Abstract
For herbivores, nutrient intake is limited by the relatively low nutritional quality of plants and high concentrations of potentially toxic defensive compounds (plant secondary metabolites, PSMs) produced by many plants. In response to phytochemical challenges, some herbivores selectively forage on plants with higher nutrient and lower PSM concentrations relative to other plants. Pygmy rabbits (Brachylagus idahoensis) are dietary specialists that feed on sagebrush (Artemisia spp.) and forage on specific plants more than others within a foraging patch. We predicted that the plants with evidence of heavy foraging (browsed plants) would be of higher dietary quality than plants that were not browsed (unbrowsed). We used model selection to determine which phytochemical variables best explained the difference between browsed and unbrowsed plants. Higher crude protein increased the odds that plants would be browsed by pygmy rabbits and the opposite was the case for certain PSMs. Additionally, because pygmy rabbits can occupy foraging patches (burrows) for consecutive years, their browsing may influence the nutritional and PSM constituents of plants at the burrows. In a post hoc analysis, we did not find a significant relationship between phytochemical concentrations, browse status and burrow occupancy length. We concluded that pygmy rabbits use nutritional and chemical cues while making foraging decisions.
Keywords: foraging, monoterpenes, nutrition, pygmy rabbit, sagebrush
Foraging theory predicts animals should make foraging decisions to regulate the excess or deficiencies of dietary components while minimizing the costs associated with foraging (Stephens and Krebs 1986). For herbivores, energy intake is partially limited by the relatively low nutritional quality of plants resulting from low protein content, physical plant defenses (e.g., cellulose, thorns) and plant secondary metabolites that serve as digestibility or intake reducers (Laca et al. 2001; Dziba and Provenza 2008). Plant protein has been associated with habitat use, reproductive success, and survival in several herbivores (Beckerton and Middleton 1982; DeGabriel et al. 2009), and it is important to mammalian herbivores (McArt et al. 2009). Plant secondary metabolites (PSMs) often result in metabolic costs that outweigh energy intake (Dearing et al. 2005). For example, energy expenditure while consuming PSMs is similar to costs of reproduction and locomotion in a generalist mammalian herbivore, the white-throated wood rat (Neotoma albigula), and cannot be compensated through increases in intake (Sorensen et al. 2005b). In response, several herbivores employ physiological mechanisms to increase tolerance of PSMs and achieve nutritional balance of protein and energy (Karban and Agrawal 2002; Sorensen and Dearing 2006). However, the physiological mechanisms that maximize tolerance to PSMs might not fully compensate for the nutritional imbalance imposed by both low nutrients and high PSMs in host plants of specialists. Selecting diets consisting of higher quality plants is a behavioral adaptation through which herbivores can maximize nutritional intake and minimize PSM intake. Many herbivores select plants with either higher crude protein content (Somers et al. 2008) or lower PSM concentrations (Guglielmo et al. 1996; Sorensen et al. 2005a), and recent studies have explored how protein content and PSMs simultaneously affect diet selection and habitat selection in free-living animals (Moore et al. 2010).
As a consequence of selective browsing pressure on more nutritious plants, herbivores have the potential to change the nutritional quality, PSM concentrations, and morphology of some plants (Bryant et al. 1992; Stolter 2008 - as an example of postive feedback loop). For example, in response to simulated and natural browsing pressure from snowshoe hares (Lepus americanus), white pine (Picea glauca) produces higher concentrations of the PSM camphor (Sharam and Turkington 2009). Such interactions are relatively understudied in natural systems, in part, because of the difficulty of reliably observing foraging choices in the field and the complex interactions between plant nutrients and PSMs. Not only did we expect pygmy rabbits to be selective while foraging, but as obligate burrowers that forage on and around the burrow systems they inhabit for extended periods of time (Green and Flinders 1980; Sanchez et al. 2009), rabbits potentially influence the phytochemistry of plants at their burrows. Burrowing animals can affect root composition, nutrient availability, and species composition of plants surrounding the burrow system (Bruun et al. 2005). Mammalian browsers often remove apical meristems of plants, which leads to rapid growth of new leaves that are frequently higher in nitrogen content (Orians et al. 2010). Additionally, constant browsing pressure may increase concentrations of PSMs, such as monoterpenes and phenolics, depending on the plant species (Bryant et al. 1992; Karban 2011).
The sagebrush (Artemisia spp.)–pygmy rabbit (Brachylagus idahoensis) system in western North America is an ideal model system for investigating how nutritional and chemical factors shape diet selection by a specialist mammalian herbivore. First, although sagebrush is highly digestible compared to other plants, it contains a diverse suite of PSMs including monoterpenes, sesquiterpene lactones, and phenolic compounds that deter herbivores (Personius et al. 1987; Frye et al. 2013). Moreover, concentrations of protein and PSMs in sagebrush vary among seasons, subspecies, and individual sagebrush plants within and among populations (Welch and McArthur 1979; 1981) and dietary selection of specific sagebrush plants in the field can be documented readily by identifying bite marks that are characteristic only of pygmy rabbits. Moreover, pygmy rabbits prefer to feed on plants that are more heavily browsed in the field; captive pygmy rabbits ate 2.4 times more leaves collected from browsed versus unbrowsed plants in a choice feeding trial (Ulappa 2011). Third, although a few mammals such as pronghorn (Antilocapra americana) and mule deer (Odocoileus hemionus), feed on sagebrush seasonally as part of their diet, the pygmy rabbit is the only facultative mammalian specialist of sagebrush (Shipley et al. 2009) as sagebrush constitutes up to 99% of its winter diet (Green and Flinders 1980). Although pygmy rabbits have a high tolerance to PSMs in sagebrush compared to other vertebrate herbivores such as cottontail rabbits (Sylvilagus floridanus), PSMs do reduce sagebrush intake by pygmy rabbits (Shipley et al. 2006). These results suggest that pygmy rabbits might not be able to meet energetic demands solely by consuming a larger quantity of sagebrush as a behavioral strategy due to the presence of relatively high concentrations of PSMs, and they may instead select higher quality plants.
