Abstract
Catel–Manzke syndrome is a rare autosomal recessive disorder characterized by Pierre Robin sequence with hyperphalangy and clinodactyly of the index finger. Recently, homozygous or compound heterozygous pathogenic variants in TGDS have been discovered to cause Catel–Manzke syndrome. Here, we describe a 12-month-old male with molecularly confirmed Catel–Manzke syndrome who presented with Pierre Robin sequence (but without cleft palate) and hyperphalangy, and we compare his phenotype with the seven previously described patients with pathogenic variants in TGDS. Our patient is on the severe end of the phenotypic spectrum, presenting with respiratory complications and failure to thrive. Furthermore, our finding of a homozygous p.Ala100Ser pathogenic variant in our patient supports that it is a common mutation in Catel–Manzke syndrome.
Keywords: Catel-Manzke syndrome, Hyperphalangy, Manzke dysostosis, Pierre Robin sequence, TGDS
1. Introduction
Catel–Manzke syndrome (MIM: 616145) is a rare genetic disorder characterized by Pierre Robin sequence with hyperphalangy and clinodactyly of the index finger [1], [2]. Affected individuals typically present with the classic features of Pierre Robin sequence including micrognathia, airway obstruction secondary to displacement of the tongue base, and often, cleft palate. The orthopedic abnormalities are the result of an accessory bone between the second metacarpal and proximal phalanx that causes radial deviation of the second digit (Manzke dysostosis). Cardiac abnormalities, facial dysmorphisms, and additional skeletal abnormalities have also been described in a subset of patients with Catel–Manzke syndrome [3], [4], [5].
Until recently, the genetic basis of Catel–Manzke syndrome was unknown, although inheritance appeared to be autosomal recessive [4], [6]. Ehmke et al. recently implicated homozygous or compound heterozygous pathogenic variants in TGDS (dTDP-d-glucose 4,6-dehydratase) as the causative factor in a series of seven unrelated patients with features of Catel–Manzke syndrome [3]. Of the seven unrelated patients reported, c.298G > T (p.Ala100Ser) was the most common pathogenic variant and was hypothesized to be a founder mutation [3]. TGDS encodes a member of the short-chain dehydrogenase/reductase (SDR) family; however the specific function of the protein in humans is unknown [3].
In the present report, we describe an additional patient with molecularly confirmed Catel–Manzke syndrome who has Pierre Robin sequence (without cleft palate) and Manzke dysostosis, and we compare his phenotype with the phenotype of the seven previously described patients with pathogenic variants in TGDS [3]. His clinical presentation was characterized by obstructive respiratory abnormalities and pharyngeal dysphagia. He required tracheostomy for severe airway hypotonia with pharyngomalacia and laryngomalacia, and gastrostomy tube placement for failure to thrive likely secondary to his pharyngeal dysphagia and airway obstruction. His severe presentation underlies the marked clinical variability associated with TGDS mutations.
2. Case report
The proband was born at 38 4/7 weeks of gestation via spontaneous vaginal delivery without complications to a 29 year-old female. Prenatal ultrasounds had been normal. The mother had a history of three prior first-trimester spontaneous abortions and one healthy son with the same partner. No prior genetic workup had been performed for the spontaneous abortions, and the cause for these spontaneous abortions was unknown. Family history was otherwise non-contributory. The father is of German ancestry, and the mother is of Czech ancestry. There is no known consanguinity. His postnatal course was complicated by significant failure to thrive noted as early as three weeks of age. His failure to thrive persisted despite increased caloric density and feeding therapy, and at two months of age, his weight Z score was − 8.22 (WHO growth chart). A modified barium swallow showed pharyngeal dysphagia, and he was noted to have significant fatigue with feeding. Thus, a gastrostomy tube was placed by 2.5 months of age. He was also diagnosed with gastroesophageal reflux.
During an admission for failure to thrive, stridor and desaturations to 85% were noted. He was diagnosed with severe pharyngomalacia and laryngomalacia by otolaryngology, and a supraglottoplasty was performed. By six months of age, he was diagnosed with mixed sleep apnea and respiratory insufficiency due to airway hypotonia and oropharyngeal weakness. These respiratory issues were suspected to contribute to his feeding difficulties and failure to thrive. Tracheostomy was ultimately placed at seven months of age, and he required nocturnal ventilator use. He also underwent myringotomy and tympanostomy for recurrent ear infections. By eight months of age, a repeat modified barium swallow still showed pharyngeal dysphagia, but his fatigue with oral feedings appeared to be improved. In addition, by this time, his weight gain slowly began to improve.
