Abstract
Objective
This study examined associations between maternal lipid levels at mid-pregnancy and preterm delivery, medically indicated or spontaneous.
Design
Prospective cohort study.
Setting
Women were recruited from 52 clinics in five Michigan, U.S.A communities (1998–2004).
Population
Pregnant women were enrolled at 15–27 weeks’ gestation and followed to delivery (n=3019).
Methods
A single blood sample was obtained at study enrollment. Blood lipids, i.e., total (TC), high-density lipoprotein (HDLc), low-density lipoprotein (LDLc) cholesterol, and triglycerides (TG), were measured on a subcohort (n=1,309).
Main Outcome Measures
There were 221 spontaneous, 100 medically indicated preterm deliveries and 988 term deliveries. Polytomous logistic regression models examined relations among cholesterol levels (Low: <10th %tile, Referent: 10th–<70th %tile, High: ≥70th %tile), quartiles of TG (Referent: first quartile) and delivery outcome (Referent: term).
Results
Odds of medically indicated preterm delivery were increased among women with low TC (adjusted odds ratio (aOR)= 2.04, 95% confidence interval (CI): 1.12,3.72), low HDLc (aOR=1.89, 95%CI: 1.04,3.42), or low LDLc (aOR=1.96, 95%CI: 1.09,3.54). Odds of spontaneous preterm delivery were increased among women with high TC (aOR=1.51, 95%CI: 1.06,2.15), high LDLc (aOR=1.42, 95%CI: 0.99,2.04) or high TG (aOR=1.90, 95%CI: 1.21,2.97 and aOR=1.72, 95%CI: 1.06,2.78 for third and fourth quartiles, respectively).
Conclusions
Extremely low TC, HDLc and LDLc were associated with a modest increase in risk of medically indicated preterm delivery, while high TC, LDLc and TG modestly increased risk of spontaneous preterm delivery. Further research is needed to uncover explanations for these associations and to identify optimal ranges for maternal lipids.
Keywords: cholesterol, hypercholesterolemia, dyslipidemia, premature birth, prenatal
Introduction
The rate of preterm delivery has steadily increased in the USA for the past two decades, but has remained at ~12–13% since 2005 (1). Preterm delivery (PTD) accounts for ~69% of perinatal mortality (2), and a substantial proportion of long-term morbidity (3, 4). Socioeconomic and racial disparities continue to exist, with African-Americans having the highest rates of PTD (18%) (1). About 30–35% of PTD are medically indicated, with the remaining precipitated by spontaneous preterm labor or premature rupture of membranes (5).
Reasons for PTD remain unclear, although maternal vascular disturbances and infection/inflammation have been implicated (5). Recently, studies have begun to examine maternal lipid profiles during pregnancy in relation to PTD. Maternal levels of total cholesterol (TC), high-density lipoprotein (HDLc), and low-density lipoprotein (LDLc) cholesterol, as well as triglycerides (TG), all increase in the 2nd and 3rd trimesters as a normal physiological response to pregnancy (6). Increased lipid levels contribute towards hormonal and nutritional support of a healthy pregnancy (7); however, extremely high levels may induce oxidative stress and have been linked to poorer birth outcomes in animal models (8) and atherosclerosis in human offspring (9, 10).
To date, seven studies have reported on maternal lipids in association with PTD risk. It was first noted that women on a low-cholesterol diet during pregnancy were significantly less likely to deliver preterm (11). Since then, studies have found that risk of spontaneous PTD was increased in association with higher levels of TC or TG and high non-HDLc (12–14), but decreased in association with high levels of HDLc (upper quartile) (15). One study also demonstrated that both low (< 10th percentile) and high (≥90th percentile) levels of TC were linked to increased odds of PTD; however, this study did not separate medically indicated and spontaneous PTD (16).
Previous work on the relation between maternal lipids and risk of PTD is limited by an almost exclusive focus on spontaneous PTD, as well as the use of differing cut-points for lipid levels, with most comparing “high” lipids to “lower” lipids despite suggestion of a U-shape effect (16). The purposes of this study were to examine the shape of the relation between mid-pregnancy levels of maternal TC, HDLc, LDLc, and TG and risk of PTD, and then to determine whether low or high levels of these lipids were associated with odds of spontaneous and/or medically indicated PTD.
Material and methods
Data from the Pregnancy Outcomes and Community Health (POUCH) Study were used to evaluate the aims. Pregnant women were enrolled during the 15th–27th week of pregnancy at 52 clinics in five Michigan (USA) communities from 1998–2004 (17). Eligibility criteria included singleton pregnancy with no known chromosomal abnormality or birth defect, maternal serum alpha-fetoprotein (MSAFP) screen at 15–22 weeks, maternal age ≥ 15 years, no pre-existing diabetes mellitus, and proficiency in English. All women with high MSAFP levels (i.e. ≥ 2 multiples of the mean) were invited to participate because this biomarker has been previously associated with PTD (18). Women with normal MSAFP levels were stratified by race/ethnicity and randomly sampled into the cohort. Institutional review boards at Michigan State University, the Michigan Department of Community Health, and nine community hospitals approved the study.
