor regressions with no regrowth of tumors during the 19 weeks of observation. Thus, RG7388 given on either schedule significantly potentiated XRT (P<0.0001 for both schedules vs control).
Figure 2.
Responses of Rh18 xenografts. Individual growth curves for Rh18 rhabdomyosarcoma xenografts treated with XRT daily × 5 (2 Gy fractions), with or without RG7388. Mice received no treatment (Control), RG7388 (80 mg/kg daily × 5; schedule 1), or 200 mg/kg weekly × 2 (Schedule 2), XRT alone (20 Gy) or XRT combined with RG7388 on either schedule. Tumor diameters were measured weekly.
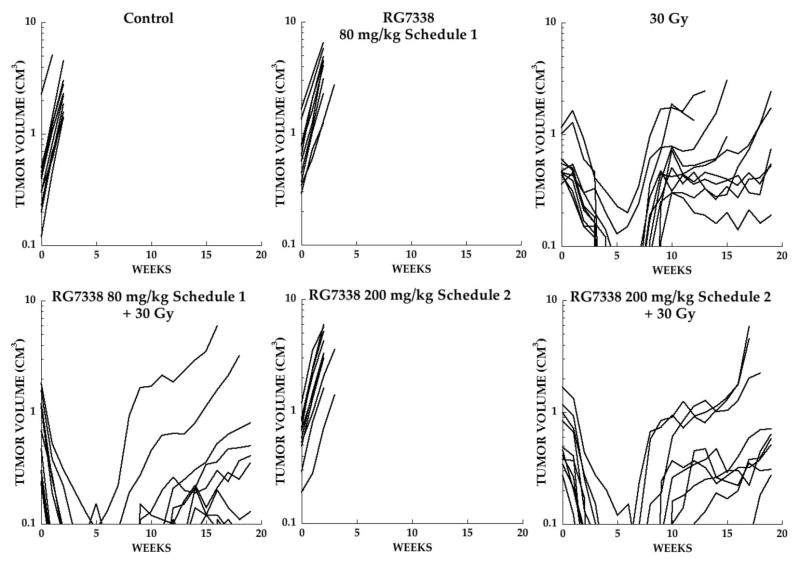
For Rh30 combination studies the dose of XRT was increased to 30 Gy. XRT alone induced CR in 8 of 10 mice with a median time to recurrence (>0.1cm3) of 7.5 weeks. In contrast, all tumors demonstrated CR (<0.1 cm3), and only 6 of 10 mice the combination group on schedule 1 showed consistent regrowth (median time to regrow ~9 weeks). The combination of XRT on Schedule 2 also showed all tumors in CR but all recurred within the period of observation (19 weeks), with a median time to recurrence of 9 weeks. As shown in Figure 3, regrowth of Rh30 tumors following 30 Gy showed a plateau in growth before tumors evented (4X pretreatment volume). Similarly tumors regrowing after combination treatment showed poor or irregular regrowth. We found that the cumulative tumor volumes at the end of the period of observation (day 134) was significantly smaller for the combination group in Schedule 1 (P=0.0005) and Schedule 2 (P=0.0029) when compared with tumors receiving 30 Gy alone, Supplemental Figure 1A. Kaplan-Meier analysis for Rh18 and Rh30 xenografts are presented in Supplemental Figure 1B.
Figure 3.
Responses of Rh30 xenografts. Individual growth curves for Rh30 rhabdomyosarcoma xenografts treated with XRT five-days per week (2 Gy fractions), with or without RG7388. Mice received no treatment (Control), RG7388 (80 mg/kg daily × 5; schedule 1), or 200 mg/kg weekly × 2 (Schedule 2), XRT alone (30 Gy) or XRT combined with RG7388 on either schedule. Tumor diameters were measured weekly.
