Abstract
Background
Although, Dan-Lou prescription (DLP) is used for antagonizing check discomfort and heartache, the pharmacological mechanism has not been clearly illustrated. Our present study aimed to design inflammatory models induced by LPS in vivo and in vitro to investigate the anti-inflammation of DLP ethanol extract (EEDL) and the potential mechanisms.
Methods
EEDL was prepared and then analyzed by high performance liquid chromatography (HPLC). Further, the anti-inflammatory effects of EEDL in vivo was evaluated by measuring inflammation-associated factors includingcytokines, chemokines and acute phase proteins in lipopolysaccharide (LPS)-induced mice serum and liver. The anti-inflammatory mechanism exploration of EEDL was performed in LPS-stimulated RAW 264.7 cells. Different effects of EEDL on nitric oxide (NO) and prostaglandin (PG)E2 secretion were investigated by Griess reagent method and enzyme-linked immunosorbent assay (ELISA) respectively. Then the mRNA and protein expression of inducible NO synthase (iNOS) and cyclooxygenase (COX)-2 were measured by real-time reverse-transcription polymerase chain reaction (RT-PCR), ELISA and Western blot. Other chemokines and acute phase proteins were determined by proteome profile array. Finally, the ELISA based transcription factor assay was applied to measure the DNA-binding activity of nuclear transcription factor (NF)-κB p65.
Results
Eight compounds from EEDL have been identified as gallic acid, salvianic acid, puerarin, daidzin, paeoniflorin, salvianolic acid B, cryptotanshinone, and tanshinone IIA, with amounts of 0.26, 9.84, 10.41, 2.55, 9.44, 3.82, 0.24 and 0.3 mg/kg, respectively. In vivo, EEDL administration antagonized the up-regulation of more than 17 kinds of cytokines, chemokines and acute phase proteins in LPS-treated mice serum, and inhibited LPS-induced IL-6 mRNA and protein expression in mice liver tissue. In vitro, LPS-induced NO and PGE2 over-productions were decreased by EEDL treatment. The mRNA and protein expression of iNOS, COX-2 and IL-6 were similarly inhibited by EEDL treatment, which might be attributed to decrease the DNA-binding activity of NF-κB p65.
Conclusion
EEDL was valid for anti-inflammation and the potential molecular mechanisms might be due to the inhibition of of LPS-induced iNOS/NO, COX-2/PGE2 and cytokines expression by antagonizing the activation of NF-κB p65.
Electronic supplementary material
The online version of this article (doi:10.1186/s12906-015-0848-4) contains supplementary material, which is available to authorized users.
Background
Coronary heart disease (CHD) is a common and frequently-occurring disease [1]. There are more than 3.8 million men and 3.4 million women die of the CHD each year and have a tendency to increase in recent years globally [2]. In clinical, Dan-Lou prescription (DLP) is given to patients with angina pectoris, and has advantages in the improvement of sputum, the lip empurples, etc. [3–5]. Researchers indicate that the pharmacological effects are attributed to increasing coronary flow [6], antagonizing platelet aggregation [7], thrombosis formation [8], hypertention [9], hyperglycemia and hyperlipidemia [10].
DLP is derived from the traditional Chinese medicine formula Gualou Xiebai Banxia Tang which is recorded in the Synopsis of Prescriptions of the Golden Chamber (Jin-Gui-Yao-Lue) and has been used for about 2000 years in China. DLP is consisted of 10 herbs, including Trichosanthes kirilowii Maxim. (Cucurbitaceae), Allium macrostemon Bunge. (Liliaceae), Pueraria lobata (Willd.) Ohwi (Leguminosae), Ligusticum chuanxiong Hort. (Umbelliferae), Salvia miltiorrhiza Bunge. (Labiatae), Paeonia lactiflora Pall. (Ranunculaceae), Alisma plantago-aquatica Linn. (Alismataceae), Astragalus membranaceus (Fisch.) Bunge. (Leguminosae), Davallia mariesii Moore ex Bak. (Davalliaceae), and Curcuma aromatica Salisb. (Zingiberaceae). To further investigate the chemical composition and pharmacological mechanism, numerous detection methods have been established such as liquid chromatography-mass spectrometry (LC-MS), gas chromatography–mass spectrometry (GC-MS), high performance liquid chromatography-chemiluminescence detection (HPLC-CL), high performance liquid chromatography-diode-array detector (HPLC-DAD) and ultra high performance liquid chromatography/diode-array detector/quadrupole time-of flight tandem mass spectrometry (UPLC-DAD-QTOF) [7, 11–14]. In this study, eight highest level ingredients of DLP ethanol extract (EEDL), gallic acid, salvianic acid, puerarin, daidzin, paeoniflorin, salvianolic acid B, cryptotanshinone and tanshinone IIA, were chosen as marker substances to investigate pharmacological mechanism.
More and more studies show that inflammation plays a vital role in CHD [15]. Atherosclerosis acts as the main cause of CHD and is formerly considered as a bland lipid storage disease. However, atherosclerosis is actually a process of inflammation invasion. In the pathological processes of atherosclerosis, all stages such as initiation, growth and complication of atherosclerotic plaque involve an inflammatory response [16]. The circulating inflammatory markers such as C-reactive protein (CRP), serum amyloid A (SAA), interleukin (IL)-6 and IL-1 receptor commonly accompany with acute coronary syndromes [17]. Pharmacological studies demonstrate that many active components of DLP such as gallic acid [18], puerarin [19], paeoniflorin [20], cryptotanshinone [21] and tanshinone IIA [22] have anti-inflammatory effects by antagonizing over-expression cyclooxygenase (COX)-2, inducible nitric oxide synthase (iNOS), IL-6, etc. In detail, gallic acid inhibits lipopolysaccharide (LPS)-induced inflammation in nuclear transcription factor (NF)-κB transgenic mice [18], cryptotanshinone suppresses LPS-induced mice cytokines secretion via inhibition of NF-κB [21], tanshinone IIA reduces LPS-induced mice acute lung injury via NF-κB inhibition [22]. For these facts, propose new strategies from anti-inflammation may be a new viewpoint for the prediction, prevention, and treatment of DLP on CHD.
The exploration of EEDL on inflammatory response is the most fundamental research to illustrate the pharmacological mechanisms. LPS-induced inflammatory models in mice and macrophages are widely used to preliminarily screen anti-inflammatory drugs [23]. In the present study, we designed inflammatory models induced by LPS in vivo and in vitro to investigate the anti-inflammation of EEDL and the potential mechanisms.