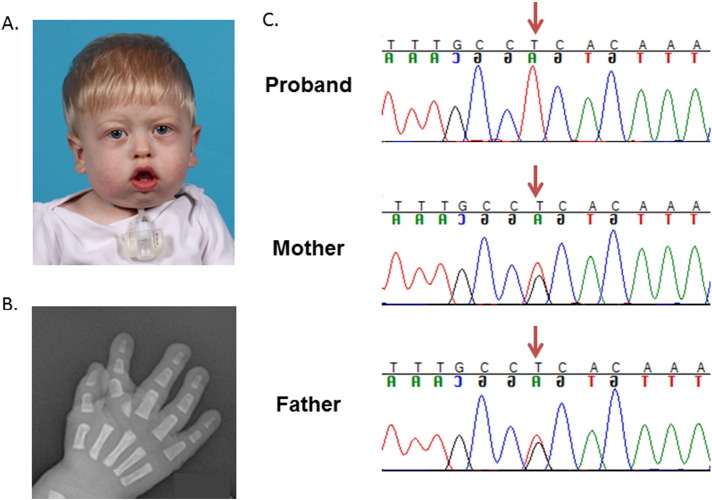
At his most recent exam at 12 months of age, his weight was 8.42 kg (Z score = − 1.22, WHO growth chart), his length was 72.4 cm (Z score = − 1.36, WHO growth chart), and his head circumference was 45.9 cm (Z score = − 0.13, WHO growth chart). He was using the gastrostomy tube for the majority of his caloric intake but was tolerating small amounts of oral feeds. He was noted to have prominent overriding sutures, a tubular-appearing nose with high nasal bridge and pinched nares, retrognathia, high and narrow arched palate with small groove of the posterior soft palate, and ankyloglossia (Fig. 1). He had mild swelling of the eyelids with proptosis but no ptosis. His fingers and toes appeared long, and his index fingers were deviated and overlapping. A mild pectus deformity was noted.
Fig. 1.
Photograph and hand X-ray. A. Photograph of proband showing facial features. B. Right hand radiograph shows a mildly hypoplastic proximal phalanx with an additional bone at the base of the second finger medially. There is also radial deviation of the index finger. C. Sanger sequencing demonstrates that the proband is homozygous for the c.298G > T (p.Ala100Ser) mutation and that parents are both heterozygous for this mutation.
On hand radiographs, the second proximal phalanges appeared hypoplastic with an accessory bone located at the base of the second digit, and there was medial deviation of the distal second digits (Fig. 1). Lateral view of the spine demonstrated anterior beaking of the L2 and L3 vertebral bodies. No other abnormalities were noted on skeletal survey. Echocardiogram at three months of age showed a small patent foramen ovale and a small patent ductus arteriosus, both with left to right flow and both of which resolved by six months of age. Neurological exam and brain MRI were normal. Routine chromosome analysis, comparative genomic hybridization microarray (Affymetrix Cytoscan HD Microarray System, Scott and White Healthcare Molecular Genetics Laboratories), very long chain fatty acids (Peroxisomal Diseases Laboratory, Kennedy Krieger Institute), and lysosomal storage panels (Lysosomal Diseases Testing Laboratory, Thomas Jefferson University) were also normal.
3. Materials and methods
3.1. Clinical study
The proband and both parents were enrolled in our skeletal dysplasia study according to the Baylor College of Medicine's Institutional Review Board. Informed consent was obtained specifically for molecular studies and for the publication of clinical information and photographs.
3.2. Sanger sequencing
DNA was extracted from peripheral blood monocytes and precipitated for polymerase chain reaction and Sanger sequencing. Polymerase chain reaction was performed using primers designed to cover the entire coding region of TGDS (Supplemental Table 1) with an annealing temperature of 55 °C. Products were verified by agarose gel electrophoresis and then sequenced by chain-termination (Sanger) sequencing using the same primers at Beckman Coulter Genomics (Danvers, MA).