Our objectives were to 1) identify if nutritional and chemical factors explain differential foraging of sagebrush by free-living pygmy rabbits, and 2) investigate whether duration of burrow system occupancy by pygmy rabbits affects sagebrush chemistry. Plant protein was used as a measure of nutritional quality. We predicted that higher protein content combined with lower concentrations of specific PSMs would best explain diet selection in pygmy rabbits, and that sagebrush plants at burrow systems occupied for longer durations would have higher concentrations of protein and/or PSMs. To test these predictions, we analyzed the phytochemistry of browsed and unbrowsed plants at burrow systems that had been occupied continuously by pygmy rabbits for shorter (1–2 years) versus longer (6–7 years) durations. We applied model selection to identify the combination of nutrient and PSM variables that best explained diet selection by pygmy rabbits. Finally, we conducted a post hoc analysis to examine the relationship between specific phytochemical variables and duration of occupancy of burrow systems by pygmy rabbits.
MATERIALS AND METHODS
Study Areas
We conducted the study at 2 sites in Idaho. The Leadore site (Leadore County, Idaho; 44°41’57” N, 113°17’12” W; elevation: 1942 m) was dominated by Wyoming big sagebrush (Artemisia tridentata wyomingensis) and included mountain big sagebrush (A. t. vasyana), black sagebrush (A. nova), and grasses. The Camas Prairie site (Lincoln County, Idaho; 44°14’28” N, 114°19’04” W; elevation: 1472 m) was dominated by Wyoming big sagebrush but also included low sagebrush (A. arbuscula) and grasses. Both sites were dominated by mima-mounds, earthen mounds with relatively large, dense sagebrush and deeper soils that pygmy rabbits used for burrow excavation and foraging.
Sample Collection
During October and November 2009, we identified burrow systems occupied by pygmy rabbits at each site. We considered burrows to be currently occupied by a pygmy rabbit if the following 3 criteria were fulfilled: 1) open and recently used burrow entrances as indicated by fresh digging; 2) fresh fecal pellets from pygmy rabbits; and 3) evidence of recent foraging on sagebrush by pygmy rabbits. At the Leadore site, we categorized duration of burrow system occupancy because monitoring of burrows began in 2002. Burrow systems inhabited continuously for 6–7 years were classified as “long occupancy”, and those used for only 1–2 years were classified as “short occupancy” (Price 2009). Occupancy data were not available for burrows at the Camas Prairie site.
To identify variables that best explained differential foraging on sagebrush by pygmy rabbits, we collected browsed and unbrowsed plants during 1 field season at 20 occupied burrow systems at the Leadore site and 15 at the Camas Prairie site. We defined browsed plants as those with ≥75% of available stems showing evidence of clipping by pygmy rabbits. We defined unbrowsed plants as those with ≤25% of stems showing evidence of clipping. Foraging by pygmy rabbits was characterized by slanted (approximately 45°) angles on the stems and lack of foliated branches left on the ground below clipped sagebrush plants (foliated branches remaining on the ground are characteristic of clipping by other lagomorph species in the area). We collected only the dominant sagebrush type at each site (Wyoming big sagebrush) for consistency. We designated a center point for each burrow system as the geometric center of all burrow entrances. Beginning at the center point and circling outward, we collected the 1st and 3rd browsed plants we encountered. The nearest unbrowsed plant of the same subspecies (generally <1 m away) was paired with each identified browsed plant (Leadore = 2 pairs from each of 20 burrows; Camas Prairie = 2 pairs from each of 15 burrows). We sampled branches from all sides of the plant from each plant with pruning shears. Branches were stored in plastic bags on ice in the field, and then stored at −20°C until analysis.
Sample Analysis
We separated sagebrush leaves from stems by freezing samples using dry ice to make them brittle, dislodging the leaves, and then removing stems, dead leaves, and debris. We ground each sample of leaves in liquid nitrogen with a mortar and pestle to an average particle size of ~2 mm. A subsample was stored at −20°C for monoterpene and total phenolic analysis, and the remaining sample was weighed and dried to a constant weight at 50°C to determine dry weight. We calculated percent dry matter and analyzed the dried sample for protein content.
We determined crude protein content and crude protein digestibility for each sample. Samples were digested with an in vitro digestion assay using pepsin and cellulase to simulate digestion in a hindgut fermenter (DeGabriel et al. 2008). Our preliminary results (Ulappa 2011) and those of Thines et al. (2007) indicated that tannins were not present in the sagebrush samples examined. Therefore, we did not include analysis of crude protein digestibility with tannin inhibitors. We used the Total Kjeldahl Nitrogen method (Dairy One Forage Labs, Ithaca, New York) to quantify protein content from a subsample before digestion (pre-digest sample) and protein content of remaining material from a subsample after digestion (post-digest sample). All pre-digestion and post-digestion samples were analyzed in duplicate and averaged. We estimated crude protein content by multiplying total nitrogen content by 6.25 (Robbins 1983). For each sample, we subtracted average post-digestion crude protein amounts from average pre-digestion crude protein amount.