Methods
Reagents
The drug powder of DLP was provided by Jilin Connell Medicine Co., Ltd (Jilin, China). Dulbecco’s modified Eagle’s medium-high glucose (DMEM), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), aspirin and LPS from Escherichia coli 0111:B4 were obtained from Sigma-Aldrich Co. (St. Louis, USA). IL-6 Mouse ELISA Kit was obtained from eBioscience (San Diedo, USA). Mammalian Cell Lysis Kit and UNIQ-10 column Trizol total RNA extraction kit were obtained from Sangon Biological Engineering Technology & Services Co., Ltd (Shanghai, China). Improm-II Reverse Transcription System was purchased from Promega Corporation (Madison, USA). FastStart Universal SYBR Green Master (ROX) kit was purchased from Roche (Mannheim, Germany). BCA Protein Assay Kit was purchased from Thermo Fisher (Rochford, USA). Dexamethasone injection was obtained from Shanghai General Pharmaceutical Co., Ltd. (Shanghai, China). 4-amino-5-methylamino-2′, 7′-difluorofluorescein diacetate (DAF-FM-DA) was obtained from Invitrogen (Carlsbad, USA). Prostaglandin (PG)E2 EIA monoclonal kit and Mouse Cytokine Array Panel A Array kit were obtained from R&D Systems, Inc. (Minneapoils, USA). Mouse anti-cyclooxygenase (COX)-2 monoclonal antibody was from BD PharMingen (San Diego, USA) and goat anti-Mouse IgG peroxidase conjugate was from Merck (Darmstadt, Germany). BAY 11–7082 and Nuclear and Cytoplasmic Protein Extraction Kit were purchased from Beyotime Institute of Biotechnology (Haimen, China). Antibody for iNOS was purchased from Cell Signaling Technology (Beverly, USA). Goat anti-rabbit IgG peroxidase conjugate was bought from Merck (Darmstadt, Germany). Transcription Factor Assay Kit for nuclear transcription factor (NF)-κB p65 was purchased from Thermo Fisher (Rochford, USA).
Extract preparation
The DLP drug powder (50 g) was extracted with 75 % ethanol (500 mL) in an ultrasonic bath for 30 min at room temperature and then filtrated. The extraction process above mentioned was repeated for three times, mixed the filtrates and concentrated to 100 mL under reduced pressure at 60 °C. Finally, the concentrate was lyophilized, sealed and stored lucifugally in 50 mL centrifuge tube at 4 °C for use. The EEDL was dissolved in DMEM to obtain the desired concentrations in experiments.
Quantitative chromatographic analysis
To ensure the consistent results, the constituents of EEDL were analyzed by HPLC using a Waters 2695 system (Waters, New York, USA), which is equipped with a quaternary pump, an autosampler, an online vacuum degasser, and an ultraviolet detector. The chromatographic analyses were performed on a Kromasil C18 Column (250 mm × 4.6 mm, 5 μm, Sweden) at 25 °C. For HPLC analysis, a 5 μL sample was injected into the column with a constant flow rate of 1.0 mL/min. The mobile phase consisted of acetonitrile (A) and water (containing 0.1 % formic acid, v/v, B). The elution was carried out as follows: 10–11 % A at 0–6 min, 11 % A at 6–8 min, 11–18 % A at 8–10 min, 18–33 % A at 10–17 min, 33 % A at 17–20 min, 33–52 % A at 20–21 min, 52–55 % A at 21–25 min, 55–75 % A at 25–26 min, 75–90 % A at 26–35 min, 90 % A at 35–40 min, 90–100 % A at 40–40.1 min. Chromatograms were monitored at a wavelength of 280 nm [7].
In vivo anti-inflammatory analysis
Animals and acute inflammatory injury
Male ICR mice (18–22 g) were obtained from Beijing HuaFuKang Bio-technology Co., Ltd. They were group-housed in cages at constant humidity (55 ± 5 %), temperature (24 ± 1 °C) and light–dark cycle (on at 7:00 a.m. and off at 7:00 p.m.). All animals were allowed ad libitum water according to the experimental protocols. Studies were performed in accordance with NIH Guide for the Care and Use of Laboratory Animals and protocol was approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine (TCM-2009-037-E12).
Forty-eight mice were randomly divided into 6 groups (8 mice per group): blank control, LPS (dissolved in sterile pyrogen-free saline solution, 1 mg/kg body weight), LPS + EEDL (0.25, 1 and 4 g/kg); positive control (LPS + dexamethasone injection, 10 mg/kg). Mice were firstly pretreated intraperitoneally (i.p.) with 0.9 % saline, different amount of EEDL and dexamethasone injection, which were dissolved in 0.9 % saline to make a total volume of 200 μL. After drug administration for 30 min, LPS were then i.p. given to mice for 90 min, while blank control mice received only 0.9 % saline. Subsequently, mice were anesthetized with ether and blood samples were collected. Serum was obtained by incubating for 1 h at 37 °C (water bath), and then centrifuged at 3000 rpm for 10 min at 4 °C and stored at −20 °C until use. Simultaneously, the liver tissues were quickly removed, frozen in liquid nitrogen immediately and stored at −80 °C until analysis.
Analysis of IL-6 protein and mRNA expression in liver
The IL-6 level in mice liver was detected according to the manufacturer’s instructions of commercially available ELISA kits. The protein was quantified by BCA method. For total RNA extraction, the mice tissues of liver samples (about 30 mg) were homogenized in 500 μL of Trizol reagent. Real-time RT-PCR was performed according to a previously described method [23]. The real-time RT-PCR oligonucleotide primers used for mouse IL-6 and β-actin (internal control) were shown in Table 1.
Table 1.
Real-time RT-PCR oligonucleotide primers
| Gene | Primer | Sequence (5′–3′) | PCR product (bp) |
|---|---|---|---|
| β-actin | Forward | TGTTACCAACTGGGACGACA | 165 |
| (NM_007393.3) | Reverse | GGGGTGTTGAAGGTCTCAAA | |
| iNOS | Forward | CACCTTGGAGTTCACCCAGT | 170 |
| (NM_010927.3) | Reverse | ACCACTCGTACTTGGGATGC | |
| COX-2 | Forward | TGAGTACCGCAAACGCTTCTC | 151 |
| (NM_011198.3) | Reverse | TGGACGAGGTTTTTCCACCAG | |
| IL-6 | Forward | TCCAGTTGCCTTCTTGGGAC | 140 |
| (NM_031168.1) | Reverse | GTGTAATTAAGCCTCCGACTTG |
Cytokine protein array analysis
To screen for different acute phase proteins, cytokines, and chemokines in mice serum, a protein profile array kit (Mouse Cytokine Array Panel A) from R&D Systems (cat.no.ARY006) was performed according to the manufacturer’s instructions. Samples of mice serum (150 μL) were separately run on the array [24–26]. The proteins were detected including: BLC, C5a, G-CSF, GM-CSF, I-309, Etoaxin, sICAM-1, IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-13, IL-12p70, IL-16, IL-17, IL-23, IL-27, IP-10, I-TAC, KC, M-CSF, JE, MCP-5, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, SDF-1, TARC, TIMP-1, TNF-α and TREM-1. Horseradish peroxidase chemiluminescent substrate (Millipore Corporation, USA) was used to detect protein blotting by exposure to Kodak BioMax Light films. Films were scanned into a computer and densitometry of the image was performed using an Image-Pro Plus Software version 6.0 (Media Cybernetics, Sliver Spring, MD, USA).