4. Results
Sanger sequencing revealed that the proband was apparently homozygous for the c.298G > T (p.Ala100Ser) pathogenic variant, the most common pathogenic variant identified by Ehmke et al. [3]. Sanger sequencing of DNA samples from the parents revealed that both parents are heterozygous for this variant and thus, confirmed that the patient is homozygous (Fig. 1).
5. Conclusions
To date, this report is only the second publication describing molecularly confirmed Catel–Manzke syndrome, and our patient is only the eighth reported patient. We compared our patient's features with the features of the seven previously reported patients in the original manuscript describing pathogenic variants in TGDS as the cause for Catel–Manzke syndrome (Table 1) [3], [4], [7], [8]. Our patient has Pierre Robin sequence and Manzke dysostosis, the two classic features of Catel–Manzke syndrome. In addition, he had a minor congenital cardiac defect that resolved and facial features (narrow nose, full cheeks) that have been described in a subset of the previously reported patients [3], [4]. Notably, he did not have a cleft palate, a feature seen in six of the seven other molecularly confirmed patients [3].
Table 1.
Phenotypic features of patients with homozygous or compound heterozygous mutations in TGDS.
| Feature | Current patient | Previous patients [2], [3], [4], [7], [8] |
|---|---|---|
| Pierre Robin sequence | + | 7/7 |
| Cleft palate | − | 6/7 |
| High-arched palate without cleft | + | 1/7 |
| Tracheostomy | + | 1/7 |
| Manzke dysostosis | + | 7/7 |
| Joint hypermobility | Not reported | 2/7⁎ |
| Pectus deformity | + | 1/7 |
| Congenital heart defect | + (PDA) | 2/7 (VSD) |
| Facial dysmorphism | + | 5/7⁎⁎ |
| Narrow nose or nostrils | + | 2/7 |
| Proptosis | + | 1/7 |
| Low set ears | − | 3/7 |
| Thin or arched eyebrows | − | 4/7 |
| Round face or full cheeks | + | 3/7 |
| Hypertelorism | − | 3/7 |
| Postnatal growth retardation | + | 2/7⁎⁎ |
Not reported in 5/7 patients.
Not reported in 2/7 patients.
Our patient's respiratory complications place him on the more severe end of the spectrum of reported patients. He required multiple procedures including supraglottoplasty and eventual tracheostomy placement with nocturnal ventilator support. In addition, he had significant failure to thrive requiring hospital admission, supplemental calories and gastrostomy tube placement. Although postnatal growth failure was noted in two of the seven previously reported patients, only one of these two patients was reported to require tube feedings [3], [4]. Although it is unclear if early postnatal growth issues and failure to thrive are primary features of Catel–Manzke syndrome, we suspect that our patient's failure to thrive was secondary to his severe complications from Pierre Robin sequence because weight gain improved with both gastrostomy and tracheostomy in place. Significant feeding difficulties and failure to thrive are common complications in patients with Pierre Robin sequence [9].
TGDS encodes dTDP-d-glucose 4,6-dehydratase/growth-inhibiting protein 21, a protein whose function remains unknown although it has been hypothesized to play a role in proteoglycan metabolism [3]. The finding of the c.298G > T (p.Ala100Ser, rs140430952) pathogenic variant in our patient supports the hypothesis of Ehmke et al. that this is the most common pathogenic variant associated with Catel–Manzke syndrome [3]. Taken together, the eight reported patients with pathogenic variants in TGDS have the c.298G > T variant at an allele frequency of 68.75%. Prior work has suggested that this variant is harbored on a 50 KB haplotype and thus suggests a common founder mutation, found primarily in the European population [3]. Our patient is of mixed European ancestry and previously described patients with this mutation were of German, French, Dutch and British/South American ancestry. A search of the ExAC database reveals that this variant has been observed 37 times in 121,256 alleles (allele frequency of 0.0003) in the heterozygous state [10]. In addition to individuals reporting European ethnicity, the variant has also been observed in two individuals with African ethnicity, and one individual with ethnicity described as “other” [10]. The amino acid substitution is present in the predicted NAD binding domain, and it is suggested that the mechanism is loss of function [3]. The absence of individuals with this variant in the homozygous state in the ExAC database provides further evidence of the pathogenicity [10].
