Abstract
CUG-BP, Elav-like family member 1 (CELF1) is a multi-functional RNA binding protein that regulates pre-mRNA alternative splicing in the nucleus, as well as polyadenylation status, mRNA stability, and translation in the cytoplasm [1]. Dysregulation of CELF1 has been implicated in cardiomyopathies in myotonic dystrophy type 1 and diabetes [2], [3], [4], [5], but the targets of CELF1 regulation in the heart have not been systematically investigated. We previously demonstrated that in the developing heart CELF1 expression is restricted to the myocardium and peaks during embryogenesis [6], [7], [8]. To identify transcripts regulated by CELF1 in the embryonic myocardium, RNA-seq was used to compare the transcriptome of primary embryonic cardiomyocytes following siRNA-mediated knockdown of CELF1 to that of controls. Raw data files of the RNA-seq reads have been deposited in NCBI's Gene Expression Omnibus [9] under the GEO Series accession number GSE67360. These data can be used to identify transcripts whose levels or alternative processing (i.e., alternative splicing or polyadenylation site usage) are regulated by CELF1, and should provide insight into the pathways and processes modulated by this important RNA binding protein during normal heart development and during cardiac pathogenesis.
Keywords: CUG-BP, Elav-like family member 1 (CELF1), Heart, Cardiomyocytes, RNA-seq, Chicken
| Specifications | |
|---|---|
| Organism/cell line/tissue | Gallus gallus/primary embryonic cardiomyocytes/heart muscle |
| Sex | Mixed pool of male and female |
| Sequencer or array type | Illumina HiSeq 2000 |
| Data format | Raw |
| Experimental factors | Mock-transfected versus siRNA-mediated knockdown of CELF1 |
| Experimental features | Primary cardiomyocytes were isolated from chicken embryos at embryonic day 8 and transfected ± siRNA against CELF1. RNA-seq was performed to identify transcripts regulated by CELF1. |
| Consent | N/A |
| Sample source location | Fertilized chicken eggs were purchased from the Department of Animal Sciences at The Ohio State University in Columbus, OH. Eggs were shipped overnight via truck to the Cleveland Clinic Lerner Research Institute in Cleveland, OH, where eggs were incubated, cardiomyocytes were isolated, transfected, and cultured, and RNA was extracted. RNA samples were then shipped to The Ohio State University Comprehensive Cancer Center in Columbus, OH, where RNA-seq was performed. |
1. Direct link to deposited data
2. Experimental design, materials and methods
2.1. Isolation and culture of chicken primary embryonic cardiomyocytes
Fertilized Hy-line W-36 White Leghorn chicken eggs were purchased from the Department of Animal Sciences at The Ohio State University. Eggs were stored at 59 °F (15 °C) for up to one week until use. Eggs were incubated at 99–100 °F (37–38 °C), 40–60% humidity in a DandyLyon floor model incubator with auto-turning (900–156/4; Lyon Electric) for eight days to obtain embryos at Hamburger and Hamilton stage 35 [10]. Eggs were sprayed with ethanol, and embryos were removed inside a laminar flow tissue culture hood under sterile conditions. Chicken embryos were euthanized by decapitation immediately upon removal from the egg, which is consistent with the recommendations of the American Veterinary Medical Association Panel on Euthanasia for birds. Hearts were removed, washed three times with Hank's Balanced Salt Solution (HBSS; 165–1000; Cleveland Clinic Media Preparation Core), and minced as finely as possible with a sterile razor blade. Minced tissue was transferred to a 50 ml conical tube and digested with 0.13% trypsin (TRL3; Worthington Biochemicals), 0.13% collagenase (CLS-2; Worthington Biochemicals), and 0.033% DNase I (D2; Worthington Biochemicals) in HBSS at 37 °C for 20 min with agitation. An equal volume of growth medium [Minimal essential medium (MEM with Earle's salts and l-glutamine; 31–500; Cleveland Clinic Media Preparation Core) supplemented with 10% horse serum (16,050–122; Life Technologies) that has been heat-inactivated (30 min at 55 °C), 5% chick embryo extract (chick embryos were collected at embryonic day 12, decapitated, and passed through a 60 ml syringe; an equal volume of MEM was added to the homogenate and incubated with rocking for two hours at room temperature; suspensions were centrifuged at 500 × g for 5 min; the supernatant was collected, passed through a 0.45 μm filter, and stored at − 80 °C until use), and 1% penicillin/streptomycin (725–100; Cleveland Clinic Media Preparation Core)] was added and the undigested tissue was separated from dissociated cells by spinning at 60 × g for 2 min at room temperature. The supernatant containing the dissociated cells was removed to a 50 ml conical tube, and cells were pelleted by centrifugation at 500 × g for 5 min at room temperature, resuspended in growth medium, and kept on ice. Meanwhile, the remaining tissue was digested again in 0.13% trypsin/0.13% collagenase/0.033% DNase I in HBSS as before. Serial digestions were performed until all heart tissue was dissociated; typically, three rounds of digestion were enough. Dissociated cells from all digestions were pooled, pelleted for 5 min at 500 × g at room temperature, and resuspended in 1.082 g/ml Percoll (17–0891–01; Pharmacia) diluted in Ads buffer (116 mM NaCl, 0.83 mM MgSO4, 5.4 mM KCl, 12.5 mM NaH2PO4, 5.6 mM dextrose, and 20 mM HEPES, pH 7.3). A Percoll gradient was established by adding 4 ml of 1.050 g/ml Percoll diluted in Ads buffer in a 15 ml conical, then using a long 15-gauge needle to load 4 ml of 1.060 g/ml Percoll diluted in Ads buffer with 0.002% phenol red under the 1.050 g/ml layer, followed by the 1.082 g/ml Percoll + cell suspension below that. The Percoll gradient was spun at 2000 × g in a fixed-angle rotor for 25 min at room temperature. Purified myocytes were isolated from the interphase between the red 1.060 g/ml layer and the clear 1.082 g/ml layer. Purified cardiomyocytes were washed three times in Ads buffer with 0.002% phenol red, spinning at 500 × g for 5 min in between each wash to pellet cells. Cells were resuspended in growth medium and counted using a hemocytometer. Cardiomyocytes were plated on 0.005% Fibronectin (F1141; Sigma) at a density of 2.5 x 105 cells per 35 mm plate. Cells were maintained in a humidified atmosphere at 37 °C and 5% CO2.
2.2. Knockdown of CELF1 in chicken primary embryonic cardiomyocytes
Cardiomyocytes were transfected 24 h after plating (when cells were approximately 50–60% confluent) using Lipofectamine 2000 transfection reagent (Life Technologies) according to the manufacturer's directions. siRNA duplexes against CELF1 (“si1”: 5′-GGGUGCUGUUUUGUUACAUdTdT-3′, “si2”: 5′-GAGCCGAGGUUGUGCAUUUdTdT-3′) [11] were used at a final concentration of 100 nM. Transfection of parallel plates with the fluorescently labeled, RISC-independent control siGLO Red confirmed transfection efficiency was nearly 100%. All siRNAs were purchased from Dharmacon (now Thermo Scientific). Samples were collected 72 h after transfection and knockdown of CELF1 was confirmed by Western blot analysis.
To collect total protein samples, cells were rinsed in PBS, scraped into protein collection buffer [0.2% Triton X-100 in PBS, supplemented with 1 × c0mplete Protease Inhibitor Cocktail (11 873 580 001; Roche)], sonicated, and stored at − 80 °C until use. Protein concentrations were quantitated using a Bio-Rad Protein Assay (500-0006; Bio-Rad) according to manufacturer's instructions. 30 μg of each protein sample was combined with an equal volume of 2 × protein loading buffer [64 mM Tris, pH 6.8, 10% glycerol, 2% sodium dodecyl sulfate (SDS), 5% β-mercaptoethanol, and 0.01% bromphenol blue], boiled for 5 min, and resolved on 10% denaturing SDS polyacrylamide gels by electrophoresis. Proteins were transferred onto Immobilon-P PVDF membrane (IPVH 000 10; Fisher Scientific), and probed as previously described [12] with the following modification: bands were visualized using Immobilon Western Chemiluminescent HRP Substrate (WBKL S0 500; Fisher Scientific). CELF1 was detected using a mouse monoclonal anti-CUG-BP1 antibody, 3B1 (sc-20,003; Santa Cruz Biotechnology), and a goat anti-mouse-HRP secondary antibody (DC02L; Calbiochem). To confirm equivalent loading, membranes were stripped using Restore Western Blot Stripping Buffer (PI-21,059; Fisher Scientific) and reprobed for GAPDH using a mouse monoclonal anti-GAPDH antibody, clone 6G5 (4699-9555; Biogenesis), and goat anti-mouse-HRP secondary antibody (DC02L; Calbiochem). Protein integrity and equivalent loading were also confirmed by Ponceau S staining. CELF1 expression was high in mock-transfected cells, consistent with high levels of expression in the embryonic myocardium [6], [7], [8]. Transfection with si1 moderately reduced CELF1 protein expression, whereas transfection with si2 produced a more robust knockdown; siGLO Red had no effect on CELF1 protein levels (Fig. 1).