The POUCH study enrolled 3038 women, and accomplished delivery follow-up for 3019 women. A sub-cohort of women (n=1371) was selected to maximize resources for more detailed study (such as biomarker assays, placental pathology) and to permit sub-analyses of strata of particular interest. The sub-cohort included all women who delivered preterm (<37 weeks), women who delivered at term but had high MSAFP levels, and a race-stratified sample of women with term deliveries and normal MSAFP levels, with oversampling of the African-American stratum. All analyses were weighted according to the probabilities of selection into the cohort and sub-cohort to remove bias due to oversampling from certain strata. Stored blood samples from 62 women in the sub-cohort lacked enough blood for lipid analyses, thus the final sample for this study included 1309 women (95% of the sub-cohort).
At enrollment, women signed consent forms, completed self-administered surveys and in-person interviews with a study nurse, and had a non-fasting venous blood draw. Prenatal and labor and delivery records were abstracted and delivered placentas were collected and stored for pathology examination.
Blood samples were drawn at a mean gestational age of 22.4 weeks (range 15–27 weeks), centrifuged within 45 minutes of collection, aliquoted (1 ml), and stored at −80◦C until analyses. Samples were shipped on dry ice to the Nutrition Laboratory in the Department of Epidemiology at the University of Pittsburgh for lipid analyses. This laboratory has been included in the CDC-NHLBI Lipid Standardization Program since 1982, is CLIA certified and participates in CAP proficiency programs. TC (mg/dL) was determined using the enzymatic method of Allain et al. (19). HDLc (mg/dL) was measured directly using a homogeneous two-reagent method with materials obtained from Equal Diagnostics. LDLc (mg/dL) was calculated indirectly using the Friedewald equation: LDLc = TC– HDLc - 0.2*(TG), except when total TG exceeded 400 mg/dL, in which case LDLc was measured directly using an automated spectrophotmetric assay (LDL Direct Liquid Select) from Equal Diagnostics (20). TG (mg/dL) were determined enzymatically using the Bucolo et. al. procedure (21). Duplicate samples with standards, control sera and serum calibrators were included in each run. The coefficients of variation ranged from 1.3 to 2.0%.
Gestational age was calculated using the last menstrual period (LMP) unless it disagreed by more than two weeks with ultrasound conducted prior to 25 weeks gestation, in which case the ultrasound value was used. Accordingly, the LMP estimate was used in 76% of the entire cohort where the two estimates agreed and in 6% of the cohort where only LMP estimates were available. Ultrasound estimates were used for the remaining 18% of the cohort with absent or conflicting LMP estimates. PTD was defined as births before 37 weeks gestation. A physician and a labor and delivery nurse independently reviewed and abstracted data from prenatal and labor and delivery records to identify clinical circumstances leading to PTD. Spontaneous preterm labor was defined as intact membranes, regular contractions and cervical changes (≥2 cm dilation) in the absence of labor induction. Preterm premature rupture of membranes (PROM) was defined as rupture of membranes before or simultaneously with the initiation of spontaneous contractions. Women with either spontaneous labor or PROM leading to preterm delivery were combined into a single spontaneous PTD (sPTD) group for analyses. Medically indicated preterm delivery (Mi PTD) included women induced or given cesarean sections before onset of labor or PROM. Primary reasons for Mi PTD included gestational hypertensive disorders (n=39), intrauterine growth restriction (n=11), oligohydramnios (n=8), abruption (n=3), other maternal conditions including previous complications and placenta previa (n=23), and other fetal conditions including signs of fetal distress (n=16).
The enrollment interview and questionnaire provided information on demographics, medical and reproductive history, pre-pregnancy weight and height (body mass index (BMI) calculated as kg/m2), and smoking and alcohol intake during pregnancy. Maternal weight at the time of the blood draw was recorded. Medical record abstraction provided information on birthweight, preeclampsia/gestational hypertension, gestational diabetes, and placental abruption as previously described (22). Births were classified as small (<10th percentile), appropriate, or large (≥90th percentile) for gestational age using sex and gestational age-specific birthweight cut-points (23). A single pathologist blinded to clinical information performed gross and microscopic evaluation of placentas. As previously described, these data were used to classify women as having histological chorioamnioitis and high vs. not high evidence of five placental vascular pathology constructs (24, 25). Placental evaluations were available for 1057 of the 1309 women with lipid levels.
Statistical analyses
Analyses were conducted with SAS (www.sas.com/statistics) version 9.2. Statistical significance was set at a two-sided alpha level of p<0.05. Sampling weights were used to remove bias due to oversampling of high MSAFP into the cohort and oversampling of high MSAFP, PTD, and African-Americans into the sub-cohort. Thus, weighted results account for the POUCH sampling scheme and should reflect the experience of the population of pregnant women that was initially sampled. Chi-squared testing was used to compare maternal characteristics by delivery status (term, sPTD Mi PTD). Lipid values were skewed, thus log-transformed values were used. Analysis of variance compared least square mean values of log transformed lipids by delivery status.
Generalized additive models (PROC GAM) with cubic spline smoothing (degrees of freedom=3) were used to investigate the shapes of relations between maternal lipids and PTD, adjusted for maternal race, parity, and gestational age at time of the blood draw (26). Plots of relations were inspected to determine decile cut-points for lipid levels. Accordingly, women were categorized as having low (<10th), referent (10–<70th), and high (≥70th percentile) TC, HDLc, and LDLc values based on the distributions of these variables among women with normal MSAFP values who gave birth at term. Quartiles of TG were calculated based on women with normal MSAFP values who gave birth at term, and the lowest quartile was designated as referent.