Pharmacodynamic studies
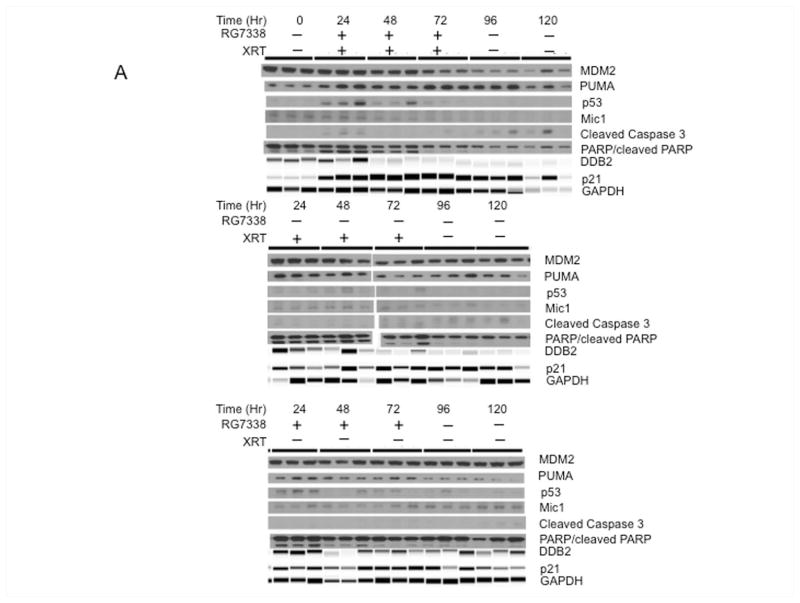
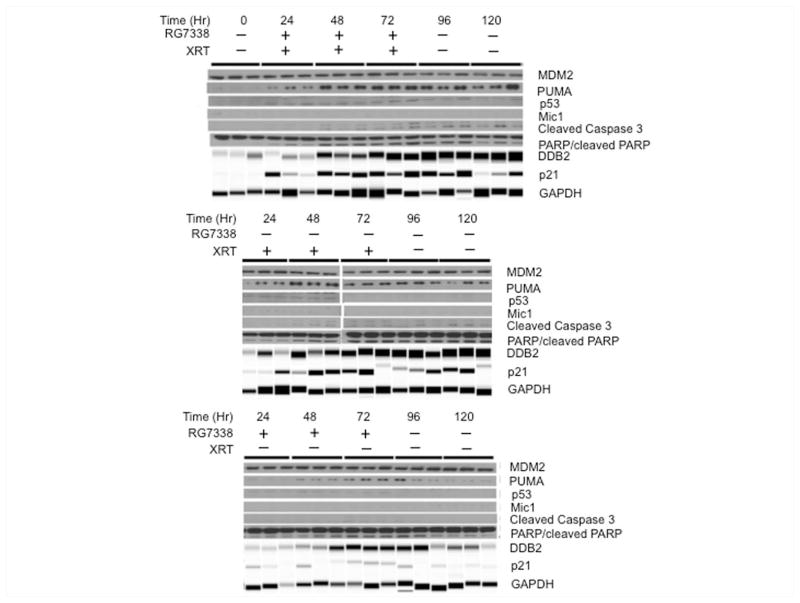
Tumor samples were derived from untreated tumors, or tumors following 2, 4 or 6 Gy XRT and 24 and 48 hr after the last XRT fraction with or without RG7388, or at the same time points from mice treated for 3 days with RG7388 alone (80 mg/kg). As the mechanism of RG7388 is to prevent MDM2-induced proteolysis of TP53, the action of this drug should manifest in upregulation of TP53 responsive gene products p21, PUMA and DDB2 [33,34]. Combination treatment slightly increased detection of TP53 at 24 Hr. DDB2, a TP53 responsive gene is reported to enhance the proteolysis of p21, and low levels of DDB2 have been reported to confer resistance to DNA damage through increased p21 accumulation [35]. In Rh18 xenografts, RG7388/XRT or XRT alone slightly induced DDB2 by day 1 of treatment, but thereafter levels were lower than controls. Levels of DDB2 slightly increased at 24 hr, but subsequently decreased slightly in tumors treated with RG7388 alone, Figure 4. RG7388/XRT increased p21 levels over the 3 days of treatment, and p21 decreased by 48 Hr after the last dose, although by this time GAPDH (and tubulin, not shown) had decreased suggesting few viable cells remained in tumor tissue following combination treatment. XRT alone induced p21 with maximal levels achieved after 6 Gy (day 3), although p21 remained relatively stable up to 48 hr post XRT, whereas p21 was maximal at 3 days in tumors treated with RG7388 alone. Both XRT and combination treatments induced PARP cleavage over the first 48 hr, although caspase 3 and cleaved caspase 3 were not detected in Rh18 xenografts. Similarly, macrophage inhibitory cytokine (Mic1), a marker of sensitivity to MDM2 inhibitors [36] was not detected.