Fig. 1.
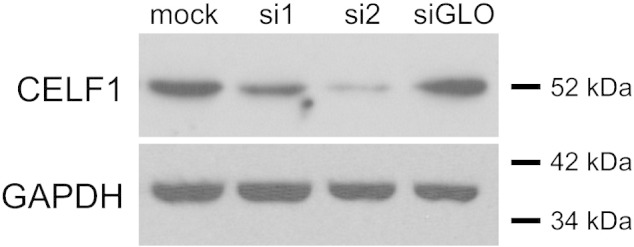
siRNA-mediated knockdown of CELF1 in chicken primary embryonic cardiomyocytes. Western blots were performed on total protein samples collected from primary embryonic cardiomyocytes transfected without (mock) or with siRNAs against CELF1 (si1 and si2) or a non-targeting control siRNA (siGLO). Protein integrity and equivalent loading was confirmed by Ponceau S staining (not shown) and by stripping and reprobing membranes for GAPDH. A representative of several independent transfections is shown.
2.3. RNA-seq and analysis
Total RNA was extracted from plates transfected in parallel with those from which total protein was collected. Cells were rinsed in PBS and scraped into Trizol (15596018; Life Technologies), and RNA was isolated according to the manufacturer's instructions. RNA samples were quantified using a NanoDrop Spectrophotometer (Thermo Scientific). Mock- and si2-transfected samples were chosen from each of three independent transfections for an n = 3 per group; si2-transfected samples were chosen to represent the CELF1-depleted state since si2 consistently gave a more robust knockdown than si1 (Fig. 1). RNA quality control, library preparation, and high-throughput sequencing were performed using an Illumina HiSeq 2000 platform through The Ohio State University Comprehensive Cancer Center. Subsequent read mapping and differential gene expression analyses were performed by the Case Western Reserve University Genomics Core. Reads that were 51 bases were trimmed to 50 bases by removing a single base from the 3′ end. The genome sequence and corresponding annotation of Gallus gallus from Ensembl release 73, which has 17,108 genes and 17,954 transcripts, were used as the reference. Reads were mapped to the reference sequence using TopHat v2.0.9 under the guidance of Ensembl annotation for more accurate mapping of the transcripts. Over 85% of the reads were successfully mapped to the genome for all six samples. The individual transcriptomes of the six samples were generated with Cufflinks v2.1.1; these were then merged to generate a comprehensive transcriptome that was used as the reference for differential gene expression analysis using CuffDiff. Using no cutoff for the scale of differences, this identified 10,021 transcripts that differed between mock- and si2-transfected samples with P values ≤ 0.05 after correction for multiple testing and accounting for false discovery rate. Using a two-fold cutoff, 3346 transcripts were up-regulated following CELF1 knockdown (including 1535 transcripts detected only in si2-transfected samples) and 1653 transcripts were down-regulated (including 308 transcripts that were detected only in mock-transfected samples). Notably, CELF1 transcript levels were reduced more than 2.5-fold in si2-transfected samples relative to controls. Raw data files of the RNA-seq reads have been deposited in NCBI's Gene Expression Omnibus [9], and are accessible through GEO Series accession number GSE67360. Further study of the transcripts regulated by CELF1 may shed light on how this RNA binding protein contributes to cardiac pathogenesis [1], [2], [3], [4], [5].