The odds of sPTD and Mi PTD by lipid categorizations were estimated using logistic and polytomous logistic regression models. Since lipid values increase with gestational age and African-Americans are known to have more favorable lipid profiles than Whites, gestational age at the time of blood draw (continuous) and race (White/Others vs. African-American) were considered as covariates in all models (27, 28). Based on previous literature the following covariates were also considered: parity, pre-pregnancy BMI, maternal weight at blood draw, Medicaid status, marital status, education level, age, and smoking and/or alcohol use during pregnancy. Any variable that altered estimates of associations between lipid levels and PTD by more than 10% was retained in the adjusted models. Secondary analyses removed groups of women with higher risk of PTD based on pre-pregnancy BMI (<18.5 kg/m2, underweight women), diagnoses of preeclampsia/gestational hypertension, presence of histological chorioamnioitis, presence of placental vascular pathology, and birth size (small-for-gestational age) to assess whether their removal changed parameter estimates for relations between lipids and odds of PTD.
Results
Spontaneous PTD accounted for 7.4% and Mi PTD 3.3% of all deliveries. Women who delivered preterm were more likely to be African-American and enrolled in Medicaid compared to women who delivered at term (Table 1). The prevalence of preeclampsia/gestational hypertension and small for gestational age births was also greater among Mi PTD vs. term deliveries.
Table 1.
Participant Characteristics by Delivery Status (n (Weighted Column Percent)), Pregnancy Outcomes and Community Health Study, 1998–2004
| Term (n=988) |
sPTD (n=221) |
Mi PTD (n=100) |
Chi-squared p-value* |
|
|---|---|---|---|---|
| Race | ||||
| White/Other | 553 (76.4) | 148 (66.3) | 71 (68.9) | <0.001 |
| African-American | 435 (23.6) | 73 (33.7) | 29 (31.1) | |
| Paritya | ||||
| Nulliparous | 409 (41.0) | 101 (46.5) | 38 (37.9) | 0.27 |
| Parous | 579 (59.0) | 119 (53.5) | 62 (62.1) | |
| Living Arrangementb | ||||
| Live alone | 351 (26.5) | 70 (32.6) | 27 (27.0) | 0.17 |
| Live with partner/spouse | 636 (73.5) | 149 (67.4) | 73 (73.0) | |
| Medicaid | ||||
| Yes | 558 (48.2) | 129 (58.4) | 52 (51.9) | 0.02 |
| Age | ||||
| ≤19 years | 151 (12.4) | 35 (16.5) | 12 (11.6) | |
| 20–29 years | 572 (57.6) | 125 (56.9) | 60 (59.8) | 0.52 |
| ≥30 years | 265 (30.0) | 61 (26.6) | 28 (28.6) | |
| Education | ||||
| < High School | 223 (18.4) | 51 (23.6) | 24 (25.3) | |
| High School | 277 (26.9) | 66 (30.0) | 28 (27.5) | 0.09 |
| > High School | 488 (54.8) | 104 (46.4) | 48 (47.2) | |
| Pre-pregnancy BMI | ||||
| ≤18.4 kg/m2 | 44 (3.6) | 14 (6.8) | 3 (2.7) | |
| 18.5–24.9 kg/m2 | 439 (46.9) | 107 (47.9) | 40 (41.4) | 0.17 |
| 25–29.9 kg/m2 | 226 (23.3) | 44 (20.5) | 20 (20.6) | |
| ≥30 kg/m2 | 279 (26.1) | 56 (24.8) | 37 (35.4) | |
| Smoking in pregnancy | ||||
| Yes | 274 (27.2) | 67 (30.9) | 28 (26.2) | 0.52 |
| Gestational Diabetes | ||||
| Yes | 46 (5.5) | 12 (4.7) | 7 (6.6) | 0.81 |
| Hypertension | ||||
| None | 901 (91.1) | 205 (92.8) | 59 (58.0) | |
| PE/Gestational Hypertension | 56 (5.8) | 4 (1.9) | 32 (33.5) | <0.001 |
| Chronic Hypertension | 31 (3.0) | 12 (5.3) | 9 (8.5) | |
| Size for Gestational Agec | ||||
| Small (<10th percentile) | 121 (9.8) | 2 (0.8) | 18 (17.8) | |
| Appropriate | 766 (77.6) | 187 (84.3) | 72 (73.6) | <0.001 |
| Large (≥90th percentile) | 100 (12.6) | 32 (14.9) | 9 (8.6) | |
| Timing of Blood Draw | ||||
| <20 weeks gestation | 148 (15.1) | 47 (20.4) | 18 (18.6) | 0.36 |
| 20–27 weeks gestation | 840 (84.8) | 174 (79.6) | 82 (81.4) |
BMI=Body mass index; Mi PTD= Medically indicated preterm delivery; PE=Preeclampsia; sPTD=Spontaneous preterm delivery
P-values indicated significant differences among Term, sPTD, and MI PTD delivery groups
n=1 woman missing information on parity
n=3 women missing information on living arrangement
n=2 women missing information on size for gestational age
Least square mean lipid values were adjusted for race, parity, and gestational week of blood draw and back-transformed from the log scale (Table 2). Lipid values for term deliveries were similar to those previously reported for second trimester measurements (6). Spontaneous PTD deliveries had significantly higher TC and TG values compared to term deliveries (p<0.05). Pre-pregnancy BMI was inversely but weakly correlated with TC (r=−0.1, p<0.001), HDLc (r=−0.2, p<0.001) and LDLc (r=−0.1, p=0.002), and directly correlated with TG (r=0.2, p<0.001).
Table 2.
