Abstract
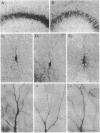
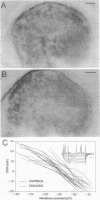
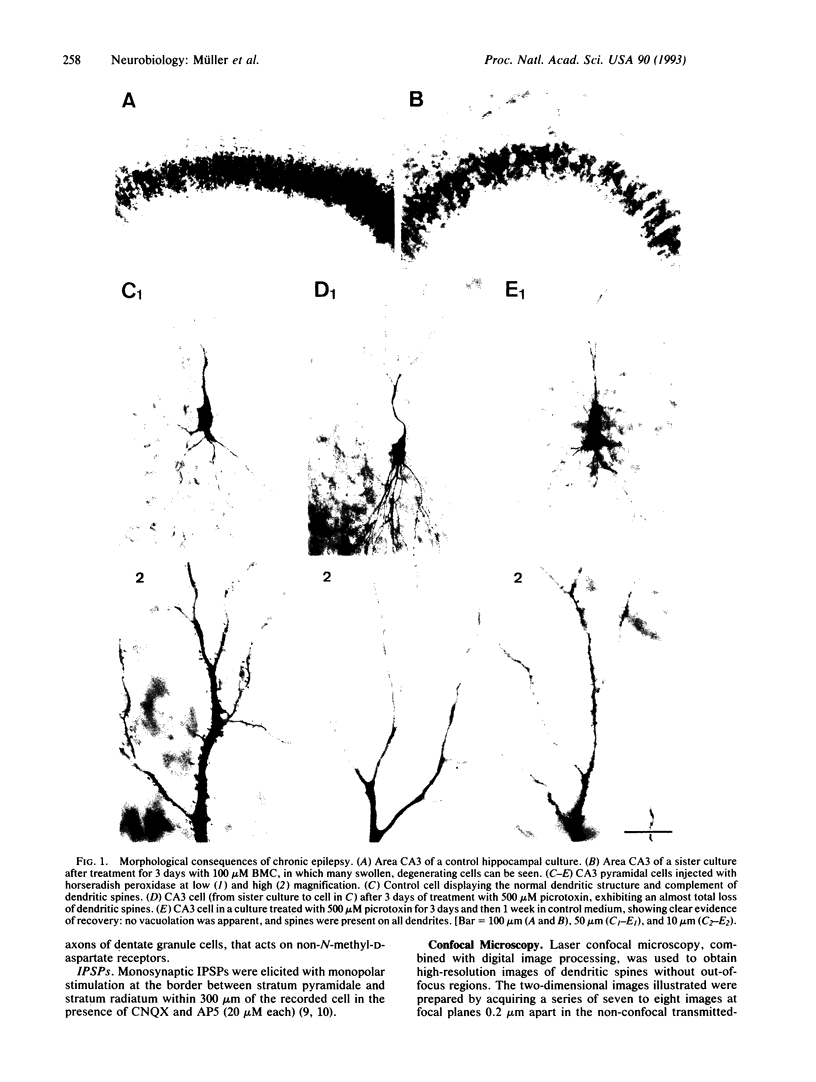
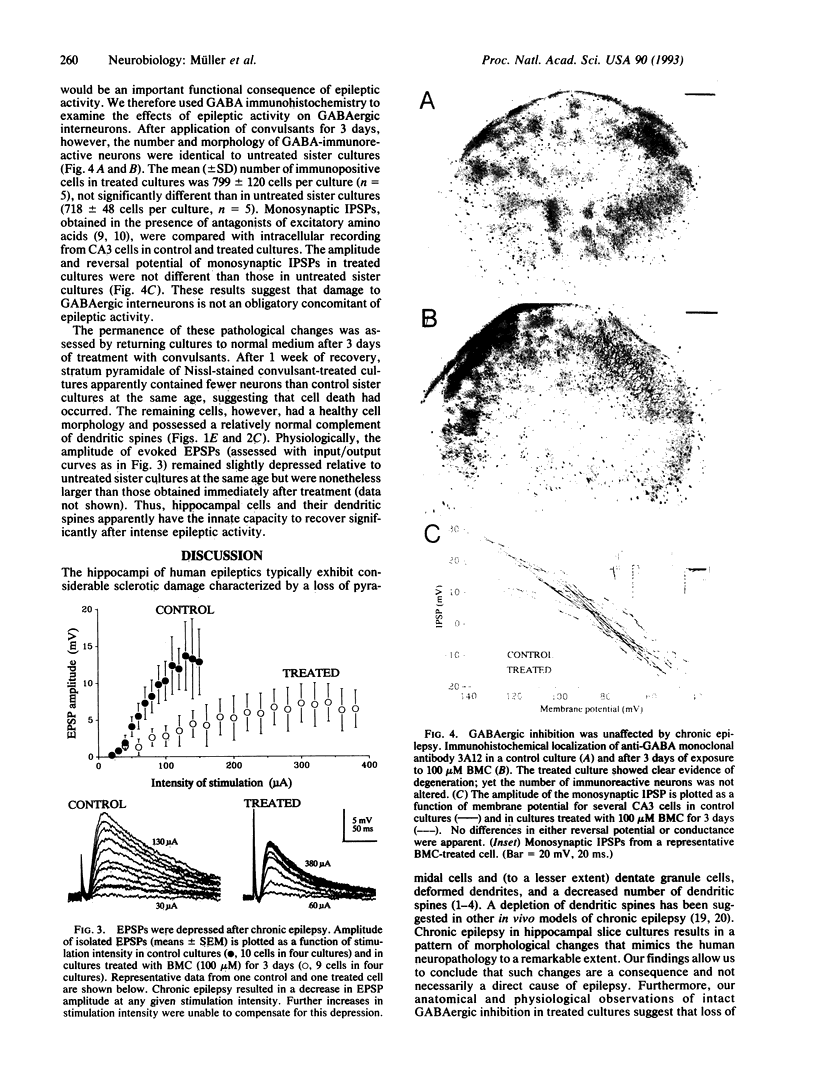
The morphological and functional consequences of epileptic activity were investigated by applying the convulsants bicuculline and/or picrotoxin to mature rat hippocampal slice cultures. After 3 days, some cells in all hippocampal subfields showed signs of degeneration, including swollen somata, vacuolation, and dendritic deformities, whereas others displayed only a massive reduction in the number of their dendritic spines. Intracellular recordings from CA3 pyramidal cells revealed a decrease in the amplitude of evoked excitatory synaptic potentials. gamma-Aminobutyric acid-releasing interneurons and inhibitory synaptic potentials were unaffected. Seven days after withdrawal of convulsants, remaining cells possessed a normal number of dendritic spines, thus demonstrating a considerable capacity for recovery. The pathological changes induced by convulsants are similar to those found in the hippocampi of human epileptics, suggesting that they are a consequence, rather than a cause, of epilepsy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babb T. L., Pretorius J. K., Kupfer W. R., Crandall P. H. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci. 1989 Jul;9(7):2562–2574. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley R. K., Jones D. G. Contributions of dendritic spines and perforated synapses to synaptic plasticity. Brain Res Brain Res Rev. 1990 Sep-Dec;15(3):215–249. doi: 10.1016/0165-0173(90)90002-6. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Rothman S. M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Corsellis J. A., Bruton C. J. Neuropathology of status epilepticus in humans. Adv Neurol. 1983;34:129–139. [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Ayala G. F. Cellular mechanisms of epilepsy: a status report. Science. 1987 Jul 10;237(4811):157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- Franck J. E., Kunkel D. D., Baskin D. G., Schwartzkroin P. A. Inhibition in kainate-lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J Neurosci. 1988 Jun;8(6):1991–2002. doi: 10.1523/JNEUROSCI.08-06-01991.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M., Gähwiler B. H. Synaptic organization of intracellularly stained CA3 pyramidal neurons in slice cultures of rat hippocampus. Neuroscience. 1988 Feb;24(2):541–551. doi: 10.1016/0306-4522(88)90348-x. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Morrell F., deToledo-Morrell L. Increase in the relative proportion of perforated axospinous synapses following hippocampal kindling is specific for the synaptic field of stimulated axons. Brain Res. 1990 Jan 22;507(2):325–331. doi: 10.1016/0006-8993(90)90291-i. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H. Development of the hippocampus in vitro: cell types, synapses and receptors. Neuroscience. 1984 Apr;11(4):751–760. doi: 10.1016/0306-4522(84)90192-1. [DOI] [PubMed] [Google Scholar]