Figure 4.
Pharmacodynamic responses for Rh18 xenografts. Tumor-bearing mice were administered RG7388 (80 mg/kg) daily for 3 days with or without XRT (2 Gy daily × 3). Tumors were harvested at the indicated times during treatment and 24 and 48 Hr after the final dose of XRT. DDB2, p21 and GAPDH were determined using a Protein Simple machine, other samples were determined using conventional immunoblotting as described in Materials and Methods.
Pharmacodynamic changes induced by each treatment were similar in Rh30 xenografts to those determined in Rh18 xenografts. One notable exception was the greater induction of DDB2 which persisted in XRT and combination treated tumors. In contrast, RG7388 induced DDB2 relatively slowly being maximal between 48–96 hr. TP53, MIC1 and Caspase 3 were not detected in Rh30 xenografts under control or treatment conditions, Figure 5.
Figure 5.
Pharmacodynamic responses for Rh30 xenografts. Tumor-bearing mice were administered RG7388 (80 mg/kg) daily for 3 days with or without XRT (2 Gy daily × 3). Tumors were harvested at the indicated times during treatment and 24 and 48 Hr after the final dose of XRT. DDB2, p21 and GAPDH were determined using a Protein Simple machine, other samples were determined using conventional immunoblotting as described in Materials and Methods.
Local XRT-induced skin toxicity was assessed using a scale of damage previously described [32]. Skin toxicity scores for treatment groups receiving cumulative doses of XRT at 10, 20, or 30 Gy were compared to mice receiving RG7388 (80 mg/kg, schedule 1 or 200 mg/kg, schedule 2) combined with 20 Gy (Rh18) or 30 Gy (Rh30). Overall, the average maximum skin toxicity scores were slightly lower in combination treatments compared to XRT alone, Figure 6.
Figure 6.
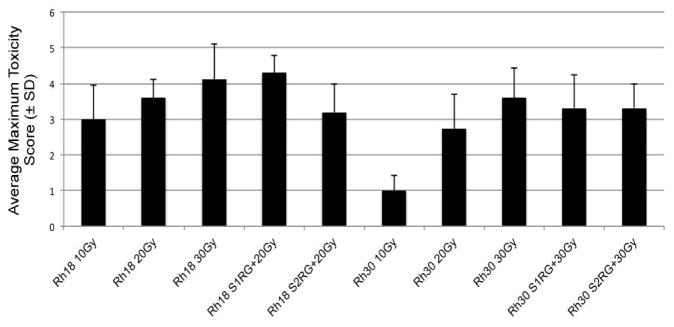
Skin toxicity was assessed using the scoring system: 0 = No visible reaction; 1 = Faint erythema and/or faint dry desquamation, 2 = Patchy dry desquamation; 3 = Confluent dry desquamation; 4 = Patchy moist desquamation; 5 = Confluent moist desquamation.
Discussion
In a previous study, we reported that RG7112, an inhibitor of MDM2-TP53 interaction, had very significant activity against xenograft models of childhood acute lymphoblastic leukemia, but rather modest activity against childhood solid tumors causing regressions in only 5 of 26 models with wild type p53. In contrast, TP53 wild type cell lines derived from childhood solid tumors were equally sensitive to leukemia cell lines to RG7112 in vitro [23]. The reason for the lack of antitumor activity for RG7112 against solid tumors in mice is unknown. Several studies have reported the synergy between MDM2-TP53 inhibitors and cytotoxic chemotherapy, however these studies were also restricted to hematopoietic cells in vitro [37–39]. In several of these studies, normal bone marrow cells appeared relatively resistant compared to the malignant cells. Against retinoblastoma cells, in vitro, nutlin-3 enhanced the cytotoxic effect of topotecan, and showed synergistic activity when administered with topotecan that was given by subconjunctival administration in a mouse model of retinoblastoma [40]. The cyclin-dependent kinase inhibitor, roscovitin, also potently synergized with nutlin-3a [41]. In mouse models nutlins and RG7112 have shown no significant toxicity. However, the phase I trial of RG7112 revealed a high incidence of thrombocytopenia and neutropenia as dose limiting toxicities [42]. Of note, this study evaluated RG7112 in patients with liposarcoma, a tumor with frequent amplification of MDM2, and having wild type TP53 [43]. Clinical benefit was modest, with one partial regression (5%) and stable disease in 14 of the 20 patients. Thus this clinical experience is similar to that predicted from pediatric preclinical solid tumor models suggesting only modest single agent activity, despite wild type TP53 status.