Overall, we describe the eighth patient with molecularly confirmed Catel–Manzke syndrome. Our patient is on the severe end of the spectrum of previously reported patients with respiratory complications and failure to thrive requiring gastrostomy tube placement, supplemental calories, and tracheostomy but without cleft palate. Our finding of a homozygous p.Ala100Ser variant in TGDS in our patient supports that this is a common mutation in Catel–Manzke syndrome, and his phenotypic presentation underlies the severe respiratory and growth complications that can occur in association with Pierre Robin sequence in this disorder.
The following is the supplementary data related to this article.
Primers used for sequencing the coding region of TGDS.
Acknowledgments
We thank Alyssa Tran and Yuqing Chen for their technical assistance. L.C.B. was supported by the National Urea Cycle Disorders Foundation Fellowship and a fellowship from the Urea Cycle Disorders Consortium (UCDC; U54HD061221), which is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the Office of Rare Diseases Research (ORDR), the National Center for Advancing Translational Science (NCATS) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). L.C.B. and M.J. were supported by the National Institutes of Health [T32 GM07526]. This work was also supported by Baylor College of Medicine's IDDRC [Grant Number 1 U54 HD083092] from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
References
- 1.Catel W. G. Thieme; Stuttgart: 1961. Differentialdiagnose von Krankheitssymptomen bei Kindern und Jugendlichen. [Google Scholar]
- 2.Manzke H. Symmetrical hyperphalangy of the second finger by a supplementary metacarpus bone. Fortschr. Geb. Rontgenstr. Nuklearmed. 1966;105:425–427. [PubMed] [Google Scholar]
- 3.Ehmke N., Caliebe A., Koenig R., Kant S.G., Stark Z., Cormier-Daire V., Wieczorek D., Gillessen-Kaesbach G., Hoff K., Kawalia A., Thiele H., Altmuller J., Fischer-Zirnsak B., Knaus A., Zhu N., Heinrich V., Huber C., Harabula I., Spielmann M., Horn D., Kornak U., Hecht J., Krawitz P.M., Nurnberg P., Siebert R., Manzke H., Mundlos S. Homozygous and compound-heterozygous mutations in TGDS cause Catel–Manzke syndrome. Am. J. Hum. Genet. 2014;95:763–770. doi: 10.1016/j.ajhg.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manzke H., Lehmann K., Klopocki E., Caliebe A. Catel–Manzke syndrome: two new patients and a critical review of the literature. Eur. J. Med. Genet. 2008;51:452–465. doi: 10.1016/j.ejmg.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram V., Taysi K., Hartmann A.F., Jr., Shackelford G.D., Keating J.P. Hyperphalangy and clinodactyly of the index finger with Pierre Robin anomaly: Catel–Manzke syndrome. A case report and review of the literature. Clin. Genet. 1982;21:407–410. doi: 10.1111/j.1399-0004.1982.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 6.Kiper P.O., Utine G.E., Boduroglu K., Alanay Y. Catel–Manzke syndrome: a clinical report suggesting autosomal recessive inheritance. Am. J. Med. Genet. A. 2011;155A:2288–2292. doi: 10.1002/ajmg.a.34163. [DOI] [PubMed] [Google Scholar]
- 7.Nizon M., Alanay Y., Tuysuz B., Kiper P.O., Genevieve D., Sillence D., Huber C., Munnich A., Cormier-Daire V. IMPAD1 mutations in two Catel–Manzke like patients. Am. J. Med. Genet. A. 2012;158A:2183–2187. doi: 10.1002/ajmg.a.35504. [DOI] [PubMed] [Google Scholar]
- 8.Kant S.G., Oudshoorn A., Gi C.V., Zonderland H.M., Van Haeringen A. The Catel–Manzke syndrome in a female infant. Genet. Couns. 1998;9:187–190. [PubMed] [Google Scholar]
- 9.Evans A.K., Rahbar R., Rogers G.F., Mulliken J.B., Volk M.S. Robin sequence: a retrospective review of 115 patients. Int. J. Pediatr. Otorhinolaryngol. 2006;70:973–980. doi: 10.1016/j.ijporl.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Exome Aggregation Consortium (ExAC), Cambridge, MA. (URL: http://exac.broadinstitute.org/) [May 2015].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for sequencing the coding region of TGDS.