Abstract
Hebbian Learning should not be reduced to contiguity since it detects contingency and causality. Hebbian Learning accounts of mirror neurons make predictions that differ from associative learning: through Hebbian Learning mirror neurons become dynamic networks that calculate predictions and prediction errors and relate to ideomotor theories. The social force of imitation is important for mirror neuron emergence and suggests canalization.
There is much to like about Cook et al.’s paper. Asking how mirror neurons emerge is indeed different from asking what mirror neurons are good for. Their richness of stimuli argument is well made. Their experimental evidence shows that experience can have an effect on sensorimotor associations. Unfortunately, it also presents the Hebbian Learning account of mirror neurons (Keysers, 2011; Keysers & Perrett, 2004) as an inferior alternative to ASL based on contiguity alone. Here we will argue instead that Hebbian Learning and ASL represent different levels of description - neural and cognitive, respectively - by showing that (a) Hebbian Learning is sensitive to contingency and causality, and (b) Hebbian Learning generates valuable predictions about the neural properties of mirror neurons.
Psychology and Physiology
ASL was proposed from a psychological perspective to explain the “causes and consequences of imitation” (Heyes, 2001). In contrast, the Hebbian Learning account was independently developed from a neurophysiological perspective to explain the emergence of mirror neurons. Single cell physiologists, Keysers and Perrett (2004), unaware of ASL, recorded neurons in the superior temporal sulcus (STS) and the premotor (PM) cortex. The unexpected similarity in the sensorimotor properties encountered in these two regions begged for a mechanistic explanation of how neurons acquire such action sensitive responses, and they harnessed a modern understanding of Hebbian Learning, based on the known physiology of Spike Time Dependent Plasticity, to explain how such neuron sensitivities could be wired up because, when you hear/see your actions or others imitate you, STS and PM neurons have the firing statistics to become interconnected (Keysers & Perrett, 2004).
Hebbian Learning is not simply contiguity, i.e. when neurons ‘fire together’ (their 2.2)
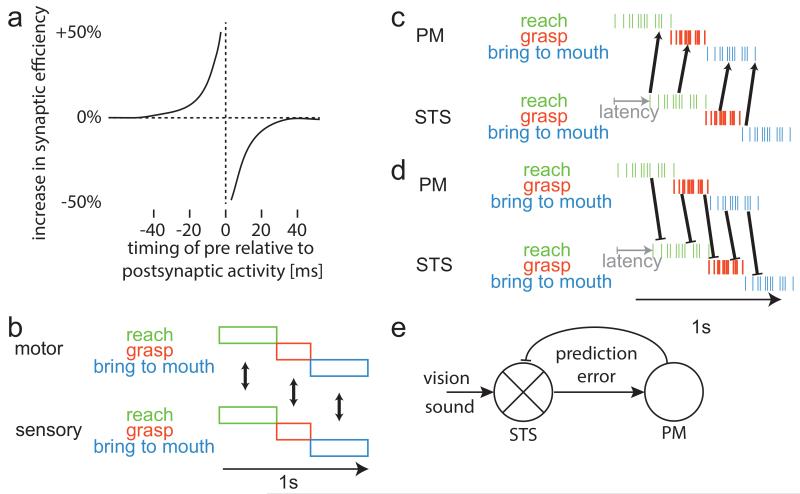
The Hebbian Learning account of mirror neurons draws on our contemporary understanding of Spike Time Dependent Plasticity (Caporale & Dan, 2008). Hebb (1949) stated that synapses become stronger “when one cell repeatedly assists in firing another” (p. 63), emphasizing causality, and neuroscience shows synapses are potentiated if the presynaptic input precedes but not follows postsynaptic activity (Fig 1a). Additionally, intermixing trials in which postsynaptic spiking occurs without presynaptic input prevents synaptic potentiation (Bauer, LeDoux, & Nader, 2001). In summary, physiologists and neuromodellers (http://lcn.epfl.ch/~gerstner/SPNM/node70.html) understand Hebbian Learning to depend on contingency/causality not simple contiguity. The authors’ critique of Hebbian Learning in this and other papers is a misunderstanding of what physiologists and modellers understand it to mean. Ironically, the authors’ new attempt to define ASL in neural terms (“The kind of learning that produces MNs occurs when there is correlated […] excitation of sensory neurons and motor neurons [… that] increases the strength of the connection between them […] when we observe our own actions”) is thus actually an adoption of a Hebbian Learning account of mirror neurons.
Figure 1.
(a) Temporal asymmetry in Hebbian Learning. (b) ASL predicts associations between corresponding phases of an action sequence. Hebbian Learning predicts associations between subsequent phases, i.e. predictions (c); utilizes inhibitory feedback (d) for prediction errors (e).