Maternal Lipids by Delivery Status Presented as Least Square Means (95% Confidence Intervals),a Pregnancy Outcomes and Community Health Study, 1998–2004
| Total (n=1309) | Term (n=988) | sPTD (n=221) | Mi PTD (n=100) | |||||
|---|---|---|---|---|---|---|---|---|
| Lipid Units (mg/dl) |
95% Confidence Interval |
Lipid Units (mg/dl) |
95% Confidence Interval |
Lipid Units (mg/dl) |
95% Confidence Interval |
Lipid Units (mg/dl) |
95% Confidence Interval |
|
| Total Cholesterol | 220.1 | 217.7, 222.5 | 219.7 | 217.1–222.4 | 226.8* | 220.9, 232.8 | 213.1 | 204.2, 222.4 |
| HDL Cholesterol | 68.3 | 67.4, 69.2 | 68.2 | 67.2–69.2 | 70.3 | 68.2, 72.4 | 65.5 | 62.5, 68.6 |
| LDL Cholesterol | 113.5 | 111.3, 115.8 | 113.5 | 111.0–116.0 | 116.4 | 111.1, 122.0 | 106.9 | 98.8, 115.6 |
| Triglycerides | 162.1 | 158.7, 165.6 | 161.1 | 157.3–164.9 | 171.1* | 163.5, 179.0 | 167.0 | 154.8, 180.2 |
HDL=High density lipoprotein; LDL=Low density lipoprotein; MI PTD= Medically indicated preterm delivery; sPTD=Spontaneous preterm delivery
Significantly different from term deliveries (P <0.05)
Least square mean lipid values are back-transformed from log lipid values and adjusted for maternal race (White/Other, African-American), parity (nulliparous, parous), and gestational age at time of blood draw (weeks)
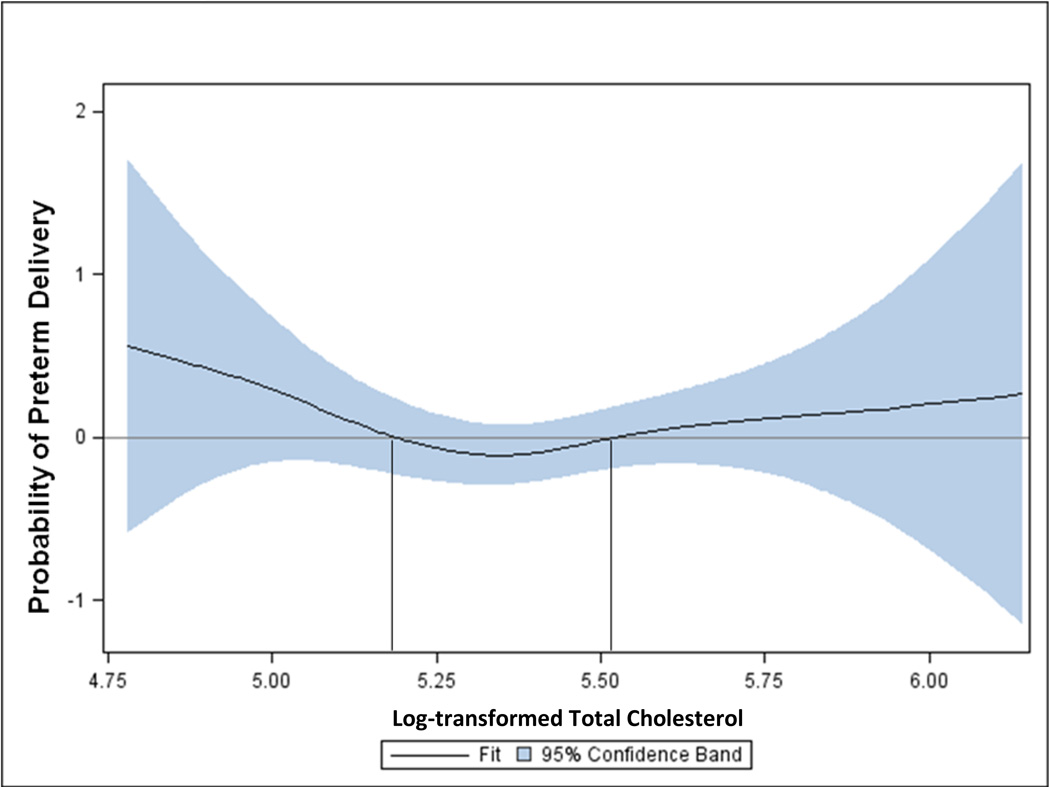
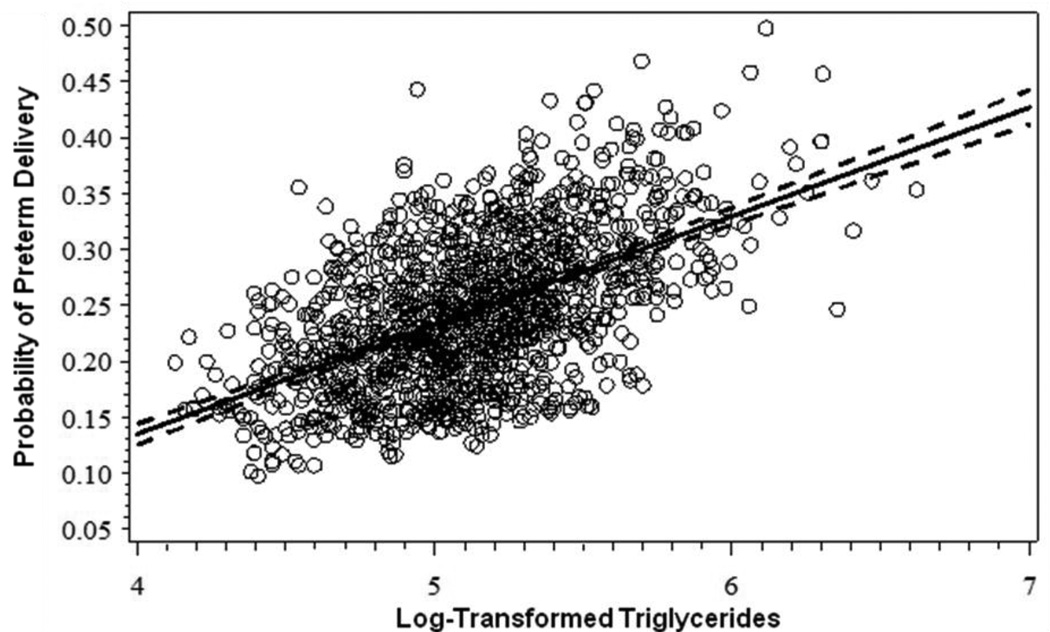
Results from the general additive modeling showed that the probability of PTD was greater than zero when log-transformed TC values were less than 5.18 or greater than 5.52, corresponding to the 10th and 70th percentiles of the distribution of TC among women with normal MSAFP values who gave birth at term (Figure 1). The shapes of the relations between HDLc or LDLc and PTD were similar and also suggested cut-points at the 10th and 70th percentiles (data not shown). In contrast, the general additive model for the relations between TG and PTD did not support a curvilinear term, but rather indicated a linear relation (Figure 2).
Figure 1.
Probability of preterm delivery by levels of log-transformed maternal total cholesterol measured at mid-pregnancy. General additive model fitted using cubic spline smoothing and adjusted for maternal race (White/Other vs. Black), parity (nulliparous vs. parous), and gestational week of blood draw (continuous). Pregnancy Outcomes and Community Health Study, 1998–2004.
Figure 2.
Probability of preterm delivery by levels of log-transformed maternal triglycerides measured at mid-pregnancy. Linear model adjusted for maternal race (White/Other vs. Black), parity (nulliparous vs. parous), and gestational week of blood draw (continuous). Pregnancy Outcomes and Community Health Study, 1998–2004.