To address the effect of monoterpenes on browsing, we analyzed monoterpene concentration using gas chromatography (GC). For each sample ~0.10 g (wet weight, ww) of sample was weighed into a 1.5 ml microcentrifuge vial and extracted for 24 h at room temperature in 1 ml of methylene chloride (HPLC grade) spiked with an internal standard of fenchone (>98% purity, CAS# 4695-62-9, not naturally present in sagebrush samples) at a concentration of 50 µg/ml. We then dried each sample for 1 h with 0.25 g of anhydrous sodium sulfate (granular). Supernatant was removed from each vial with a glass Pasteur pipette and filtered through glass wool into 1.5 ml amber vials. Each vial was processed by a HP 7673 controller and sampled by a HP 6890 series II injector. Two µl of each sample were injected into the HP 5890 series II gas chromatograph with flame ionization detector. The stationary phase was a DB-5 Agilent silica column (30 m × 0.25 mm) with a 0.25 µm coating. The initial oven temperature was 40°C for 2 min, then increasing 3 degrees per min until reaching 60°C, increasing at 5 degrees until 120°C, and finally increasing at 20 degrees per min to 300°C and held for 7 min. Injector and detector temperatures were 250°C and 300°C, respectively. The make-up and carrier gases were helium. The inlet pressure was 80 KPa with a flow rate of 1.00 ml/min.
Monoterpene retention times and peak areas were calculated by HP ChemStation version B.01.00. To identify individual compounds for our analysis, we integrated all peaks on chromatograms from a random subset of browsed and unbrowsed samples to derive estimates of area under the peak curve (AUC) for each peak. Peaks that were greater than 1% of the total AUC and present in 4 of the 6 plants in each subset (browsed and unbrowsed) were selected for further analysis. Peaks were identified by running 1 representative sample from each site through a Hewlett-Packard 5972 Series Mass Selective Detector using the same column and temperature program used previously to separate and quantify compounds. Mass spectral data were generated for each peak and compared to a published library of mass spectra (NIST 2008). To ensure correct peak identification, we verified the relative retention times of compounds with co-chromatography using standards of camphene (CAS # 79-92-5), camphor (76-22-2), terpineol (98-55-5), borneol (464-45-9), cymene (99-87-6), 1,8-cineole (470-67-7), α-pinene (7785-26-4), and β-pinene (18172-67-3). Monoterpene concentrations were quantified as fenchone equivalents. Amounts were calculated for each monoterpene in each sample by dividing the AUC of the monoterpene by the AUC of fenchone, and then multiplying by the concentration of fenchone present in the sample (50 µg/ml). The concentration of compound extracted was then divided by the amount of dry matter extracted. Monoterpene amounts are expressed as µg of compound in fenchone equivalents per g dry weight of plant sample (µg/g dry weight).
To address the effect of phenolics on browsing, we analyzed each sample for total phenolic content using a colorimetric assay (Ainsworth and Gillespie 2007). Each sample was extracted in 95% methanol for 24 h. From the supernatant, 50 µl of sample was diluted 1:5 and then mixed with 200 µl of Folin-Ciocalteu reagent (20%) and 800 µl of 700 mM sodium bicarbonate in distilled water. The color intensity was immediately measured using a BioTek Synergy MX multi-mode plate reader (BioTek, Winooski, Vermont) at an absorbance of 765 nm at room temperature. Values were quantified in gallic acid equivalents (µmol/g dry weight).
Statistical Analysis
We analyzed data from the 2 field sites independently because (1) they were separated by approximately 180 km, (2) the dominant sagebrush at each site had different chemical profiles (Ulappa 2011), and (3) pygmy rabbits from each site were far enough apart to be considered genetically distinct (Estes-Zumpf et al. 2010). We analyzed all 30 pairs of plants from the Camas Prairie site, whereas we analyzed 35 of the 40 pairs from the Leadore site because 5 pairs from the latter were damaged during Total Kjeldahl Nitrogen analysis. We used conditional logistic regression in a discrete choice analysis of plant selection within burrows (Hosmer and Lemeshow 2000; Compton et al. 2002). We modeled browse status (browsed or unbrowsed) as a function of protein and PSM variables, with data stratified by burrow.
Prior to building models, we used a variable screening procedure to reduce the number of variables under consideration. Specifically, we fit univariate conditional logistic regression models for each nutrient (Leadore: n = 2 variables; Camas Prairie: n = 2 variables) and PSM (Leadore: n = 16 variables; Camas Prairie: n = 7 variables) predictor and then ranked them, along with their respective null models, using Akaike’s Information Criterion (AIC, Akaike 1981) with sample size bias adjustment (AICc; Burnham and Anderson 2002). This is equivalent to ranking these models by their likelihoods because the number of parameters was the same in each univariate model (i.e., K = 1). Predictor variables from univariate models that were ranked below null models (i.e., those that did not improve model likelihood over a model with a constant predictor) were removed from further analysis. Similarly, so as not to include both crude protein and digestible protein in the same models, we compared univariate AICc values and removed the one with the lower rank (digestible protein) from further consideration.
After screening variables, we used correlation coefficients (r) to identify correlated pairs of predictor variables (|r| > 0.6), and we removed the variable with the higher univariate AICc value from each correlated pair. To check for multicollinearity among predictors, we examined variance inflation factors (VIFs) for all possible subsets of remaining variables. Variance inflation factors did not indicate problems with multicollinearity (i.e., all VIFs < 3.0). Finally, we used all possible combinations of the remaining variables (Table 3) to fit binary conditional logistic regression models. We included these models in an information-theoretic analysis using AICc as the selection criterion. We then weighted final models within 2 AICc units of the top model (i.e., ΔAICc ≤ 2) by Akaike model weight (wi) to derive model-averaged parameter estimates, and we used unconditional variance for estimating 95% confidence intervals (Burnham and Anderson 2002).
Table 3.