2.4. Validation of differential gene expression by real-time polymerase chain reaction
CELF1 and three other differentially expressed transcripts (CELF2, MBNL1, and HOPX) were chosen for validation by qRT-PCR. Total RNA was extracted from primary embryonic cardiomyocytes that were mock-transfected or transfected with si1, si2, or siGLO siRNA as described above. Reverse transcription reactions were performed using the Superscript-VILO cDNA Synthesis Kit (Life Technologies, formerly Invitrogen) in a 20 μl reaction volume with 2 μg of total RNA. cDNA was quantified using the Quant-iT OliGreen ssDNA Assay Kit (Life Technologies), and diluted to 5 ng/μl in water. All cDNA samples were stored at − 20 °C until use. Real-time PCR reactions were performed using the StepOnePlus platform (Life Technologies, formerly Applied Biosystems), using the following program: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. CELF1, CELF2, and MBNL1 transcript levels were determined using TaqMan probes (Life Technologies) for chicken CELF1 (FAM-labeled, Gg03340922_m1) CELF2 (FAM-labeled, Gg03364304_m1), and MBNL1 (FAM-labeled, Gg03356554_m1) normalized against GUSB or GAPDH (VIC-labeled, Gg03346982, primer limited). Each reaction consisted of 1 × TaqMan Gene Expression Master Mix, 1 × endogenous control probe, 0.25 × target gene probe, 5 ng cDNA, and water to 20 μl final volume. HOPX transcript levels were determined using SYBR Green Master Mix (Life Technologies) and normalized to GAPDH. Primer sequences: HOPX = AGGAGCAGCATCCTCTAGTCC and TTGCAAGGTGAACAAGCATC (product size = 140 bp); GAPDH = GATACACAGAGGACCAGGTTG and ACGGTTGCTGTATCCAAACTC, or CAAGAGGGTAGTGAAGGCTG and AATGGTCATTCAGTGCAATGC (primer pairs were used interchangeably; product size = 146 bp for both primer pairs). Samples from at least three independent transfections were each run with 3–4 technical replicates. Data were analyzed with StepOne software v2.1 using the comparative CT (∆∆CT) method, normalizing target gene levels to the endogenous control values. Melt curves were generated after each PCR run to ensure that only a single amplicon was present in each reaction. Statistical comparisons of means were performed via one-tailed t-tests assuming unequal variances using Microsoft Excel software. Differences were considered statistically significant when P ≤ 0.05. Each of the four transcripts showed a significant difference in expression level between mock- and si2-transfected samples that was similar in direction and order of magnitude to the difference observed in the RNA-seq data. Expression levels in siGLO-transfected samples resembled those of mock-transfected controls, while si1-transfected cells showed responses similar to si2-transfected cells, although in some cases to a lesser degree. Thus, overall there is strong agreement between the RNA-seq and qRT-PCR results.
Acknowledgments
This work was supported by a grant to A.N.L. from the National Institutes of Health (1R01HL089376). Y.B-H. was supported in part by a National Institutes of Health training grant (5T32GM008056). The National Institutes of Health had no involvement in the study design, collection, analysis, or interpretation of the data, or the writing or submission of this article for publication.
References
- 1.Dasgupta T., Ladd A.N. The importance of CELF control: molecular and biological roles of the CUG-BP, Elav-like family of RNA-binding proteins. Wiley Interdiscip. Rev. RNA. 2012;3:104–121. doi: 10.1002/wrna.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philips A., Timchenko L., Cooper T. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–741. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- 3.Timchenko N., Cai Z.-J., Welm A., Reddy S., Ashizawa T., Timchenko L. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J. Biol. Chem. 2001;276:7820–7826. doi: 10.1074/jbc.M005960200. [DOI] [PubMed] [Google Scholar]
- 4.Wang G.S., Kearney D.L., De Biasi M., Taffet G., Cooper T.A. Elevation of RNA-binding protein CUGBP1 is an early event in an inducible heart-specific mouse model of myotonic dystrophy. J. Clin. Invest. 2007;117:2802–2811. doi: 10.1172/JCI32308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma S.K., Deshmukh V., Liu P., Nutter C.A., Espejo R., Hung M.L. Reactivation of fetal splicing programs in diabetic hearts is mediated by protein kinase C signaling. J. Biol. Chem. 2013;288:35372–35386. doi: 10.1074/jbc.M113.507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladd A., Stenberg M., Swanson M., Cooper T. A dynamic balance between activation and repression regulates pre-mRNA alternative splicing during heart development. Dev. Dyn. 2005;233:783–793. doi: 10.1002/dvdy.20382. [DOI] [PubMed] [Google Scholar]
- 7.Blech-Hermoni Y., Stillwagon S.J., Ladd A.N. Diversity and conservation of CELF1 and CELF2 RNA and protein expression patterns during embryonic development. Dev. Dyn. 2013;242:767–777. doi: 10.1002/dvdy.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brimacombe K.R., Ladd A.N. Cloning and embryonic expression patterns of the chicken CELF family. Dev. Dyn. 2007;236:2216–2224. doi: 10.1002/dvdy.21209. [DOI] [PubMed] [Google Scholar]
- 9.Edgar R., Domrachev M., Lash A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 11.Vajda N.A., Brimacombe K.R., LeMasters K.E., Ladd A.N. Muscleblind-like 1 is a negative regulator of TGF-beta-dependent epithelial-mesenchymal transition of atrioventricular canal endocardial cells. Dev. Dyn. 2009;238:3266–3272. doi: 10.1002/dvdy.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladd A., Charlet-B N., Cooper T. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol. Cell. Biol. 2001;21:1285–1296. doi: 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