- Isokawa M., Levesque M. F. Increased NMDA responses and dendritic degeneration in human epileptic hippocampal neurons in slices. Neurosci Lett. 1991 Nov 11;132(2):212–216. doi: 10.1016/0304-3940(91)90304-c. [DOI] [PubMed] [Google Scholar]
- Kamphuis W., Wadman W. J., Buijs R. M., Lopes da Silva F. H. Decrease in number of hippocampal gamma-aminobutyric acid (GABA) immunoreactive cells in the rat kindling model of epilepsy. Exp Brain Res. 1986;64(3):491–495. doi: 10.1007/BF00340486. [DOI] [PubMed] [Google Scholar]
- Kapur J., Stringer J. L., Lothman E. W. Evidence that repetitive seizures in the hippocampus cause a lasting reduction of GABAergic inhibition. J Neurophysiol. 1989 Feb;61(2):417–426. doi: 10.1152/jn.1989.61.2.417. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Dou P., Kater S. B. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988 Jun;8(6):2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum B. Possible therapeutic applications of antagonists of excitatory amino acid neurotransmitters. Clin Sci (Lond) 1985 Feb;68(2):113–122. doi: 10.1042/cs0680113. [DOI] [PubMed] [Google Scholar]
- Paul L. A., Scheibel A. B. Structural substrates of epilepsy. Adv Neurol. 1986;44:775–786. [PubMed] [Google Scholar]
- Popov V. I., Bocharova L. S., Bragin A. G. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48(1):45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Bradburne R. M., Harris A. B. A preferential loss of GABAergic, symmetric synapses in epileptic foci: a quantitative ultrastructural analysis of monkey neocortex. J Neurosci. 1982 Dec;2(12):1725–1735. doi: 10.1523/JNEUROSCI.02-12-01725.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak C. E., Reiffenstein R. J. Selective inhibitory synapse loss in chronic cortical slabs: a morphological basis for epileptic susceptibility. Can J Physiol Pharmacol. 1982 Jun;60(6):864–870. doi: 10.1139/y82-122. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Crandall P. H., Scheibel A. B. The hippocampal-dentate complex in temporal lobe epilepsy. A Golgi study. Epilepsia. 1974 Mar;15(1):55–80. doi: 10.1111/j.1528-1157.1974.tb03997.x. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987 Jan 2;235(4784):73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the "dormant basket cell" hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991 Jan;1(1):41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Slater N. T., ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987 Apr 16;326(6114):698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Streit Peter, Thompson Scott M., Gähwiler Beat H. Anatomical and Physiological Properties of GABAergic Neurotransmission in Organotypic Slice Cultures of Rat Hippocampus. Eur J Neurosci. 1989 Jan;1(6):603–615. doi: 10.1111/j.1460-9568.1989.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Rate and extent of recovery from dark rearing in the visual cortex of the mouse. Brain Res. 1971 Oct 8;33(1):1–11. doi: 10.1016/0006-8993(71)90302-7. [DOI] [PubMed] [Google Scholar]