Retention times (min), and mass (µg/g dry weight, mean ± SE) of major monoterpenes (in fenchone equivalents) and total phenolics (in gallic acid equivalents) from 35 browsed and 35 unbrowsed plants from the Leadore site.
| Variable | Retention Time (min) | Browsed | Unbrowsed |
|---|---|---|---|
| Methacrolein a,b | 3.24 | 3041 ± 127 | 3391 ± 193 |
| Unknown 2 | 12.24 | 1013 ± 135 | 1266 ± 156 |
| *Unknown 3 | 12.45 | 771 ± 71 | 947 ± 80 |
| Camphene a,b | 14.1 | 2722 ± 411 | 2540 ± 379 |
| *Artemiseole a | 15.06 | 6014 ± 367 | 7242 ± 449 |
| 1,8-Cineole a,b | 17.2 | 1774 ± 210 | 1523 ± 209 |
| *Unknown 7 | 17.5 | 511 ± 54 | 606 ± 64 |
| Unknown 8 | 18.65 | 1028 ± 99 | 1128 ± 93 |
| Unknown 10 | 21.09 | 3837 ± 234 | 4426 ± 268 |
| Camphor a,b | 21.30 | 2592 ± 331 | 2607 ± 407 |
| Unknown 13 | 21.64 | 984 ± 54 | 1039 ± 53 |
| Unknown 14 | 23.40 | 3560 ± 259 | 3537 ± 225 |
| Unknown 15 | 23.49 | 484 ± 29 | 567 ± 36 |
| Unknown 16 | 23.65 | 621 ± 54 | 740 ± 72 |
| Unknown 17 | 23.95 | 1160 ± 58 | 1182 ± 66 |
| *Total Phenol Content (µmol/g) | 129.5 ± 4.4 | 140.7 ± 5.3 | |
Identified with mass spectroscopy;
Identified with co-chromatography
Indicates chemical variables used in the information-theoretic analysis.
In addition to modeling phytochemical differences among plants within a given burrow system, we conducted a post hoc analysis to evaluate effects of duration of burrow system occupancy on the 2 variables with the best parameter estimates from the Leadore site. We constructed a 2-way ANOVA that included the main effect of browse status (browsed or unbrowsed) and occupancy duration, short (15 burrows) or long (20 burrows) and their interaction on plant phytochemistry, with variables accounting for burrow system identification and the paired sample design nested within the burrow system included as random effects. We focused on crude protein and artemiseole because they were the phytochemicals identified as most influential in the first portion of the analysis. We compared mean values for browsed and unbrowsed plants at short-occupancy burrow systems (i.e., occupied for 1–2 years) to those at long-occupancy burrow systems (i.e., those occupied for 6–7 years). Statistical analyses were conducted in R version 2.14 (R Development Core Team 2011).
RESULTS
Protein Content
Sagebrush plants that were higher in crude protein content were more likely to be browsed by pygmy rabbits (Leadore: browsed = 13.47 ± 0.26% (SE), unbrowsed = 12.49 ± 0.22%, n = 35; Camas Prairie: browsed = 11.61± 0.18%, unbrowsed = 11.23 ± 0.21%, n = 30). At the Leadore site, 4 models (including the top model with the lowest AICc) predicting the probability of a plant being browsed (out of 33 possible models) had ΔAICc ≤ 2, and all 4 contained total crude protein as a covariate (Table 1). Model averaged parameter estimates indicated that the odds of browsing increased by a factor of 1.70 with every 1% increase in crude protein (Table 2).
Table 1.
Model selection results for plant characteristics influencing browsing by pygmy rabbits on sagebrush at the Leadore study site, Idaho, and Camas Prairie study site, Idaho. Number of parameters (K), Akaike’s Information Criterion corrected for small sample size (AICc), change in AICc from the top model (ΔAICc), and model weights (wi) from top conditional logistic regression models and the null model.
| Site | Model | K | AICc | ΔAICc | wi |
|---|---|---|---|---|---|
| Leadore | Crude protein + Artemiseole | 2 | 51.64 | 0 | 0.23 |
| Crude protein + Artemiseole + unknown #7 | 3 | 53.52 | 1.87 | 0.09 | |
| Crude protein + Artemiseole + total phenolics | 3 | 53.59 | 1.94 | 0.09 | |
| Crude protein + Artemiseole + unknown #3 | 3 | 53.61 | 1.96 | 0.09 | |
| Null | 0 | 61.50 | 9.85 | 0.00 | |
| Camas Prairie | Crude protein | 1 | 53.04 | 0 | 0.17 |
| Crude protein + β-pinene | 2 | 53.72 | 0.68 | 0.12 | |
| Null | 0 | 53.75 | 0.71 | 0.12 | |
| β-pinene | 1 | 53.94 | 0.90 | 0.11 |
Table 2.
Model-averaged odds ratio estimates and 95% CI for covariates in top conditional logistic regression models of pygmy rabbit forage selection at the Leadore site, Idaho, and the Camas Prairie site, Idaho. Covariates with confidence intervals overlapping 1.0 were considered to lack explanatory value.
| Site | Covariate | Odds Ratio | Lower 95% CI |
Upper 95% CI |
|---|---|---|---|---|
| Leadore | Crude protein (%) | 1.70 | 1.10 | 2.63 |
| Artemiseole | 0.97 | 0.94 | 0.99 | |
| Total phenolics | 0.99 | 0.96 | 1.02 | |
| Unknown #3 | 0.97 | 0.84 | 1.11 | |
| Unknown #7 | 0.94 | 0.76 | 1.16 | |
| Camas Prairie | Crude protein (%) | 1.64 | 0.87 | 3.06 |
| β-pinene | 0.94 | 0.85 | 1.05 |
At the Camas Prairie site, 3 models (out of 16 possible models) had ΔAICc ≤ 2. However, only 2 of those models were ranked above the null model and thus used to derive model-averaged parameter estimates; both of these models contained crude protein as a covariate (Table 1). Model-averaged parameter estimates suggested that the odds of browsing increased by a factor of 1.64 with every 1% increase in crude protein. However, parameter estimates were less precise than at the Leadore site, with 95% CI for odds ratios overlapping 1 (Table 2).