In vitro anti-inflammatory activities
Cells and cell culture
The murine macrophage cell line RAW 264.7 was obtained from Cell Culture Center of Chinese Academy of Medical Sciences (Beijing, China). Cells were cultured in DMEM supplemented with 10 % HI-FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a fully humidified incubator containing 5 % CO2. For all experiments, cells were grown to a confluence of 80–90 %, and were subjected to no more than seven cell passages.
Drug dilution
EEDL was prepared as above mentioned. Then a series of concentrations (200, 100, 50 and 25 μg/mL) were obtained by diluting with DMEM (without phenol red) which was supplemented with 5 % heat inactivated fetal bovine serum (HI-FBS) and 0.2 μg/mL LPS.
Analysis of cell viability
RAW 264.7 cells were distributed into 96-well plates (1.0 × 104 cells per well) for 24 h, and treated with various concentrations of EEDL (200, 100, 50, and 25 μg/mL), L-NAME (100 μM), and aspirin (100 μM). In this assay, both L-NAME and aspirin work as positive control drugs. L-NAME is an inhibitor of NOS, and aspirin is a commonly used anti-inflammatory drug which prevent the transformation of arachidonic acid to both PGE and thromboxanes by inhibiting COX activity. After incubated for 20 h, MTT reagent was added to a final concentration of 0.5 mg/mL and incubated for an additional 2 h at 37 °C and 5 % CO2. After discarding the medium, the formazan precipitate was solubilized in 100 μL DMSO, and the absorbance was recorded at 570 nm using a multifunctional microplate reader (FlexStation 3, Molecular Devices, USA).
Determination of nitrite secretion, intracellular NO production, iNOS mRNA and protein expression
Nitrite is the stable product from NO and is used as an indicator of NO secreted into the culture medium. RAW 264.7 cells, cultured in 96-well plates (5.0 × 104 cells per well) overnight were pretreated with various concentrations of EEDL (200, 100, 50 and 25 μg/mL), L-NAME (100 μM) or aspirin (100 μM) containing 0.2 μg/mL LPS. The LPS-treated group and blank control group was given equal volume of medium with or without LPS (0.2 μg/mL), respectively. After 24 h, the nitrite concentration accumulated in the culture supernatants was monitored by Griess reagent system (Promega, USA). Intracellular NO was detected by using the NO-sensitive fluorescent dye DAF-FM-DA. In detail, the RAW 264.7 cells were seeded in 35-mm dishes for 24 h at 37 °C and 5 % CO2, and stimulated with EEDL (200 μg/mL) and L-NAME (100 μM) in the present of LPS (0.2 μg/mL) for an additional 24 h. Similarly, LPS-treated group and blank control group was given equal volume of medium with or without LPS (0.2 μg/mL), respectively. Subsequently, the culture medium was discarded and cells were fixed with 4 % paraformaldehyde. Finally, 10 μM DAF-FM-DA was incubated for 1 h and washed with PBS (pH 7.4) for three times. Digitized images were picked with a Zeiss LSM 710 confocal laser scanning microscope with an excitation wavelength of 495 nm and an emission wavelength of 515 nm. For the detection of iNOS mRNA expression, cells were collected from 6-well plates with or without the stimulation of LPS, and performed as earlier described [23].
Assay for PGE2 production, COX-2 mRNA and protein expression
RAW 264.7 cells were treated with the indicated concentrations of EEDL (200, 100 and 50 μg/mL) containing 0.2 μg/mL LPS for 24 h, in addition to the LPS-treated group and blank control group which was given equal volume of medium with or without LPS (0.2 μg/mL), respectively. The cell supernatants were drawn off for the detection of PGE2 using the PGE2 EIA monoclonal kit (Minneapoils, USA), and cells were used to measure the expression of COX-2 protein with cell-based ELISA as previously reported [23, 27]. The COX-2 mRNA expression was conducted similarly as earlier described.
Determination of IL-6 mRNA expression and protein secretion
To determine the level of IL-6 production in cell supernatants, cells were plated at 1 × 105 cells per well in 48-well plate and stimulated with various concentrations of EEDL (200, 100 and 50 μg/mL) in the presence of 0.2 μg/mL LPS for an additional 24 h. Similarly, the LPS-treated group and blank control group was given equal volume of medium with or without LPS (0.2 μg/mL), respectively. For the detection of IL-6 mRNA, cells were cultured in 6-well plate with the density of 1 × 106 cells per well. After stimulation with LPS, cells were harvested for real-time RT-PCR as earlier described [23].
Cytokine protein array analysis and western blot
To screen for different acute phase proteins, cytokines, and chemokines in RAW 264.7 cell lysate, a total protein content of 20 μg was run on the array and performed as earlier described. Cell lysate was prepared with a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s protocol. For the protein analysis of iNOS, cells were seeded in the 6-well plates at a density of 1 × 106 cells per well. After stimulation with LPS for 24 h, cell lysate was prepared as above mentioned. Subsequently, BCA method was employed to determine the protein concentration, and equal amounts (40 μg) of protein were subjected to SDS-PAGE and boiled for 5 min. following transferring samples onto polyvinylidene fluoride (PVDF) membrane, TTBS (0.5 % Tween 20, 10 mM Tris–HCl, pH 7.5, 150 mM NaCl) containing 5 % nonfat milk was used for blocking at room temperature for 1 h. One hour later, blots were incubated with antibodies against iNOS (1:500), or β-actin (1:5000) overnight at 4 °C. After washing, appropriate secondary antibodies were selected for incubation at room temperature for another 1 h. Finally, the redundant secondary antibodies were washed and the protein bands were detected by a chemiluminescence detection kit (Millipore, Billerica, MA, USA) and exposure to Kodak BioMax Light films. After the films were scanned and saved as tagged image file format (TIFF) image files, images were quantified using Image-Pro Plus version 6.0 (Media Cybernetics, Sliver Spring, MD, USA).
DNA-binding activity of NF-κB p65
RAW 264.7 cells, cultured in 60-mm dishes with a density of 1.5 × 106 cells per well, were pretreated with EEDL (200, 100 and 50 μg/mL) or BAY 11–7082 for 2 h. BAY 11–7082 is a commonly used inhibitor of NF-κB. Then LPS (with a final concentration of 0.2 μg/mL) was added for an additional 30 min. The LPS-treated group and blank control group was given equal volume of medium with or without LPS (0.2 μg/mL), respectively. Then the cell lysate was prepared with a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s protocol. For the DNA-binding activity of NF-κB p65, BCA method was applied for the quantification of protein concentration. Finally, 3 μg protein was added as the manufacture’s instruction of Transcription Factor Assay Kit (Thermo Fisher Scientific Inc., USA).