Odds of Mi PTD were increased among women with low TC, HDLc, or LDLc, while odds of sPTD were increased among women with high TC or LDLc (Table 3), as compared to women with referent cholesterol values. Associations were adjusted for maternal race, gestational week of blood draw, and parity. No other covariate, including maternal weight or pre-pregnancy BMI, met criteria for inclusion in the cholesterol models. TG levels in the upper two quartiles (first quartile as referent) were also associated with increased odds of sPTD. TG models additionally adjusted for maternal weight at blood draw.
Table 3.
Associations among maternal lipid percentile groups and odds of spontaneous/PROM or medically indicated preterm delivery (n=1309), Pregnancy Outcomes and Community Health Study, 1998–2004
| Term Delivery (Ref) |
Preterm Delivery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Models | Adjusted Modelsa | ||||||||||
| Spontaneous | Medically Indicated | Spontaneous | Medically Indicated | ||||||||
| n | n | OR | 95% CI | n | OR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| Total Cholesterol | |||||||||||
| <10th % | 111 | 25 | 1.30 | 0.79, 2.14 | 19 | 2.09 | 1.16,3.75* | 1.10 | 0.67, 1.82 | 2.04 | 1.12, 3.72* |
| 10–<70th % | 603 | 119 | 1.0 | 56 | 1.0 | 1.0 | 1.0 | ||||
| ≥ 70th % | 274 | 77 | 1.25 | 0.90, 1.75 | 25 | 0.90 | 0.54, 1.49 | 1.51 | 1.06, 2.15* | 0.91 | 0.55, 1.51 |
| HDL Cholesterol | |||||||||||
| <10th % | 92 | 25 | 1.14 | 0.69, 1.89 | 19 | 1.86 | 1.02, 3.38* | 1.17 | 0.70, 1.95 | 1.89 | 1.04, 3.42* |
| 10–<70th % | 595 | 126 | 1.0 | 55 | 1.0 | 1.0 | 1.0 | ||||
| ≥ 70th % | 301 | 70 | 1.10 | 0.78, 1.54 | 26 | 0.97 | 0.58, 1.60 | 1.10 | 0.78, 1.55 | 0.96 | 0.58, 1.59 |
| LDL Cholesterol | |||||||||||
| <10th % | 107 | 29 | 1.50 | 0.93, 2.43 | 18 | 2.04 | 1.13, 3.67* | 1.37 | 0.85, 2.21 | 1.96 | 1.09, 3.54* |
| 10–<70th % | 590 | 120 | 1.0 | 59 | 1.0 | 1.0 | 1.0 | ||||
| ≥ 70th % | 291 | 72 | 1.21 | 0.86, 1.70 | 23 | 0.80 | 0.48, 1.36 | 1.42 | 0.99, 2.04 | 0.80 | 0.47, 1.34 |
| Triglycerides | |||||||||||
| 1st Quartile | 291 | 50 | 1.0 | 23 | 1.0 | 1.0 | 1.0 | ||||
| 2nd Quartile | 249 | 52 | 0.99 | 0.64, 1.55 | 19 | 0.82 | 0.43, 1.58 | 1.27 | 0.81, 2.01 | 0.88 | 0.45, 1.73 |
| 3rd Quartile | 231 | 66 | 1.26 | 0.83, 1.91 | 25 | 1.00 | 0.54, 1.85 | 1.90 | 1.21, 2.97* | 1.11 | 0.58, 2.13 |
| 4th Quartile | 217 | 53 | 1.03 | 0.67, 1.57 | 33 | 1.30 | 0.73, 2.32 | 1.72 | 1.06, 2.78* | 1.50 | 0.79, 2.83 |
aOR=Adjusted odds ratio; CI=Confidence interval; HDL=High density lipoprotein; LDL=Low density lipoprotein; OR=odds ratio
P<0.05
Total, HDL, and LDL Cholesterol models adjusted for maternal race (White/Other, African-American), parity (nulliparous, parous), and gestational age at time of blood draw (weeks); Triglycerides model adjusted for all of the above as well as maternal weight at time of blood draw (lbs).