Monoterpene and Phenolic Content
We detected 17 major monoterpenes from the Leadore site, of which we were able to identify 5 (Table 3), and we detected and identified 6 major monoterpenes from the Camas Prairie site (Table 4). Total phenolic content was not an important covariate at either site (Tables 3 and 4). At the Leadore site, artemiseole was negatively associated with the odds of a plant being browsed (Table 2). Unknown monoterpene #3, unknown monoterpene #7, and total phenolic content appeared in top models but had 95% confidence intervals that overlapped 1 (Table 2), so we did not consider them to have explanatory value. Model-averaged parameter estimates indicated that for every 100 µg/g dry weight increase in artemiseole, a plant was 1.03 times less likely to be browsed by pygmy rabbits. At the Camas Prairie site, β-pinene was the only PSM covariate in the top models; however, it was poorly estimated, with 95% confidence intervals overlapping 1, so we did not consider it to have explanatory value (Table 2).
Table 4.
Retention times (min), and mass (µg/g dry weight, mean ± SE) of major monoterpenes (in fenchone equivalents) and total phenolics (in gallic acid equivalents) from 30 browsed and 30 unbrowsed plants from the Camas Prairie site.
| Variable | Retention Time (min) | Browsed | Unbrowsed |
|---|---|---|---|
| α-pinene a,b | 13.34 | 1506 ± 120 | 1369 ± 109 |
| Camphene a,b | 13.97 | 8109 ± 409 | 8106 ± 552 |
| *β-pinene a,b | 15.07 | 1179 ± 55 | 1417 ± 176 |
| 1,8-Cineole a,b | 17.23 | 7361 ± 758 | 6595 ± 510 |
| Camphor a,b | 21.41 | 41147 ± 1874 | 40805 ± 2673 |
| Borneol b | 21.90 | 928 ± 73 | 999 ± 90 |
| Total Phenol Content (µmol/g) | 111.2 ± 2.9 | 114.1 ± 3.9 | |
Identified with mass spectroscopy;
Identified with co-chromatography
Indicates chemical variable used in the information-theoretic analysis.
Effects of Occupancy
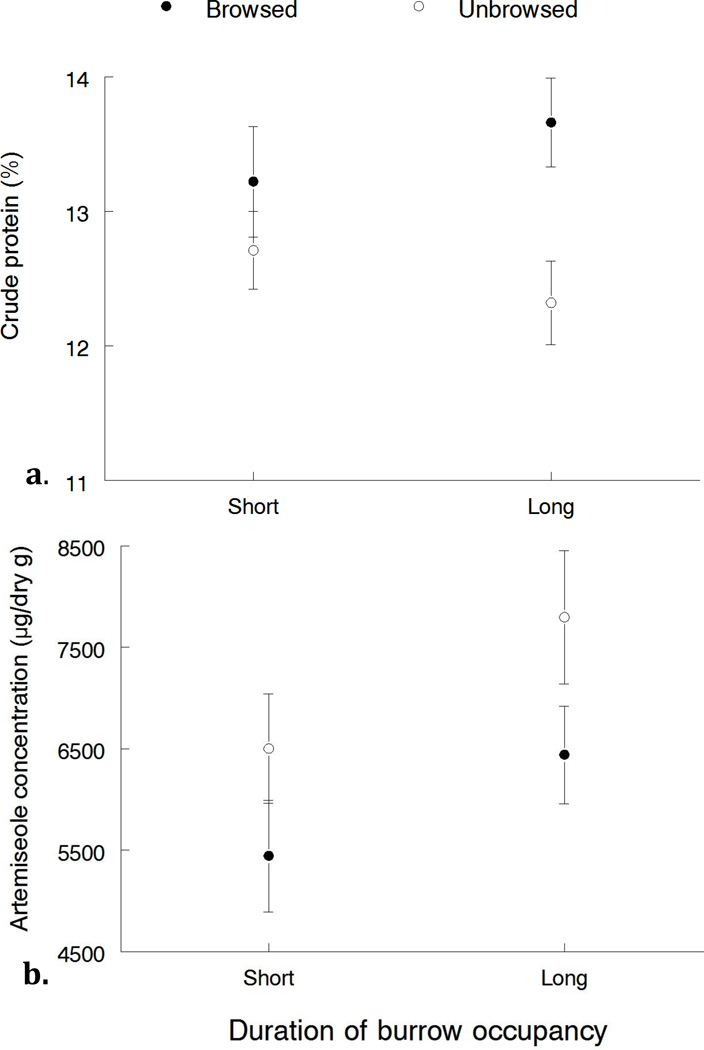
Mean levels of crude protein were higher in browsed than unbrowsed plants, and there was a significant effect of browse status (χ21(deviance) = 10.7, P = 0.001) on protein content but not an effect of the duration of burrow system occupancy (χ21(deviance) = 0.01, P = 0.937) or an interaction between the two (χ21(deviance) = 1.9, P = 0.168, Fig. 1a). Likewise, concentrations of artemiseole were significantly higher in unbrowsed plants than browsed plants (χ21(deviance) = 9.92, P = 0.002) but did not differ by occupancy length (χ21(deviance)=2.21, P = 0.137), nor was there an interaction between the two (χ21(deviance)=0.14, P = 0.706, Fig. 1b).
Fig. 1.
A) Average crude protein (%, ± SE) and B) artemiseole (µg/g dry weight, ± SE) between browsed (closed circles) and unbrowsed plants (open circle) on burrows that have been occupied by pygmy rabbits for short (1–2 years) and long (6–7 years) amounts of time in October 2009 at the Leadore site in Idaho. Crude protein (%) for short occupancy, browsed: 13.22 ± 0.41; short occupancy, unbrowsed: 12.71 ± 0.29; long occupancy, browsed: 13.66 ± 0.33; long occupancy, unbrowsed: 12.32 ± 0.31. Artemiseole (µg/g dry weight) for short occupancy, browsed: 5444.77 ± 550; short occupancy, unbrowsed: 6502.81 ± 537; long occupancy, browsed: 544 6441.15 ± 481; long occupancy, unbrowsed: 7796.31 ± 658.