Statistical analysis
The Origin 7.5 software (MicroCal, USA) was applied to perform statistical analysis. N indicated the number of animals in each group or the number of wells studied in each category. In vivo experiments, data were expressed as means ± SE, and in vitro, means ± SD were calculated. For statistical comparisons, the results were analyzed using one-way analysis of variance (ANOVA) and a P < 0.05 was considered statistically significant.
Results
Primary chemical ingredients analysis of EEDL
Analysis of various chemical compounds in EEDL was performed by HPLC. The typical chromatogram of EEDL is shown in Fig. 1 and eight compounds from EEDL have been identified as gallic acid (1), salvianic acid (2), puerarin (3), daidzin (4), paeoniflorin (5), salvianolic acid B (6), cryptotanshinone (7), and tanshinone IIA (8). According to the calibration curves, the contents of the eight compounds above mentioned in EEDL were quantified. The amounts of gallic acid, salvianic acid, puerarin, daidzin, paeoniflorin, salvianolic acid B, cryptotanshinone, and tanshinone IIA were 0.26, 9.84, 10.41, 2.55, 9.44, 3.82, 0.24 and 0.3 mg/kg, respectively.
Fig. 1.
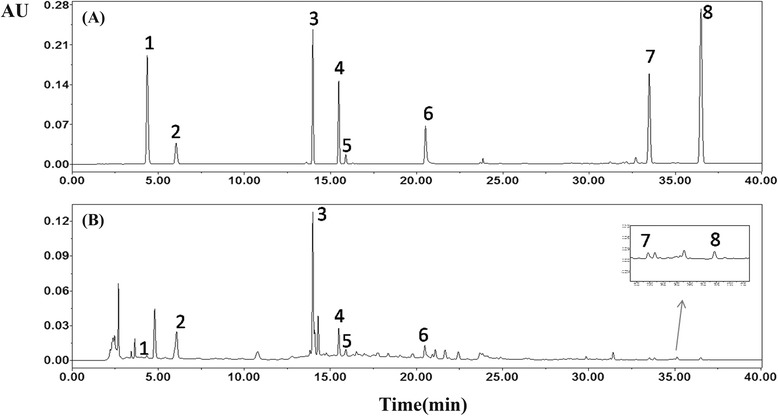
HPLC analysis of (a) standard solutions and (b) sample of EEDL. “1” gallic acid; “2” salvianic acid; “3” puerarin; “4” daidzin; “5” paeoniflorin; “6” salvianolic acid B; “7” cryptotanshinone; and “8” tanshinone IIA
Anti-inflammation of EEDL in vivo
EEDL attenuates the IL-6 mRNA and protein expression in liver
As a pro-inflammatory cytokine, IL-6 plays a vital role in the process of inflammation. When mice were i.p. injected with LPS (1 mg/kg), the IL-6 mRNA (Fig. 2a) and protein (Fig. 2b) level in mice liver were significantly increased up to hundreds folds comparing with the blank control group (P < 0.01). However, dexamethasone injection or EEDL (0.25, 1 and 4 mg/kg) treatment down-regulated the aberrant increase of IL-6 mRNA and protein expression with varying degrees (P < 0.05 or P < 0.01).
Fig. 2.
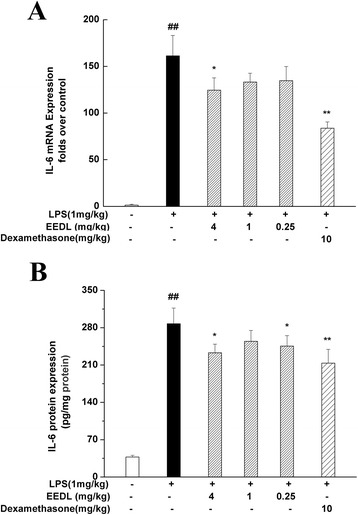
Effects of EEDL on LPS-induced IL-6 mRNA and protein expressions in mice liver. After mice were i.p. administrated with LPS (1 mg/kg) for 90 min, mice livers were selected for this assay. The mRNA and protein expressions of IL-6 (a and b) were measured with real-time RT-PCR and ELISA, respectively. Values are means ± SE (n = 3 for real-time RT-PCR, n = 6 for ELISA) and significant difference compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, *P < 0.05, **P < 0.01 vs. LPS-treated group
Effects of EEDL on multiple cytokines in serum
Previously, we showed that the inflammation induced by LPS (1 mg/kg) in mice was an inflammatory network composed of cytokines, chemokines and so on [23]. To further investigate the anti-inflammation of EEDL, the proteome profiler array was employed. It allows a simultaneous analysis of 40 proteins in a single experiment. As shown in Fig. 3, LPS resulted in a burst of 17 inflammation-related proteins, including C5/C5a, G-CSF, I-309, sICAM-1, IL-1ra, IL-6, IP-10, KC, M-CSF, MCP-1, MIP-1α, MIP-1β, MIP-2, RANTES, SDF-1, TIMP-1 and TNF-α. Also, LPS decreased the expression of IL-10, an anti-inflammatory cytokine. However, EEDL administration (4 mg/kg) not only notably suppressed the over-expressions of C5/C5a, G-CSF, I-309, sICAM-1, IL-1ra, IL-6, IP-10, KC, M-CSF, MCP-1, MIP-1α, MIP-1β, MIP-2, RANTES, SDF-1, TIMP-1, and TNF-α (P < 0.01), but increased the IL-10 protein levels (P < 0.01).
Fig. 3.