The marked thrombocytopenia and neutropenia manifest in the clinical trial of liposarcoma, raises concerns regarding the combination of MDM2 inhibitors with chemotherapeutic agents that induce hematopoietic toxicity. Similarly, there is the potential that stabilizing TP53 may induce toxicity in radiosensitive tissues [24], although it has been postulated that the effectiveness of ionizing radiation could be improved by inhibition of MDM2 [25]. Antisense MDM2 enhanced radiation sensitivity [26], and the MDM2 inhibitor PXN727 radio-sensitized HCT-116 TP53 wild type cells but not those deleted for TP53 [44]. Our study would support the concept that a small molecule inhibitor of MDM2 can enhance XRT given in clinically relevant daily fractions. Consistent with our previous study with RG7112, there was little single agent activity for RG7388 on either the 5-day or weekly schedule against Rh18 (MDM2 amplified, wild type TP53) or Rh30 (wild type TP53) rhabdomyosarcoma models. RG7388 is approximately 10-fold more potent in inhibiting MDM2 interaction with TP53 [28], thus the failure to demonstrate single agent activity for both RG7112 and RG7388 requires further study in these models. However, the potentiation of XRT by RG7388 was quite dramatic in Rh18 xenografts, on both schedules, whereas it was statistically significant but less obvious in the Rh30 model. Pharmacodynamic results indicate that the induction of TP53 genes in tumors treated with RG7112 + XRT was significantly greater than after XRT or RG7388. The induction of p21 was greatest in combination treated tumors, although it is difficult to assess the magnitude in Rh18 xenografts due to cell death during the initial period of treatment. We used an index for skin toxicity to see if the effects of XRT were exacerbated by concurrent RG7388 treatment. Mouse skin has long been used to study basic radiation biology principles - such as the shape of the radiation survival curve at low radiation doses [45] or the effect of changes in repopulation during fractionated irradiation [46]. These principles apply to human skin treated with radiotherapy. The skin of severe combined immune-deficient mice, in which these xenografts are propagated, is also hypersensitive to XRT [47]. Thus, although extrapolation from mouse toxicity data to clinical application can be problematic, the data showing no enhancement of toxicity to skin in a mouse strain where skin is hypersensitive to XRT, suggests that this may not be an issue in human trials.
In summary, RG7388 treatment enhanced the antitumor activity of daily-fractionated radiation treatments in two models of childhood rhabdomyosarcoma without exacerbating radiation-induced skin toxicity. Although further preclinical studies are required to extend these results to other tumors, the combination may increase the local control for rhabdomyosarcoma compared to XRT alone.
Supplementary Material
Supplemental Figure 1 A. Volumes of tumors at day 134 in groups treated with XRT alone (30 Gy), or XRT with RG7388 on schedule 1 or schedule 2. The line marks the mean tumor volume in each group. B. Kaplan-Meier plots for event-free survival in Rh18 and Rh30 experiments.
Acknowledgments
This work was supported by Hoffmann-La Roche Inc, and awards CA165995 and CA169368 for the NCI. We thank Christopher E. Pelloski, MD for valuable discussions in planning these studies, and valuable input from J. Martin Brown, PhD (Stanford University)
Footnotes
Conflict of Interest: Dr. Stephen Middleton is an employee of Hoffmann LaRoche Inc. Other authors have no conflict of interest.