DISCUSSION
Results from this study suggest that both crude protein content and individual PSMs in sagebrush influence browsing choices for foraging pygmy rabbits. Sagebrush with higher crude protein content and lower concentrations of specific monoterpenes were more likely to have been browsed by pygmy rabbits. Our data did not support the hypothesis that long-term browsing alters the phytochemistry of sagebrush or that browse status and burrow occupancy length interact. In fact, browsed plants all showed evidence of recent and chronic browsing, indicating that they had not induced responses as a defensive strategy and had always been preferred to the unbrowsed plants.
As predicted, crude protein content of sagebrush influenced diet selection in free-living pygmy rabbits. Plants browsed by pygmy rabbits had higher concentrations of crude protein among the existing variation at a given site. Although that pattern was less clear at the Camas Prairie site, the trend was in the same direction and supported by the significant effect at the Leadore site. Variation at both sites (Leadore: range 9.46–16.53, mean 12.97% and Camas Prairie: range 9.43–14.92, mean 11.42%) was consistent with known variation of protein in sagebrush (Welch and McArthur 1979). In captivity, pygmy rabbits require 3.45 g of crude protein/day to maintain nitrogen balance (Shipley et al. 2006). Based on observed values of crude protein in browsed and unbrowsed plants plus the actual digestibility of protein, if pygmy rabbits only consumed unbrowsed plants at either site, they would need to eat nearly 3 g more of plant matter per day to meet protein requirements than if they ate browsed plants. By foraging more efficiently on plants with more protein (browsed plants), pygmy rabbits may spend less time exposed to predation, which can account for up to 88.9% of mortality (Crawford et al. 2010). In addition, pygmy rabbits are constrained by high energy needs and strict energy budgets (Katzner et al. 1997; Shipley et al. 2006), and decreasing the number and duration of foraging bouts could play an important role in reducing energy expended on foraging and associated energetically expensive activities like evading predators while active (Camp et al. 2012).
Individual monoterpene compounds from the Leadore site seemed to influence browsing by pygmy rabbits, whereas none were tightly linked to foraging at the Camas Prairie site. The Leadore site had a more diverse monoterpene profile and contained specific chemicals absent at the Camas Prairie site, and the monoterpenes identified and the concentrations measured at both sites were comparable to previous studies (Welch and McArthur 1981; Utz 2012; Frye et al. 2013). Further studies examining variation in intake, absorption, and metabolism of PSMs identified in our models would contribute to a better understanding of mechanisms by which these chemicals affect diet selection by pygmy rabbits and other herbivores. It is possible that concentrations of specific, individual monoterpenes influence browsing more than total monotoerpene concentration. In addition to monoterpenes, PSMs such as phenolics and sesquiterpene lactones are present in plants and may affect animal behavior. Individual phenolic compounds or sesquiterpene lactones, which were not measured, may influence browsing by pygmy rabbits and warrant further investigation. Other factors that we did not measure in this study, such as protective cover, interact with the phytochemistry of foraging patches to influence plant use by pygmy rabbits (Utz 2012).
Although herbivores seem to browse selectively to maximize protein acquisition and minimize exposure to PSMs, some plants are capable of chemically responding to browsing pressure, such as birch (Bryant et al. 1992) and willow (Stolter et al. 2005). In contrast, we observed lower or equal concentrations of most PSMs in browsed sagebrush plants compared to unbrowsed plants, suggesting that sagebrush did not induce immediate chemical defenses in response to pygmy rabbit browsing. Although sagebrush plants induce PSMs in response to leaf damage (Shiojiri and Karban 2008), the type of damage caused by pygmy rabbit browsing may not induce plant chemical defenses because they do not damage leaves. In general, sagebrush does not respond to damage by browsers through vascular signaling. Instead, leaves release volatile organic compounds (VOCs) when damaged, and nearby branches detect VOCs released into the air and subsequently induce defenses (Karban et al. 2006; Shiojiri and Karban 2008). Pygmy rabbits typically remove an entire branch while foraging and eat all the leaves and branch that they clip off the plant. In contrast to direct leaf damage, stem clipping by rabbits may reduce the release of volatiles so that remaining branches may not detect the damage, and thus plants may not immediately induce PSMs in response to short-term browsing by pygmy rabbits. Although not statistically significant, our results showed a trend that certain volatile compounds in sagebrush may be induced over an extended long-term period (>6 years) in response to burrow occupancy length (Fig. 1b).
Pygmy rabbits repeatedly forage on the same plants, which may subject the plant to a state of chronic stress as it replaces the large amount of biomass repeatedly removed by pygmy rabbits over time. Some plants preferentially allocate resources to new growth over elevated PSMs when resources are limited (Orians et al. 2010) and animal browsing has been shown to increase nitrogen content in the new growth (Danell and Huss-Danell 1985). Although not statistically significant, browsed plants at long occupancy burrows tended to have higher amounts of crude protein and these plants may be responding non-defensively with increased growth and higher nitrogen. The PSM concentrations trended higher in long occupancy compared to short occupancy burrows. The sagebrush–pygmy rabbit system offers an excellent stage to further investigate tradeoffs in resource allocation between growth and PSMs under acute and long-term chronic browsing pressure that varies in a natural system.
Results from our study offer insights into the importance of diet quality for specialist mammalian herbivores. Nutrient and PSM content of plants interact to influence diet selection. Both nutritional and defensive phytochemicals should be considered when comparing the quality of plants for foraging herbivores. For instance, in habitats dominated by lower quality plants, herbivores may need to increase plant consumption to maintain energy budgets, but consequently increase exposure to PSMs. Researchers and land managers should consider the importance of dietary quality to specialist herbivores when studying habitat use or prioritizing land for species conservation. Searle et al. (2007) argue that animals likely perceive landscapes on gradients of differing forage quality. Scaling diet selection at the plant scale up to the landscape scale is critical for conservation and restoration of habitat for specialized herbivores inhabiting chemically complex landscapes.