Results were not appreciably altered by removing women who were underweight pre-pregnancy (n=61), diagnosed with preeclampsia/gestational hypertension (n=92), displayed histological chorioamnioitis (n=128), had high ratings of placental vascular pathologies (n=120–435 depending on the construct), or gave birth to small-for-gestational age infants (n=141). Removal of women with PROM (n= 88) attenuated associations between sPTD and high TC (aOR=1.40, 95% CI: 0.91–2.16) and higher quartiles of TG (aOR=1.61, 95% CI: 0.91–2.83 and aOR=1.70, 95% CI: 0.94–3.07 for the third and fourth quartiles, respectively) to statistical non-significance, but the direction of effects remained the same. Removal of women whose blood draw occurred prior to 20 weeks of gestation also did not affect the reported associations.
Discussion
Our analyses revealed that PTD had a U-shaped relation with TC, HDLc, and LDLc, but a linear relation with TG. When PTD was categorized by clinical circumstances we observed that high TC and LDLc values were associated with increased odds of sPTD while extremely low TC, HDLc, and LDLc values were associated with increased odds of Mi PTD. Higher TG (third or fourth quartiles) were also linked to increased odds of sPTD/PROM compared to low values. Removal of subgroups of women at higher risk for PTD did not appreciably alter findings.
A body of literature on lipids and PTD is accumulating; however, differences in gestational age at sampling, choice of referent group, and lipid cut-points challenge comparisons across studies. In the only previous study that reported a U-shape relation between TC and PTD, Edison et al. found that TC values in the lowest or highest 10th percentiles were associated with a 2–3 fold increase in odds of PTD (16). The range of TC values in the Edison study (93–435 mg/dl) were similar to those seen here (119–464 mg/dl). While “low” and “high” TC cutpoints differed slightly, our results are markedly similar and extend their findings by separately considering sPTD and MI PTD. Another investigation among 651 Greek women examined maternal lipids in relation to sPTD and Mi PTD separately (29). The Greek study found no associations between sPTD and linear increases in maternal TC, HDLc, LDLc, or TG, but a significantly increased risk of Mi PTD with increasing TC levels (29). These authors did not report on effects of low lipid values, nor did they provide information on the range of lipid values for their sample (29).
Others have focused on maternal lipids in relation to sPTD only (12–15). A study of 651 Canadian women examined odds of sPTD in relation to maternal lipids with the lowest quartile designated as referent (15). They found that increasing quartiles of TC and LDLc were unrelated to sPTD, but that being in the highest quartile of HDLc significantly decreased odds of sPTD (15). Once again, it is difficult to compare these results to ours as the lowest lipid values were used as the referent group. A series of studies by Catov et al. consistently showed that “dyslipidemia” was associated with more than twice the odds of sPTD (12–14). In these studies, dyslipidemia was defined as having elevated (>1 standard deviation above the mean) TC or TG (13, 14), or as having elevated (≥90th percentile vs. 10–<90th percentile) non-HDL cholesterol (12). Catov et al. also showed that the dyslipidemia-sPTD association persisted in the presence or absence of inflammation (i.e. high C-reactive protein) (14), or of markers of the fibrinolytic cascade (i.e. elevated thrombin-antithombin III complex) (12). These findings parallel ours showing minimal impact of removing women with clinical complications or a particular set of placental pathologies. Taken together, the results of these studies with ours point towards the robustness of the association between elevated maternal lipids and sPTD in samples drawn from USA populations.
The mechanisms linking low or high maternal lipid levels and PTD risk remain poorly understood. Physiological increases in maternal TC, HDLc and LDLc are important for fetal development of cell membranes, steroid synthesis, cell proliferation and differentiation, and metabolic regulation, and increases in maternal TG provide a reservoir of fatty acids for fetal growth (7). We found that low lipids increased odds of Mi PTD, which is somewhat surprising given that high lipids (TC and TG) are associated with preeclampsia (30, 31). However, low third trimester maternal TC and LDLc levels have been associated with delivering intrauterine growth restricted infants (32, 33). We removed small-for-gestational age deliveries because that group had particularly low lipid levels; however, the associations among low TC and LDLc and Mi PTD remained. It is possible that abnormal fetal development resulting in suboptimal growth (birthweight in the lower range of being appropriate-for-gestational age) and Mi PTD may also reduce the stimulus for cholesterol synthesis during pregnancy leading to our observed associations. Evidence among non-pregnant adults suggest that serum lipid levels are significantly lower during acute illness (34), thus low cholesterol levels may also mark presence of a medical condition that is the underlying cause of Mi PTD. On the opposite end, we saw that high lipids increased odds of sPTD. High lipids have been associated with oxidative stress and systemic inflammation, and may also be related to placental dysfunction or microvascular injuries that lead to sPTD (12, 35). Importantly, results remained after removing women with established risk factors for PTD (such as underweight pre-pregnancy, preeclampsia/gestational hypertension, histological chorioamnioitis, placental vascular pathology, and small-for-gestational age deliveries), indicating that atypical maternal lipid levels may mark alternative pathways to sPTD or Mi PTD that are not well described.