References
- 1.Ognjanovic S, Linabery AM, Charbonneau B, Ross JA. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975–2005. Cancer. 2009;115(18):4218–4226. doi: 10.1002/cncr.24465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Missiaglia E, Williamson D, Chisholm J, Wirapati P, Pierron G, Petel F, Concordet JP, Thway K, Oberlin O, Pritchard-Jones K, Delattre O, Delorenzi M, Shipley J. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(14):1670–1677. doi: 10.1200/JCO.2011.38.5591. [DOI] [PubMed] [Google Scholar]
- 3.Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, Heyn R, Lawrence W, Newton W, Ortega J, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61(2):209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Davicioni E, Anderson MJ, Finckenstein FG, Lynch JC, Qualman SJ, Shimada H, Schofield DE, Buckley JD, Meyer WH, Sorensen PH, Triche TJ. Molecular classification of rhabdomyosarcoma--genotypic and phenotypic determinants of diagnosis: a report from the Children’s Oncology Group. The American journal of pathology. 2009;174(2):550–564. doi: 10.2353/ajpath.2009.080631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scrable HJ, Witte DP, Lampkin BC, Cavenee WK. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Sijmons C, Bras J, Arceci RJ, Godfried M, Valentijn LJ, Voute PA, Baas F. Allelotype of pediatric rhabdomyosarcoma. Oncogene. 1997;15(11):1309–1314. doi: 10.1038/sj.onc.1201302. [DOI] [PubMed] [Google Scholar]
- 7.Zhan S, Shapiro DN, Helman LJ. Activation of an imprinted allele of the insulin-like growth factor II gene implicated in rhabdomyosarcoma. The Journal of clinical investigation. 1994;94(1):445–448. doi: 10.1172/JCI117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglass EC, Valentine M, Etcubanas E, Parham D, Webber BL, Houghton PJ, Houghton JA, Green AA. A specific chromosomal abnormality in rhabdomyosarcoma. Cytogenetics and cell genetics. 1987;45(3–4):148–155. doi: 10.1159/000132446. [DOI] [PubMed] [Google Scholar]
- 9.Biegel JA, Meek RS, Parmiter AH, Conard K, Emanuel BS. Chromosomal translocation t(1;13)(p36;q14) in a case of rhabdomyosarcoma. Genes, chromosomes & cancer. 1991;3(6):483–484. doi: 10.1002/gcc.2870030612. [DOI] [PubMed] [Google Scholar]
- 10.Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer research. 1994;54(11):2869–2872. [PubMed] [Google Scholar]
- 11.Diller L, Sexsmith E, Gottlieb A, Li FP, Malkin D. Germline p53 mutations are frequently detected in young children with rhabdomyosarcoma. The Journal of clinical investigation. 1995;95(4):1606–1611. doi: 10.1172/JCI117834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor AC, Shu L, Danks MK, Poquette CA, Shetty S, Thayer MJ, Houghton PJ, Harris LC. P53 mutation and MDM2 amplification frequency in pediatric rhabdomyosarcoma tumors and cell lines. Medical and pediatric oncology. 2000;35(2):96–103. doi: 10.1002/1096-911x(200008)35:2<96::aid-mpo2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Shangary S, Wang S. Small-Molecule Inhibitors of the MDM2-p53 Protein-Protein Interaction to Reactivate p53 Function: A Novel Approach for Cancer Therapy. Annual Review of Pharmacology and Toxicology. 2009;49(1):223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In Vivo Activation of the p53 Pathway by Small-Molecule Antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 15.Stoll R, Renner C, Hansen S, Palme S, Klein C, Belling A, Zeslawski W, Kamionka M, Rehm T, Mühlhahn P, Schumacher R, Hesse F, Kaluza B, Voelter W, Engh RA, Holak TA. Chalcone Derivatives Antagonize Interactions between the Human Oncoprotein MDM2 and p53a. Biochemistry. 2000;40(2):336–344. doi: 10.1021/bi000930v. [DOI] [PubMed] [Google Scholar]
- 16.Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S. Structure-Based Design of Spiro-oxindoles as Potent, Specific Small-Molecule Inhibitors of the MDM2-p53 Interaction. Journal of Medicinal Chemistry. 2006;49(12):3432–3435. doi: 10.1021/jm051122a. [DOI] [PubMed] [Google Scholar]