ACKNOWLEDGMENTS
This research was funded by the Bureau of Land Management (#LO9AC16253 to JSF), the National Science Foundation (DEB-1146194 to JSF, and IOS-1258217 to JSF), the NSF Idaho EPSCoR (#EPS-0814387), Sigma-Xi Aid-in-Research Grant 2011, Michael Butler Ecological Award 2009, the Idaho Department of Fish and Game, and Boise State University. We appreciate the additional assistance provided by the United States Forest Service and the USFS Pacific Northwest Research Station. Special thanks to P. Campos, K. Gelken, K. DeYoung, and E. Pankau for help in the lab and field and to N. Wiggins and L. Felicetti for preliminary review of the manuscript. This research was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health under Awards Numbers P20GM109095 and P20GM103408.
Contributor Information
Amy C. Ulappa, Washington State University, School of the Environment.
Rick G. Kelsey, USDA Forest Service Pacific Northwest Research Station, Corvallis OR
Graham G. Frye, Institute of Arctic Biology, University of Alaska Fairbanks, Fairbanks AK
Janet L. Rachlow, University of Idaho, Moscow ID
Lisa A. Shipley, Washington State University, School of the Environment
Laura Bond, Boise State University, Boise ID.
Xinzhu Pu, Boise State University, Boise ID.
Jennifer Sorensen Forbey, Boise State University, Boise ID.
LITERATURE CITED
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols. 2007;4:875–887. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Akaike H. Likelihood of a model and information criteria. Journal of Econometrics. 1981;16:3–14. [Google Scholar]
- Beckerton PR, Middleton ALA. Effects of dietary protein levels on ruffed grouse reproduction. Journal of Wildlife Management. 1982;46:569–579. [Google Scholar]
- Bruun HH, Österdahl S, Moen J, Angerbjörn A. Distinct patterns in alpine vegetation around dens of the Arctic fox. Ecography. 2005;28:81–87. [Google Scholar]
- Bryant JP, Reichardt PB, Clausen TP. Chemically mediated interactions between woody plants and browsing mammals. Journal of Range Management. 1992;45:18–24. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer-Verlag; 2002. [Google Scholar]
- Camp MJ, Rachlow JL, Woods BA, Johnson TR, Shipley LA. When to run and when to hide: the influence of cover distance to refugia on perception of predation risk. Ethology. 2012;118:1010–1017. [Google Scholar]
- Compton BW, Rhymer JM, McCollough M. Habitat selection by wood turtles (Clemmys insculpta): an application of paired logistic regression. Ecology. 2002;83:833–843. [Google Scholar]
- Crawford JA, Anthony RG, Forbes JT, Lorton GA. Survival and causes of mortality for pygmy rabbits (Brachylagus idahoensis) in Oregon and Nevada. Journal of Mammalogy. 2010;91:838–847. [Google Scholar]
- Danell K, Huss-Danell K. Feeding by insects and hares on birches earlier affected by moose browsing. Oikos. 1985;44:75–81. [Google Scholar]
- Dearing MD, McLister JD, Sorenson JS. Woodrat (Neotoma) herbivores maintain nitrogen balance on a low-nitrogen, high-phenolic forage, Juniperus monosperma. Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology. 2005;175:349–355. doi: 10.1007/s00360-005-0491-3. [DOI] [PubMed] [Google Scholar]
- Degabriel JL, Moore BD, Foley WJ, Johnson CN. The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology. 2009;90:711–719. doi: 10.1890/08-0940.1. [DOI] [PubMed] [Google Scholar]
- DeGabriel JL, Wallis IR, Moore BD, Foley WJ. A simple, integrative assay to quantify nutritional quality of browses for herbivores. Oecologia. 2008;156:107–116. doi: 10.1007/s00442-008-0960-y. [DOI] [PubMed] [Google Scholar]
- Dziba LE, Provenza FD. Dietary monoterpen concentrations influence feeding patterns of lams. Applied Animal Behaviour Science. 2008;109:49–57. [Google Scholar]
- Estes-Zumpf WA, Rachlow JL, Waits LP, Warheit KI. Dispersal, gene flow, and population genetic structure in the pygmy rabbit (Brachylagus idahoensis) Journal of Mammalogy. 2010;91:208–219. [Google Scholar]
- Frye GG, Connelly JW, Musil DD, Forbey JS. Phytochemistry predicts habitat selection by an avian herbivore at multiple spatial scales. Ecology. 2013;94:308–314. doi: 10.1890/12-1313.1. [DOI] [PubMed] [Google Scholar]
- Green JS, Flinders JT. Habitat and dietary relationships of the pygmy rabbit. Journal of Range Management. 1980;22:136–142. [Google Scholar]
- Guglielmo CG, Karasov WH, Jakubas WJ. Nutritional costs of a plant secondary metabolite explain selective foraging by ruffed grouse. Ecology. 1996;77:1103–1115. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- Karban R. The ecology and evolution of induced resistance against herbivores. Functional Ecology. 2011;25:339–347. [Google Scholar]
- Karban R, Agrawal AA. Herbivore offense. Annual Review of Ecology and Systematics. 2002;33:641–664. [Google Scholar]
- Karban R, Shiojiri K, Huntzinger M, McCall AC. Damage-induced resistance in sagebrush: Volatiles are key to intra- and interplant communication. Ecology. 2006;87:922–930. doi: 10.1890/0012-9658(2006)87[922:drisva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Katzner TE, Parker KL, Harlow HH. Metabolism and thermal response in winter-acclimatized pygmy rabbits (Brachylagus idahoensis) Journal of Mammalogy. 1997;78:1053–1062. [Google Scholar]
- Laca EA, Shipley LA, Reid ED. Structural anti-quality characteristics of range and pasture plants. Journal of Range Management. 2001;54:413–419. [Google Scholar]