Several limitations should be noted. Similar to previous studies on pregnancy lipids and PTD (12, 14–16, 29), maternal lipids were sampled only once in pregnancy, thus we were unable to describe the trajectory of lipid levels or compare pre-pregnancy and pregnancy levels. It is important to note that our results were not affected by timing of the blood draw as associations were unchanged by restricting analyses only to women whose lipids were measured in the 20–27th week of gestation (data not shown). Also similar to previous reports (12–15), our participants were not fasted, thus lipid levels may have been influenced by the woman’s last meal. However, studies comparing fasting versus non-fasting lipid levels show minimal differences (<5%) for TC, HDLc, and LDLc values, while TG are ~15% higher in the non-fasted state (36, 37). Extra variation induced by using non-fasted lipid levels should affect women with term and preterm deliveries similarly and thus have a non-differential effect, most likely attenuating results. It is surprising that TC and LDLc values were inversely, albeit weakly, correlated with pre-pregnancy BMI given that hypercholesterolemia is associated with overweight/obesity (38). It is possible that pregnancy-related increases in lipid levels disrupted expected correlations with pre-pregnancy BMI. Our internal cut-points for low and high lipid levels may not generalize to other populations. Finally, the use of last menstrual period to measure gestational age at delivery may be considered a limitation. However, we used a standard protocol of only using last menstrual period dating if it agreed within two weeks of ultrasound dating, thus we are reasonably confident in our assessment of gestational age.
Despite these limitations our results add to the existing literature on maternal lipids and PTD. Nonparametric modeling allowed us to detect a U-shape relationship between TC, HDLc, and LDLc and risk of PTD, perhaps reconciling inconsistencies across studies that focused on only one end of the continuum. Unlike most previous literature on lipids and PTD (11–16), we also considered clinical subtypes, and were able to shed more light on a previously documented U-shape relation between TC and PTD (16). Modeling each lipid individually with PTD also provided greater specificity than past studies which combined women with high TC or high TG (13, 14). As clinicians move towards measuring more biomarkers during pregnancy to assess risk of PTD, lipids may turn out to be a useful component of a biomarker risk profile.
More research is needed to identify the biological mechanisms leading to increased risk of PTD and to determine healthy ranges for lipids during pregnancy that optimize birth outcomes. Ideally, both pre-pregnancy and pregnancy lipid profiles should be measured in order to determine if one or both are associated with risk of PTD. Future studies should also measure markers of inflammation and/or oxidative stress to determine whether high lipids are related to these sPTD pathways, as well as other indicies of placental/fetal health to better evaluate mechanisms linking low lipids to Mi PTD.
Conclusions
In conclusion, low lipid values (TC, HDLc, and LDLc) were uniquely associated with a modestly increased risk of medically indicated preterm delivery while high lipids (TC, LDLc, and TG) were associated with an increased risk of spontaneous preterm delivery. Given that one in six women in the United States are dyslipidemic, it is important to more fully understand the impact of maternal lipids on pregnancy outcomes (39).
Acknowledgements
The authors thank the community research study nurses for careful data collection and Bertha Bullen for her role in study logistics and data management.
Funding
This work was supported by the Perinatal Epidemiological Research Initiative Program Grant from the March of Dimes Foundation [Grants 20FY01-38 and 20-FY04-37]; the National Institute of Child Health and Human Development and the National Institute of Nursing Research [Grant R01 HD34543]; the Thrasher Research Foundation [Grant 02816-7]; and the Centers for Disease Control and Prevention [Grant U01 DP000143-01].