- 17.Parks DJ, LaFrance LV, Calvo RR, Milkiewicz KL, Gupta V, Lattanze J, Ramachandren K, Carver TE, Petrella EC, Cummings MD, Maguire D, Grasberger BL, Lu T. 1,4-Benzodiazepine-2,5-diones as small molecule antagonists of the HDM2-p53 interaction: discovery and SAR. Bioorganic & Medicinal Chemistry Letters. 2005;15(3):765–770. doi: 10.1016/j.bmcl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LGGC, Masucci M, Pramanik A, Selivanova G. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10(12):1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 19.Tovar C, Graves B, Packman K, Filipovic Z, Higgins B, Xia M, Tardell C, Garrido R, Lee E, Kolinsky K, To KH, Linn M, Podlaski F, Wovkulich P, Vu B, Vassilev LT. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer research. 2013;73(8):2587–2597. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 20.Kurzrock R, Blay J-Y, Nguyen BB, Wagner AJ, Maki RG. A phase I study of MDM2 antagonist RG7112 in patients (pts) with relapsed/refractory solid tumors. J Clinical Oncology. 2012;30(Suppl):abstract 13600. [Google Scholar]
- 21.Yee k, Martinelli G, Assouline S, Kasner M. Phase 1b Study Of The MDM2 Antagonist RG7112 In Combination With 2 Doses/Schedules Of Cytarabine. Blood. 2013;122(21) [Google Scholar]
- 22.Iancu-Rubin C, Mosoyan G, Glenn K, Gordon RE, Nichols GL, Hoffman R. Activation of p53 by the MDM2 inhibitor RG7112 impairs thrombopoiesis. Experimental hematology. 2014;42(2):137–145. e135. doi: 10.1016/j.exphem.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Carol H, Reynolds CP, Kang MH, Keir ST, Maris JM, Gorlick R, Kolb EA, Billups CA, Geier B, Kurmasheva RT, Houghton PJ, Smith MA, Lock RB. Initial testing of the MDM2 inhibitor RG7112 by the Pediatric Preclinical Testing Program. Pediatric blood & cancer. 2013;60(4):633–641. doi: 10.1002/pbc.24235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443(7108):214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 25.Impicciatore G, Sancilio S, Miscia S, Di Pietro R. Nutlins and ionizing radiation in cancer therapy. Current pharmaceutical design. 2010;16(12):1427–1442. doi: 10.2174/138161210791033932. [DOI] [PubMed] [Google Scholar]
- 26.Luo H, Yount C, Lang H, Yang A, Riemer EC, Lyons K, Vanek KN, Silvestri GA, Schulte BA, Wang GY. Activation of p53 with Nutlin-3a radiosensitizes lung cancer cells via enhancing radiation-induced premature senescence. Lung Cancer. 2013;81(2):167–173. doi: 10.1016/j.lungcan.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Wang H, Prasad G, Li M, Yu D, Bonner JA, Agrawal S, Zhang R. Radiosensitization by antisense anti-MDM2 mixed-backbone oligonucleotide in in vitro and in vivo human cancer models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(4):1263–1273. doi: 10.1158/1078-0432.ccr-0245-03. [DOI] [PubMed] [Google Scholar]
- 28.Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, Chu XJ, Bartkovitz D, Podlaski F, Janson C, Tovar C, Filipovic ZM, Higgins B, Glenn K, Packman K, Vassilev LT, Graves B. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. Journal of medicinal chemistry. 2013;56(14):5979–5983. doi: 10.1021/jm400487c. [DOI] [PubMed] [Google Scholar]
- 29.Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Tajbakhsh M, Reynolds CP, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Zeidner J, Wu J, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatric blood & cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 30.Kaplon R, Hadziahmetovic M, Sommerfeld J, Bondra K, Lu L, Leasure J, Nguyen P, McHugh K, Li N, Chronowski C, Sebastian N, Singh M, Kurmasheva R, Houghton P, Pelloski CE. The application of radiation therapy to the Pediatric Preclinical Testing Program (PPTP): results of a pilot study in rhabdomyosarcoma. Pediatric blood & cancer. 2013;60(3):377–382. doi: 10.1002/pbc.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu L, Bondra K, Gupta N, Sommerfeld J, Chronowski C, Leasure J, Singh M, Pelloski CE. NanoDots Dosimetry for RS 2000 X-Ray Biological Irradiator. Int J Radiat Biol. 2013 doi: 10.3109/09553002.2013.817703. [DOI] [PubMed] [Google Scholar]