Hebbian Learning and ASL are not “synonyms” (7.1)
ASL takes a holistic, systems perspective. When I reach for a peanut, grasp it, and bring it to my mouth, I have three separate episodes of correlated sensorimotor experiences. ASL predicts associations within action phases (Fig. 1b and their fig 1c and vertical connections in Heyes, 2001). In contrast, Hebbian Learning takes the microscopic perspective of individual neurons and their spiking (Fig. 1c). STS neurons start firing ~100ms after their favorite stimulus (Keysers, Xiao, Foldiak, & Perrett, 2001) and hundreds of milliseconds lapse between PM spiking and overt movement (and even more before imitation by others); the assumption that sensory and motor representations are simultaneous is therefore an approximation (Keysers, 2011) – STS activity occurs ~250ms after PM activity for the same action (gray arrows in Fig. 1c). With synaptic plasticity temporally asymmetric (Fig. 1a and 1c), Hebbian Learning, unlike ASL, predicts that synaptic plasticity will also occur between action phases, connecting reach-STS to grasp-PM neurons, and grasp-STS to bring-to-mouth-PM neurons. Viewing reaching should then activate grasp-PM neurons. Predictive coding (Keysers, 2011; Keysers & Perrett, 2004) and active inference (Friston, Mattout, & Kilner, 2011) are fascinating outcomes of Hebbian Learning. Indeed, predictive coding is apparent: still images of reaching increase the excitability of muscles involved in grasping (Urgesi et al., 2010) and grasping mirror neurons respond to the sight of reaching behind an opaque screen (Umilta et al., 2001).
Connections from PM to STS also exist and have a net inhibitory influence. The Hebbian Learning account suggests the information flow from PM back to STS may cancel predicted sensory consequences and thereby compute action prediction errors (Fig. 1d) (Keysers & Perrett, 2004). Indeed, we showed that as people increasingly predict the gestures of others, the flow of activation indeed shifts from STS->PM to PM->STS (Schippers & Keysers, 2011).
Hence, unlike ASL, Hebbian Learning predicts the dynamic details of the neural circuitry that emerge during self-observation and imitation. The Hebbian Learning account matches modern theories of predictive coding or active inference (Fig 1e). While ASL refers to sensory or motor representations, Hebbian Learning describes a flow of information between STS and PM with both coming to contain hybrid sensorimotor representations. This opens intriguing parallels with ideomotor theories (Hommel, Musseler, Aschersleben, & Prinz, 2001)(and Brass, this volume)
ASL and Hebbian Learning are descriptions at different levels, and arguing that ASL accounts for mirror neurons better than Hebbian Learning seems as idle as arguing that psychology is better than neuroscience. Instead, asking how the circuitry-level predictions of contemporary Hebbian Learning relate to, implement and inform the systems-level predictions of ASL are more fruitful approaches.
Finally, the authors argue mirror neurons are not a social adaptation because domain-general mechanisms suffice to explain them - yet, parents’ peculiar motivation to imitate their child’s facial expressions, a domain-specific social behavior, is argued to be essential for, hence to canalize (Del Giudice, Manera, & Keysers, 2009) mirror neurons. This social force merits more analysis before accepting the argument against hybrid models.
References
- Bauer EP, LeDoux JE, Nader K. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nat Neurosci. 2001;4(7):687–688. doi: 10.1038/89465. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Manera V, Keysers C. Programmed to learn? The ontogeny of mirror neurons. Dev Sci. 2009;12(2):350–363. doi: 10.1111/j.1467-7687.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- Friston K, Mattout J, Kilner J. Action understanding and active inference. Biol Cybern. 2011;104(1-2):137–160. doi: 10.1007/s00422-011-0424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. The organisation of behaviour. Wiley; New York: 1949. [Google Scholar]
- Heyes C. Causes and consequences of imitation. Trends Cogn Sci. 2001;5(6):253–261. doi: 10.1016/s1364-6613(00)01661-2. [DOI] [PubMed] [Google Scholar]
- Hommel B, Musseler J, Aschersleben G, Prinz W. The Theory of Event Coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences. 2001;24(5):849–+. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Keysers C. The Empathic Brain. Social Brain Press; Amsterdam: 2011. [Google Scholar]
- Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends in Cognitive Sciences. 2004;8(11):501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Keysers C, Xiao DK, Foldiak P, Perrett DI. The speed of sight. J Cogn Neurosci. 2001;13(1):90–101. doi: 10.1162/089892901564199. [DOI] [PubMed] [Google Scholar]
- Schippers MB, Keysers C. Mapping the flow of information within the putative mirror neuron system during gesture observation. Neuroimage. 2011;57(1):37–44. doi: 10.1016/j.neuroimage.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. a neurophysiological study. Neuron. 2001;31(1):155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Maieron M, Avenanti A, Tidoni E, Fabbro F, Aglioti SM. Simulating the future of actions in the human corticospinal system. Cereb Cortex. 2010;20(11):2511–2521. doi: 10.1093/cercor/bhp292. [DOI] [PubMed] [Google Scholar]