Abbreviations
- BMI
body mass index
- CAP
College of American Pathologists
- CDC-NHLBI
Centers for Disease Control and Prevention - National Heart, Lung, and Blood
- Institute CLIA
Clinical laboratory Improvement Act
- HDLc
high-density lipoprotien
- LDLc
low-density lipoprotein
- Mi PTD
medically indicated preterm delivery
- MSAFP
maternal serum alpha-fetoprotein
- POUCH
Pregnancy Outcomes and Community Health
- PROM
premature rupture of membranes
- PTD
preterm delivery
- sPTD
spontaneous preterm delivery
- TC
total cholesterol
- TG
triglycerides
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2007. Natl Vital Stat Rep. 2009;57(12):1–23. [PubMed] [Google Scholar]
- 2.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008 Jul 30;57(2):1–32. [PubMed] [Google Scholar]
- 3.Lindstrom K, Winbladh B, Haglund B, Hjern A. Preterm infants as young adults: a Swedish national cohort study. Pediatrics. 2007 Jul;120(1):70–77. doi: 10.1542/peds.2006-3260. [DOI] [PubMed] [Google Scholar]
- 4.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000 Aug 10;343(6):378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008 Jan 5;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basaran A. Pregnancy-induced hyperlipoproteinemia: review of the literature. Reprod Sci. 2009 May;16(5):431–437. doi: 10.1177/1933719108330569. [DOI] [PubMed] [Google Scholar]
- 7.Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002 Oct;19(1):43–55. doi: 10.1385/ENDO:19:1:43. [DOI] [PubMed] [Google Scholar]
- 8.De Assis SMA, Seguro AC, Helon CMB. Effects of maternal hypercholesterolemia on pregnancy and development of offspring. Pediatr Nephrol. 2003;18:328–334. doi: 10.1007/s00467-003-1082-8. [DOI] [PubMed] [Google Scholar]
- 9.Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997 Dec 1;100(11):2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palinski W, D'Armiento FP, Witztum JL, de Nigris F, Casanada F, Condorelli M, et al. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ Res. 2001 Nov 23;89(11):991–996. doi: 10.1161/hh2301.099646. [DOI] [PubMed] [Google Scholar]
- 11.Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. Am J Obstet Gynecol. 2005 Oct;193(4):1292–1301. doi: 10.1016/j.ajog.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Catov JM, Bodnar LM, Hackney D, Roberts JM, Simhan HN. Activation of the fibrinolytic cascade early in pregnancy among women with spontaneous preterm birth. Obstet Gynecol. 2008 Nov;112(5):1116–1122. doi: 10.1097/AOG.0b013e31818aa5b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catov JM, Bodnar LM, Kip KE, Hubel C, Ness RB, Harger G, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007 Dec;197(6):610 e1–610 e7. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007 Dec 1;166(11):1312–1319. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 15.Kramer MS, Kahn SR, Rozen R, Evans R, Platt RW, Chen MF, et al. Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. Int J Epidemiol. 2009 Jun;38(3):715–723. doi: 10.1093/ije/dyp167. [DOI] [PubMed] [Google Scholar]
- 16.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, et al. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007 Oct;120(4):723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 17.Holzman C, Bullen B, Fisher R, Paneth N, Reuss L. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr Perinat Epidemiol. 2001 Jul;15(Suppl 2):136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 18.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand. 2005 Jun;84(6):516–525. doi: 10.1111/j.0001-6349.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 19.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 21.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- 22.Gargano JW, Holzman CB, Senagore PK, Reuss ML, Pathak DR, Friderici KH, et al. Polymorphisms in thrombophilia and renin-angiotensin system pathways, preterm delivery, and evidence of placental hemorrhage. Am J Obstet Gynecol. 2009 Sep;201(3):317 e1–317 e9. doi: 10.1016/j.ajog.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbuckle TE, Wilkins R, Sherman GJ. Birth weight percentiles by gestational age in Canada. Obstet Gynecol. 1993;81(1):39–48. [PubMed] [Google Scholar]
- 24.Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol. 2007 Oct 1;166(7):786–794. doi: 10.1093/aje/kwm168. [DOI] [PubMed] [Google Scholar]
- 25.Kelly R, Holzman C, Senagore P, Wang J, Tian Y, Rahbar MH, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009 Jul 15;170(2):148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hastie T, Tibshirani R. Generalized Additive Models. New York: Chapman and Hall; 1990. [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2008 Dec 9;140(2):226–235. doi: 10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003 May 6;107(17):2185–2189. doi: 10.1161/01.CIR.0000066320.27195.B4. [DOI] [PubMed] [Google Scholar]
- 29.Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, et al. Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol. 2009 Oct 1;170(7):829–836. doi: 10.1093/aje/kwp211. [DOI] [PubMed] [Google Scholar]
- 30.Baker AM, Klein RL, Moss KL, Haeri S, Boggess K. Maternal serum dyslipidemia occurs early in pregnancy in women with mild but not severe preeclampsia. Am J Obstet Gynecol. 2009 Sep;201(3):293 e1–293 e4. doi: 10.1016/j.ajog.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. 2009 Nov;201(5):482 e1–482 e8. doi: 10.1016/j.ajog.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sattar N, Greer IA, Galloway PJ, Packard CJ, Shepherd J, Kelly T, et al. Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab. 1999 Jan;84(1):128–130. doi: 10.1210/jcem.84.1.5419. [DOI] [PubMed] [Google Scholar]
- 33.Wadsack C, Tabano S, Maier A, Hiden U, Alvino G, Cozzi V, et al. Intrauterine growth restriction is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition. Am J Physiol Endocrinol Metab. 2007 Feb;292(2):E476–E484. doi: 10.1152/ajpendo.00547.2005. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs DR, Jr, Hebert B, Schreiner PJ, Sidney S, Iribarren C, Hulley S. Reduced cholesterol is associated with recent minor illness: the CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1997 Oct 1;146(7):558–564. doi: 10.1093/oxfordjournals.aje.a009314. [DOI] [PubMed] [Google Scholar]
- 35.Napoli C, Lerman LO. Involvement of oxidation-sensitive mechanisms in the cardiovascular effects of hypercholesterolemia. Mayo Clin Proc. 2001 Jun;76(6):619–631. doi: 10.4065/76.6.619. [DOI] [PubMed] [Google Scholar]
- 36.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008 Sep 2;118(10):993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008 Nov 11;118(20):2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 38.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. Jama. 2005 Apr 20;293(15):1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 39.National Center for Health Statistics. Health, United States, 2008. Hyattsville (MD): 2009. DHHS Publication #2009-1232; [PubMed] [Google Scholar]