- 32.Singh M, Leasure JM, Chronowski C, Geier B, Bondra K, Duan W, Hensley LA, Villalona-Calero M, Li N, Vergis AM, Kurmasheva RT, Shen C, Woods G, Sebastian N, Fabian D, Kaplon R, Hammond S, Palanichamy K, Chakravarti A, Houghton PJ. FANCD2 is a potential therapeutic target and biomarker in alveolar rhabdomyosarcoma harboring the PAX3-FOXO1 fusion gene. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(14):3884–3895. doi: 10.1158/1078-0432.CCR-13-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 34.Tan T, Chu G. p53 Binds and activates the xeroderma pigmentosum DDB2 gene in humans but not mice. Molecular and cellular biology. 2002;22(10):3247–3254. doi: 10.1128/MCB.22.10.3247-3254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoyanova T, Roy N, Kopanja D, Bagchi S, Raychaudhuri P. DDB2 decides cell fate following DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(26):10690–10695. doi: 10.1073/pnas.0812254106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chawla SP, Blay J-Y, Italiano A, Gutierrez M, Le Cesne A. Phase 1b study of RG7112 with doxorubicin (D) in advanced soft tissue sarcoma (ASTS) Jounal of Clinical Oncology. 2013;31(Suppl):abstract 10514. [Google Scholar]
- 37.Stuhmer T, Chatterjee M, Hildebrandt M, Herrmann P, Gollasch H, Gerecke C, Theurich S, Cigliano L, Manz RA, Daniel PT, Bommert K, Vassilev LT, Bargou RC. Nongenotoxic activation of the p53 pathway as a therapeutic strategy for multiple myeloma. Blood. 2005;106(10):3609–3617. doi: 10.1182/blood-2005-04-1489. [DOI] [PubMed] [Google Scholar]
- 38.Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, Ruvolo V, Tsao T, Zeng Z, Vassilev LT, Andreeff M. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106(9):3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de Frias M, Castano E, Campas C, Barragan M, de Sevilla AF, Domingo A, Vassilev LT, Pons G, Gil J. MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood. 2006;107(10):4109–4114. doi: 10.1182/blood-2005-08-3273. [DOI] [PubMed] [Google Scholar]
- 40.Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, Teunisse A, Lam S, Ramos Y, Mohan A, Johnson D, Wilson M, Rodriguez-Galindo C, Quarto M, Francoz S, Mendrysa SM, Guy RK, Marine JC, Jochemsen AG, Dyer MA. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444(7115):61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- 41.Cheok CF, Dey A, Lane DP. Cyclin-dependent kinase inhibitors sensitize tumor cells to nutlin-induced apoptosis: a potent drug combination. Molecular cancer research : MCR. 2007;5(11):1133–1145. doi: 10.1158/1541-7786.MCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 42.Ray-Coquard I, Blay JY, Italiano A, Le Cesne A, Penel N, Zhi J, Heil F, Rueger R, Graves B, Ding M, Geho D, Middleton SA, Vassilev LT, Nichols GL, Bui BN. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. The Lancet Oncology. 2012;13(11):1133–1140. doi: 10.1016/S1470-2045(12)70474-6. [DOI] [PubMed] [Google Scholar]
- 43.Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic acids research. 1998;26(15):3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schilling D, Duwel M, Molls M, Multhoff G. Radiosensitization of wildtype p53 cancer cells by the MDM2-inhibitor PXN727 is associated with altered heat shock protein 70 (Hsp70) levels. Cell stress & chaperones. 2013;18(2):183–191. doi: 10.1007/s12192-012-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas BG, Fowler JF. The effect of multiple small doses of x rays on skin reactions in the mouse and a basic interpretation. Radiation research. 1976;66(2):401–426. [PubMed] [Google Scholar]
- 46.Denekamp J. Changes in the rate of repopulation during multifraction irradiation of mouse skin. The British journal of radiology. 1973;46(545):381–387. doi: 10.1259/0007-1285-46-545-381. [DOI] [PubMed] [Google Scholar]
- 47.Biedermann KA, Sun JR, Giaccia AJ, Tosto LM, Brown JM. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 A. Volumes of tumors at day 134 in groups treated with XRT alone (30 Gy), or XRT with RG7388 on schedule 1 or schedule 2. The line marks the mean tumor volume in each group. B. Kaplan-Meier plots for event-free survival in Rh18 and Rh30 experiments.