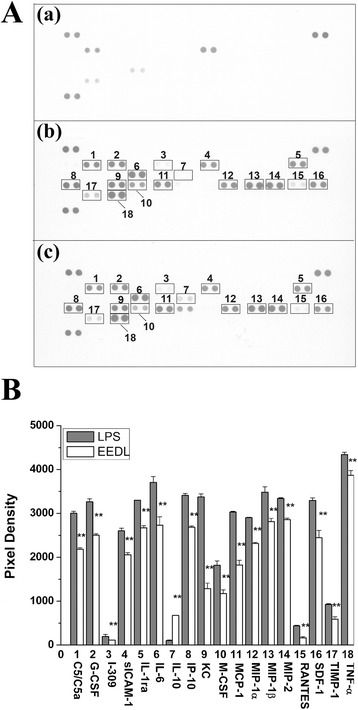
Effects of EEDL on LPS-induced multiple cytokines production in mice serum. After mice were pre-treated with EEDL (4 mg/kg) or 0.9 % saline for 30 min, LPS (1 mg/kg) or 0.9 % saline were injected, respectively, for an additional 90 min. a Expression of multiple proteins in the acute inflammation was measured by proteome profile array. (a) Blank control; (b) mice were administrated (i.p.) with LPS alone; (c) mice were administrated (i.p.) with LPS + EEDL. Numbers marked on the membranes are presented as: “1” C5/C5a; “2” G-CSF; “3” I-309; “4” sICAM-1; “5” IL-1a; “6” IL-6; “7” IL-10; “8” IP-10; “9” KC; “10” M-CSF; “11” MCP-1; “12” MIP-1α; “13” MIP-1β; “14” MIP-2; “15” RANTES; “16” SDF-1; “17” TIMP-1; “18” TNF-α. b Analysis of optical density was captured by the Image-Pro Plus version 6.0. Values are means ± S.D. and significant difference compared with LPS treated alone, **P < 0.01
Anti-inflammation of EEDL in vitro
Cytotoxicity of EEDL on RAW 264.7 cells
The cytotoxicity of EEDL on RAW 264.7 cells was detected with MTT assay. Comparing with the blank control group, no significant variation of optical density was observed among each group (Additional file 1). It suggested that all concentrations of EEDL, L-NAME and aspirin used were not toxic to RAW 264.7 cells.
Effect of EEDL on NO production, iNOS mRNA and protein expression
To estimate how EEDL modulated inflammation responses, we first investigated the effect of EEDL on LPS-induced nitrite accumulation in LPS-induced RAW 264.7 cells. Results in Fig. 4a showed the nitrite concentrated in culture medium was increased 4 times after stimulated by LPS (P < 0.01), and a dose-dependent antagonism was observed in EEDL treatment group (P < 0.01). Moreover, the effectiveness of the highest concentration was better than aspirin but far less than that of L-NAME. Similarly, confocal scanning micrographs displayed EEDL was a potential anti-inflammation agent by reducing the intracellular NO production stimulated by LPS alone (Fig. 4c).
Fig. 4.
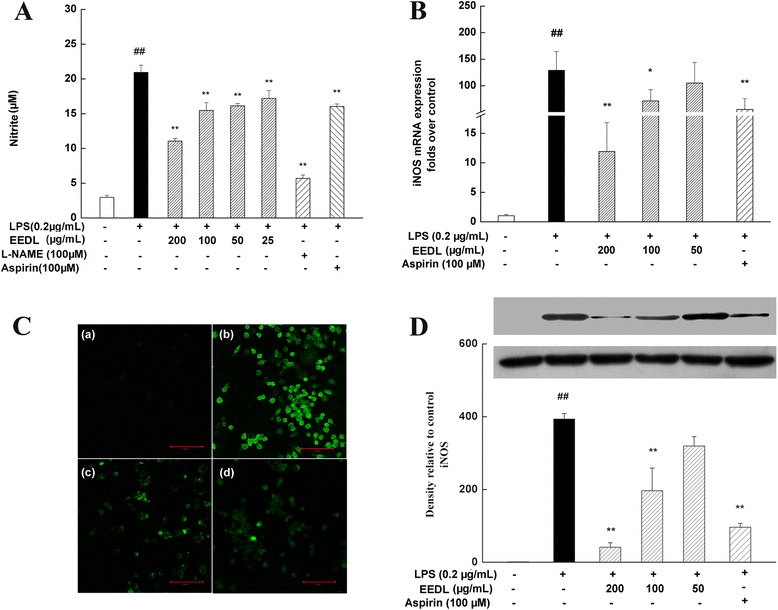
Effects of EEDL on NO and iNOS levels in LPS-stimulated RAW 264.7 cells. a Nitrite production secreted in the culture medium was monitored by the Griess reagent system. Values are means ± SD (n = 6) from three independent experiments and significant difference compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, **P < 0.01 vs. LPS-treated group. b RAW 264.7 cells were pretreated with EEDL (200 μg/mL) and 0.2 μg/mL LPS for 18 h. The mRNA expression of iNOS was detected by real-time RT-PCR. Values are means ± SD (n = 3) and significance compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, *P < 0.05, **P < 0.01 vs. LPS-treated group. c Intracellular NO was detected by confocal laser scanning microscope: (a) blank control; (b) cells pretreated with LPS; (c) cells pretreated with EEDL in the present of LPS; (d) cells pretreated with 100 μM L-NAME in the present of LPS. d The protein expression of iNOS was measured with Western blot. The optical density was captured by the Image-Pro Plus version 6.0. Values are means ± SD (n = 3) and significant difference compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, **P < 0.01 vs. LPS-treated group
For the detections of iNOS mRNA and protein expression, EEDL concentration-dependently decreased the abnormal iNOS mRNA (Fig. 4b) and protein (Fig. 4d) (P < 0.01 or P < 0.05) expression. Especially, administrated with the high dose of EEDL (200 μg/mL) displayed more potently anti-inflammatory effect (inhibitory rate was about 90 %) on reducing the mRNA and protein expressions of iNOS compared with positive control group, pretreated with aspirin.
Effect of EEDL on PGE2 production, COX-2 mRNA and protein expression
PGE2 is another important inflammatory mediator. Its production is regulated by COX-2 which catalyzed the metabolism of arachidonic acid. The high concentration of PGE2 increases vascular permeability and evokes the inflammatory reaction. As shown in Fig. 5a, EEDL at 200 and 100 μg/mL possessed significant inhibitory activities on LPS-induced PGE2 over-production (P < 0.05 or P < 0.01) and stronger than that of aspirin. For COX-2 mRNA expression (Fig. 5c), a dose-dependent inhibition was observed (200 μg/mL, P < 0.01). To evaluate the regulation of EEDL on COX-2 protein expression, cell-based ELISA (Fig. 5b) was performed. Results depicted that EEDL markedly reduced the LPS-induced COX-2 protein over-expression (P < 0.05 or P < 0.01).
Fig. 5.
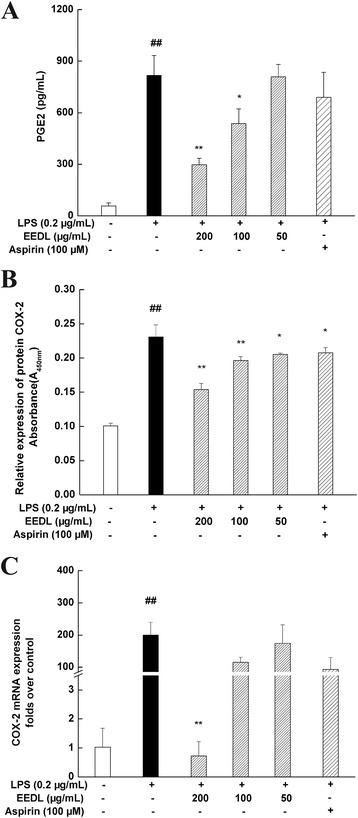
Effects of EEDL on levels of PGE2 and COX-2 in LPS-stimulated RAW 264.7 cells. PGE2 production (a) and COX-2 protein (b) and mRNA expression (C) were detected by ELISA and real-time RT-PCR. The protein expression of COX-2 (b) was detected by cell-based ELISA. Values are means ± SD (n = 6 for ELISA, n = 3 for real-time RT-PCR) and significant difference compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, *P < 0.05, **P < 0.01 vs. LPS-treated group
Effects of EEDL on IL-6 mRNA expression and protein secretion
As shown in Fig. 6, the dramatic increase of pro-inflammatory cytokine IL-6 was generally observed following the stimulation with LPS for 24 h. With the intervention of EEDL, however, IL-6 mRNA and protein levels were concentration-dependently decreased (P < 0.05 or P < 0.01). The inhibitory rate of IL-6 protein secretion was above 50 %.