- Mcart SH, Spalinger DE, Collins WB, Schoen ER, Stevenson T, Bucho M. Summer dietary nitrogen availability as a potential bottom-up constraint on moose in south-central Alaska. Ecology. 2009;90:1400–1411. doi: 10.1890/08-1435.1. [DOI] [PubMed] [Google Scholar]
- Moore BD, Lawler IR, Wallis IR, Beale CM, Foley WJ. Palatability mapping: a koala's eye view of spatial variation in habitat quality. Ecology. 2010;91:3165–3176. doi: 10.1890/09-1714.1. [DOI] [PubMed] [Google Scholar]
- National Institute of Standards and Technology. Release of NIST 08 and Robert P. Adams library accompanying text titled "Identification of essential oil components by gas chromatography/mass spectrometry. 4th Edition. Illinois: Allured Publishing Corporation; 2008. [Google Scholar]
- Orians CM, Hochwender CG, Fritz RS, Snäll T. Growth and chemical defense in willow seedlings: trade-offs are transient. Oecologia. 2010;163:283–290. doi: 10.1007/s00442-009-1521-8. [DOI] [PubMed] [Google Scholar]
- Personius TL, Wambolt CL, Stephens JR, Kelsey RG. Crude terpenoid influence on mule deer preference for sagebrush. Journal of Range Management. 1987;40:84–88. [Google Scholar]
- Price AJ. Masters thesis. Moscow: University of Idaho; 2009. Survival and burrowing ecology of pygmy rabbits: implications for sagebrush habitat and estimation of abundance. [Google Scholar]
- R Development Core Team. R, a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Robbins CT. Wildlife Feeding and Nutrition. New York: Academic Press Inc.; 1983. [Google Scholar]
- Sanchez DM, Rachlow JL, Robinson AP, Johnson TR. Survey indicators for pygmy rabbits: temporal trends of burrow systems and pellets. Western North American Naturalist. 2009;69:426–436. [Google Scholar]
- Searle KR, Hobbs NT, Gordon IJ. It’s the “foodscape”, not the landscape: Using foraging behavior to make functional assessments of landscape condition. Israel Journal of Ecology and Evolution. 2007;53:297–316. [Google Scholar]
- Sharam GJ, Turkington R. Growth, camphor concentration, and nitrogen responses of white spruce (Picea glauca) leaves to browsing and fertilization. Ecoscience. 2009;16:258–264. [Google Scholar]
- Shiojiri K, Karban R. Vascular systemic induced resistance for Artemisia cana and volatile communication for Artemisia douglasiana. American Midland Naturalist. 2008;159:468–477. [Google Scholar]
- Shipley LA, Davila TB, Thines NJ, Elias BA. Nutritional requirements and diet choices of the pygmy rabbit (Brachylagus idahoensis): A sagebrush specialist. Journal of Chemical Ecology. 2006;32:2455–2474. doi: 10.1007/s10886-006-9156-2. [DOI] [PubMed] [Google Scholar]
- Shipley LA, Forbey JS, Moore BD. Revisiting the dietary niche: When is a mammalian herbivore a specialist? Integrative and Comparative Biology. 2009;49:274–290. doi: 10.1093/icb/icp051. [DOI] [PubMed] [Google Scholar]
- Somers N, D’Haese B, Bossuyt B, Lens L, Hoffman M. Food quality affects diet preference of rabbits: experimental evidence. Belgian Journal of Zoology. 2008;138:170–176. [Google Scholar]
- Sorensen JS, Dearing MD. Efflux transporters as a novel herbivore countermechanism to plant chemical defenses. Journal of Chemical Ecology. 2006;32:1181–1196. doi: 10.1007/s10886-006-9079-y. [DOI] [PubMed] [Google Scholar]
- Sorensen JS, Heward E, Dearing MD. Plant secondary metabolites alter the feeding patterns of a mammalian herbivore (Neotoma lepida) Oecologia. 2005a;146:415–422. doi: 10.1007/s00442-005-0236-8. [DOI] [PubMed] [Google Scholar]
- Sorensen JS, McLister JD, Dearing MD. Plant secondary metabolites compromise the energy budgets of specialist and generalist mammalian herbivores. Ecology. 2005b;86:125–139. [Google Scholar]
- Stephens DW, Krebs JR. Foraging Theory. New Jersey: Princeton University Press; 1986. [Google Scholar]
- Stolter C. Intra-individual plant response to moose browsing: Feedback loops and impacts on multiple consumers. Ecological Monographs. 2008;78:167–183. [Google Scholar]
- Stolter C, Ball JP, Julkunen-Tiitto R, Lieberei R, Ganzhorn JU. Winter browsing of moose on two different willow species: food selection in relation to plant chemistry and plant response. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2005;83:807–819. [Google Scholar]
- Thines NJ, Shipley LA, Bassman JH, Fellman JK, Mattison DS, Slusser JR, Gao W. Effects of enhanced UV-B radiation on plant chemistry: nutritional consequences for a specialist and generalist lagomorph. Journal of Chemical Ecology. 2007;33:1025–1039. doi: 10.1007/s10886-007-9280-7. [DOI] [PubMed] [Google Scholar]
- Ulappa AC. Masters thesis. Boise: Boise State University; 2011. Nutritional and chemical factors shaping diet selection for two sagebrush specialists. [Google Scholar]
- Utz JL. Masters thesis. Boise: Boise State University; 2012. Understanding the tradeoff between safety and food quality in a mammalian herbivore specialist, the pygmy rabbit. [Google Scholar]
- Welch BL, McArthur ED. Variation in winter levels of crude protien amont Artemisis-tridentata subspecies grown in a uniform garden. Journal of Range Management. 1979;32:467–469. [Google Scholar]
- Welch BL, McArthur ED. Variation of monoterpenoid content among subspecies and accessions of Artemisia-tridentata grown in a uniform garden. Journal of Range Management. 1981;34:380–384. [Google Scholar]