Fig. 6.
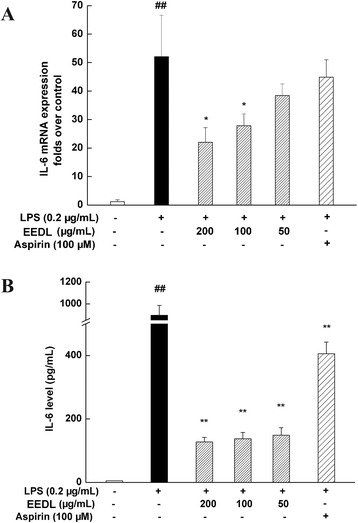
Effects of EEDL on IL-6 mRNA expression and protein secretion in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with the indicated concentrations of EEDL in the presence of LPS (0.2 μg/mL) for 24 h. The mRNA expression of IL-6 (a) was determined by real-time RT-PCR. Values are means ± SD (n = 3) and significant difference compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, *P < 0.05 vs. LPS-treated group. The protein secretion of IL-6 (b) was measured with ELISA. Values are means ± SD (n = 6) and significant difference compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, **P < 0.01 vs. LPS-treated group
Effects of EEDL on multiple cytokines in LPS-induced RAW 264.7 cells
As shown in Fig. 7, LPS resulted in a burst of 15 inflammation-related proteins in cell lysate, including C5/C5a, G-CSF, IL-1α, IL-1β, IL-1ra, IP-10, KC, MCP-1, MIP-1α, MIP-1β, MIP-2, RANTES, SDF-1, TIMP-1, and TNF-α. However, EEDL treatment (200 μg/mL) notably alleviated the over-expressions of C5/C5a, G-CSF, IL-1α, IL-1β, IL-1ra, IP-10, KC, MCP-1, MIP-1α, MIP-2, RANTES, SDF-1, TIMP-1, and TNF-α (P < 0.01 or P < 0.05), but with no significant influence on MIP-1β (P > 0.05).
Fig. 7.
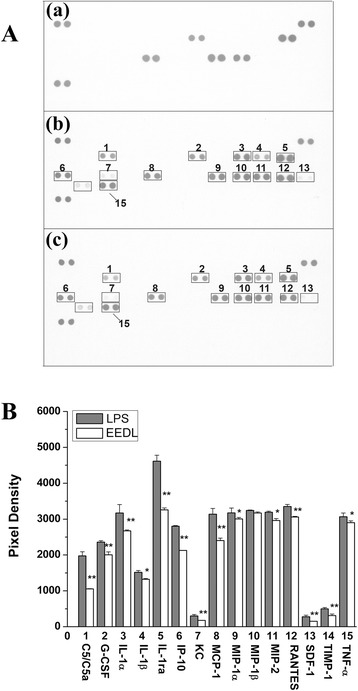
Effects of EEDL on LPS-induced multiple cytokines production in RAW 264.7 cells. a Expression of multiple proteins in the acute inflammation was measured by proteome profile array. (a) Blank control; (b) RAW 264.7 cells were treated with LPS alone; (c) RAW 264.7 cells were treated with LPS + EEDL. Numbers marked on the membranes are presented as: “1” C5/C5a; “2” G-CSF; “3” IL-1α; “4” IL-1β; “5” IL-1ra; “6” IP-10; “7” KC; “8” MCP-1; “9” MIP-1α; “10” MIP-1β; “11” MIP-2; “12” RANTES; “13” SDF-1; “14” TIMP-1; “15” TNF-α. b Analysis of optical density was captured by the Image-Pro Plus version 6.0. Values are means ± SD and significant difference compared with LPS treated alone, *P < 0.05, **P < 0.01
Effects of EEDL on the DNA-binding activity of NF-κB p65
The activation of NF-κB signaling pathway has been known to play a critical role in the inflammation response. As illustrated in Fig. 8, LPS induced a one-fold increase on the DNA-binding activity of NF-κB p65. After intervened with EEDL for 30 min, however, the over-binding was decreased by EEDL in a dose-dependent manner (P < 0.01 or P < 0.05).
Fig. 8.
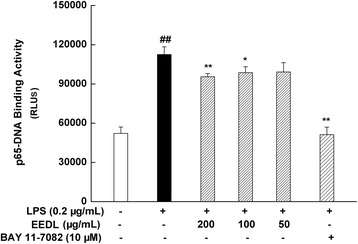
Effects of EEDL on LPS-induced DNA-binding activity of NF-κB p65 in RAW 264.7 cells. RAW 264.7 cells cultured in 6-well plate were pre-treated with EEDL (200, 100 and 50 μg/mL) for 2 h, then the LPS (with a final concentration of 0.2 μg/mL) was added for another 30 min, then the cell lysates were extracted and quantified. The DNA-binding activity of NF-κB p65 was measured by transcription factor assay. Values are means ± SD (n = 6) and significant difference compared with blank control or LPS treated alone, ## P < 0.01 vs. blank control group, *P < 0.05, **P < 0.01 vs. LPS-treated group
Discussion
Mounting evidence highlights that a systemic inflammatory state contributes to the cardiovascular risk [28]. The circulating inflammation mediators take part in the mechanisms of vascular insult and atheromatous changes. As a cardiovascular protective agent, many components of DLP have been proved to have the capacity of anti-inflammation such as gallic acid [29], salvianic acid [30], puerarin [13], daidzin [31], paeoniflorin [32], salvianolic acid B [33], cryptotanshinone [21], and tanshinone IIA [34]. Here, we presented results from the investigation on the efficacy of EEDL on inflammation induced by LPS in vivo and in vitro.
Studies have shown that LPS injection in mice caused significant inflammatory damage (such as biochemical and histological damage in lung and liver) [23, 35]. In the present study, we used the mice model to investigate anti-inflammation of EEDL. Results showed that EEDL possessed a strong and wide anti-inflammatory effect. In detail, EEDL was effective for antagonizing the over-expression of C5/C5a, G-CSF, I-309, sICAM-1, IL-1ra, IL-6, IP-10, KC, M-CSF, MCP-1, MIP-1α, MIP-1β, MIP-2, RANTES, SDF-1, TIMP-1, and TNF-α (P < 0.01), and increasing IL-10 protein level in mice serum (P < 0.01). The in vivo experiments preliminarily indicated that EEDL might be an effective anti-inflammatory agent.
According to the animal experiments, we tried to further explore the anti-inflammation mechanisms on RAW 264.7 cells, which depicted that treatment of RAW 264.7 cells with EEDL could modulate the iNOS/NO, COX-2/PGE2 and cytokine levels by declining the DNA-binding activity of NF-κB p65. iNOS is an important enzyme for mediating inflammatory processes. Generally, NO is at low concentration under physiological conditions which acts as a vasodilator by inhibiting the adhesion of neutrophils to the vascular endothelium [36]. However, large amounts of NO are produced in the inflammatory tissue by the activation of iNOS in both resident tissue cells and infiltrating leucocytes when inflammation was triggered [37]. In this study, we found that the production of NO, intracellular NO, as well as the mRNA and protein expression of iNOS were all up-regulated by LPS, whereas they were attenuated by EEDL treatment. These results indicated that the inhibition of iNOS/NO might be one of the anti-inflammatory targets of EEDL.
In addition to iNOS, COX-2, which is an inducible and isoform enzyme in inflammatory cells [38], is another vital enzyme for mediating inflammatory processes. COX-2 is activated by physiologic stimuli such as inflammation, and is involved in the production of PGs that mediate pain and support the inflammatory process [39]. The PGE2, produced by the enzymatic oxidation of arachidonic acid, has been implicated as inflammatory mediators for many years [40]. Additionally, PGs have wide-ranging effects in the body [38]. In this work, we found that EEDL could remarkably decrease the burst production of PGE2 based on the detection of EEDL on LPS-induced inflammation.
Moreover, the cell-based ELISA showed that EEDL could dose-dependently antagonize the over-expression of COX-2, which depicted that inhibition of COX-2/PGE2 might be another anti-inflammatory target.
Numerous pro-inflammatory cytokines are present in the inflammation process, such as IL-1, TNF, macrophage inflammatory protein (MIP). Firstly, we found that EEDL effectively attenuated the over-expression of IL-6 mRNA and protein induced by LPS in a concentrate-dependent manner. In addition to the ELISA method, protein profile array analysis, a high-throughput way to screen the different acute phase proteins, cytokines, and chemokines in a particular inflammatory process, further indicated that EEDL suppressed LPS-induced inflammation via decreasing multiple pro-inflammatory cytokines such as the common IL-1β, TNF-α, MIP-2.
Studies have demonstrated that NF-κB is involved in the regulation of COX-2 and iNOS expression, namely, blocking the improper NF-κB activation could inhibit the expression of COX-2 and iNOS [41]. Syntheses of cytokines, such as IL-1β, TNF-α, IL-6, are also mediated by NF-κB [42]. Various proteins in NF-κB family play an important role in the defense of the host against certain pathogens [42]. For example, studies have shown that NF-κB p65 plays a role in constitutive IL-6 production in rheumatoid arthritis synovial fibroblasts. In this study, we designed experiment to investigate the DNA-binding activity of NF-κB p65 by transcription factor assay, which showed that the LPS-stimulated NF-κB activation was notably inhibited by EEDL, as indicated by a decreased DNA-binding activity of NF-κB p65.
Conclusions
We have unraveled a novel pharmacological effect that EEDL effectively suppressed the inflammation induced by LPS in mice. The anti-inflammation of EEDL may be one of the mechanisms of cardiovascular and cerebrovascular disease. Meanwhile, we have preliminarily deciphered the potential molecular mechanisms of EEDL, namely, inhibiting the LPS-induced iNOS/NO, COX-2/PGE2 and cytokines levels by decreasing the DNA-binding activity of NF-κB p65. However, we also aware that further researches are needed to obtain more definitive evidence for the deeper exploration.
Acknowledgements
This work was supported by the National Key Basic Research Program (973 project, No. 2014CB542902) and the National Natural Science Foundation of China (No. 81403198).
Additional file
Cytotoxicity of EEDL on RAW 264.7 cells. Cells cultured in the 96-well plate for 24 h were incubated with L-NAME (100 μM), aspirin (100 μM) and indicated dilutions of EEDL (200, 100, 50 and 25 μg/mL) in the presence of LPS (0.2 μg/mL) for 20 h. After MTT reagent was added for an additional 2 h, the absorbance was recorded at 570 nm. Values are means ± SD (n=6) from three independent experiments and there is no significant difference compared with LPS treated cells alone. (TIFF 284 kb)
Footnotes
Li-Na Gao and Xin Zhou contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The study concepts and design were provided by CYL and YCQ. GLN, ZX and ZY contributed to experiments performance and data collection. GLN, ZX and GS participated in data evaluation. The manuscript was drafted by GLN and ZX. Manuscript editing and review was performed by CYL and GLN. GLN and ZX contributed equally to this paper. All authors read and approved the final manuscript.
Contributor Information
Li-Na Gao, Email: linagao228@126.com.
Xin Zhou, Email: xx2015_hi@163.com.
Yi Zhang, Email: 36700259@qq.com.
Yuan-Lu Cui, Email: cuiyl@tju.edu.cn.
Chun-Quan Yu, Email: ycq-4@163.com.
Shan Gao, Email: bianjibugs@163.com.
References
- 1.Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.Zhao N, Liu YY, Wang F, Hu BH, Sun K, Chang X, et al. Cardiotonic pills, a compound Chinese medicine, protects ischemia-reperfusion-induced microcirculatory disturbance and myocardial damage in rats. Am J Physiol Heart Circ Physiol. 2010;298:H1166–76. doi: 10.1152/ajpheart.01186.2009. [DOI] [PubMed] [Google Scholar]
- 3.Wang SH, Wang J, Li J. Efficacy assessment of treating patients with coronary heart disease angina of phlegm and stasis mutual obstruction syndrome by Danlou tablet. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1051–5. [PubMed] [Google Scholar]
- 4.Xu XK, Yang Q. The clinical curative effect of Danlou tablet in the treatment of phlegm and blood stasis type transresistance senile coronary heart disease angina pectoris. Chin J Gerontol. 2013;20:5126–7. [Google Scholar]
- 5.Zhou MJ. The clinical curative effect observation of Danlou tablet in the treatment of 70 cases of patients with phlegm and blood stasis type transresistance coronary heart disease angina. Chin J Ethnomed Ethnopharm. 2013;3:98. [Google Scholar]
- 6.Fang ZF, Zhou SH, Hu XQ. Changes of plasma adhesion molecule levels of coronary circulation in patient with acute coronary syndrome. Chin J Arterial Lerosis. 2005;2:215–7. [Google Scholar]
- 7.Dong J, Zhu Y, Gao X, Chang Y, Wang M, Zhang P. Qualitative and quantitative analysis of the major constituents in Chinese medicinal preparation Dan-Lou tablet by ultra high performance liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. 2013;80:50–62. doi: 10.1016/j.jpba.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Lu YT, Du DT. GW24-e2170 impact of different dose rosuvatation on the expression of CD147 in atherosclerotle plaque of rabbit. Heart. 2013;99:A91. doi: 10.1136/heartjnl-2013-304226. [DOI] [Google Scholar]
- 9.Ji RZ, Yu CH, He ZQ, Wu F, Liang C, Wu ZG. GW24-e1008 the hypolipidaemic activity of danlou tablet extract in triton WR-1339 induced hyperlipidaemic rats: a comparison with rosuvastatin. Heart. 2013;99:A90–1. [Google Scholar]
- 10.Xie S, Wang Y, Li B, Nan X, Liu Y, Zhang B, et al. GW24-e2125 protective effects of a prescription of JIASHEN on myocardial infarction via inhibition of inflammation in rats. Heart. 2013;99:A91. doi: 10.1136/heartjnl-2013-304613.246. [DOI] [Google Scholar]
- 11.Prasain JK, Reppert A, Jones K, Moore I, Ray D, Barnes S, et al. Identification of isoflavone glycosides in Pueraria lobata cultures by tandem mass spectrometry. Phytochem Anal. 2007;18:50–9. doi: 10.1002/pca.951. [DOI] [PubMed] [Google Scholar]
- 12.Yi T, Leung KS-Y, Lu G-H, Chan K, Zhang H. Simultaneous qualitative and quantitative analyses of the major constituents in the rhizome of Ligusticum chuanxiong using HPLC-DAD-MS. Chem Pharm Bull. 2006;54:255–9. doi: 10.1248/cpb.54.255. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y, Hsieh CY, Peng ZA, Yen TL, Hsiao G, Chou DS, et al. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J Biomed Sci. 2009;16:1–13. doi: 10.1186/1423-0127-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang YX, Ding XP, Qi J, Cao J, Kang LY, Zhu DN, et al. Determination of phenolic acids in danshen preparations by LC with chemiluminescence detection. Chromatographia. 2009;69:319–23. doi: 10.1365/s10337-008-0876-z. [DOI] [Google Scholar]
- 15.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Eng J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 16.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 17.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.CIR.104.3.365. [DOI] [PubMed] [Google Scholar]
- 18.Hsiang CY, Hseu YC, Chang YC, Kumar KJ, Ho TY, Yang HL. Toona sinensis and its major bioactive compound gallic acid inhibit LPS-induced inflammation in nuclear factor-κB transgenic mice as evaluated by in vivo bioluminescence imaging. Food Chem. 2013;136:426–34. doi: 10.1016/j.foodchem.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Hu WZ, Yang XJ, Zhe C, Zhang Q, Sun L, Cao KJ. Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-B activation in LPS-induced RAW264.7 macrophage cells. Pharmacol Rep. 2011;63:781–9. doi: 10.1016/S1734-1140(11)70590-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang QS, Gao T, Cui YL, Gao LN, Jiang HL. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol. 2014;52:1189–95. doi: 10.3109/13880209.2014.880490. [DOI] [PubMed] [Google Scholar]
- 21.Tang S, Shen XY, Huang HQ, Xu SW, Yu Y, Zhou CH, et al. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW 264.7 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation. 2011;34:111–8. doi: 10.1007/s10753-010-9214-3. [DOI] [PubMed] [Google Scholar]
- 22.Xu M, Cao FL, Zhang YF, Shan L, Jiang XL, An XJ, et al. Tanshinone IIA therapeutically reduces LPS-induced acute lung injury by inhibiting inflammation and apoptosis in mice. Acta Pharmacol Sin. 2015;36:179–87. doi: 10.1038/aps.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao LN, Cui YL, Wang QS, Wang SX. Amelioration of Danhong injection on the lipopolysaccharide-stimulated systemic acute inflammatory reaction via multi-target strategy. J Ethnopharm. 2013;149:772–82. doi: 10.1016/j.jep.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 24.Feron M, Guevel L, Rouger K, Dubreil L, Arnaud MC, Ledevin M, et al. PTEN contributes to profound PI3K/Akt signaling pathway deregulation in dystrophin-deficient dog muscle. Am J Pathol. 2009;174:1459–70. doi: 10.2353/ajpath.2009.080460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, et al. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67:3094–105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci. 2008;105:2865–70. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raspotnig G, Fauler G, Jantscher A, Windischhofer W, Schachl K, Leis HJ. Colorimetric determination of cell numbers by Janus green staining. Anal Biochem. 1999;275:74–83. doi: 10.1006/abio.1999.4309. [DOI] [PubMed] [Google Scholar]
- 28.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 29.Kroes B, Van den Berg A, Van Ufford HQ, Van Dijk H, Labadie R. Anti-inflammatory activity of gallic acid. Planta Med. 1992;58:499–504. doi: 10.1055/s-2006-961535. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Wu X, Yao Z, Li H. The influence of emodin and danshensu on monocyte^ s secretion of inflammatory cytokines. Chin J Immunol. 1994;11:370–2. [Google Scholar]
- 31.Kim SH, Heo JH, Kim YS, Kang SS, Choi JS, Lee SM. Protective effect of daidzin against d‐galactosamine and lipopolysaccharide‐induced hepatic failure in mice. Phytother Res. 2009;23:701–6. doi: 10.1002/ptr.2710. [DOI] [PubMed] [Google Scholar]
- 32.Wang QS, Gao T, Cui YL, Gao LN, Jiang HL. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol. 2014;1–7. [DOI] [PubMed]
- 33.Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, et al. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem. 2001;82:512–21. doi: 10.1002/jcb.1176. [DOI] [PubMed] [Google Scholar]
- 34.Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM, et al. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol. 2009;113:275–80. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–8. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 36.Brown G. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc T. 2007;35:1119–21. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- 37.Downey D, Elborn J. Nitric oxide, iNOS, and inflammation in cystic fibrosis. J Pathol. 2000;190:115–6. doi: 10.1002/(SICI)1096-9896(200002)190:2<115::AID-PATH491>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 38.Dinchuk JE, Car BD, Focht RJ, Johnson JJ, Jaffee BD, Covington MB, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–9. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 39.Seibert K, Masferrer J. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1993;4:17–23. [PubMed] [Google Scholar]
- 40.Kuehl FA, Jr, Egan RW. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210:978–84. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- 41.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001;480:243–68. doi: 10.1016/S0027-5107(01)00183-X. [DOI] [PubMed] [Google Scholar]
- 42.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]


