Abstract
Background:
Effective management of postoperative pain leads to increased patient satisfaction, earlier mobilization, reduced hospital stay and costs. One of the methods used for management of postoperative pain is preemptive analgesia-blockade of afferent nerve fibers before a painful stimulus. It modifies peripheral and central nervous system processing of noxious stimuli and reduces postoperative opioid consumption. In this study, we sought to determine whether the preoperative use of pregabalin reduced postoperative pain and morphine consumption in thyroidectomy.
Materials and Methods:
The observation was conducted on patients undergoing thyroidectomy surgery in two groups of 30 each. Of the two groups, one received a single oral dose of pregabalin 1 h preoperatively. Both the group of patients undergoes anesthesia in a similar manner. Following surgery the efficacy of the preoperative dose of pregabalin is observed by measuring the total opioid consumption 6 h postoperatively and assessing verbal numeric pain scales.
Results:
The mean time to request of rescue analgesia in pregabalin group was 322.07 ± 69.106 min when compared to morphine group 256.33 ± 111.978 min (P < 0.05). The mean pain scores in the postoperative period were also significantly lower in patients receiving pregabalin.
Conclusion:
Single oral dose of pregabalin was effective in reducing acute postoperative pain in thyroidectomy patients. It prolongs the time to the request of rescue analgesia and also results in lower postoperative pain scores in the immediate postoperative period. However a statistically significant low opioid consumption could not be proved.
Keywords: Preemptive analgesia, pregabalin, verbal numeric scores
INTRODUCTION
Postoperative pain remains a challenging problem, which requires a pro-active approach using a variety of treatment modalities to obtain an optimal outcome with respect to enhancing patient comfort and facilitating the recovery process. The literature indicates that up to 75% of postsurgical patients have reported pain, and 80% of these patients experienced severe acute pain during their hospital stay.[1] The perception of pain varies enormously between individuals. Patients experiencing worsening pain after surgery are at risk of delayed discharge from hospital and may go on to develop chronic pain. Improving postoperative pain control accelerates the ability of patients to resume their activities of daily living.[2] Many patients undergoing surgery continue to experience unacceptably high levels of pain after their operation.[3,4] Despite recent advances in our knowledge of multimodal analgesic therapies and progress in our understanding of the pathophysiologic basis of acute pain, there remains a need for clinicians to implement evidence-based, procedure-specific multimodal analgesic protocols, which are modified to meet the needs of individual patients and to enhance the quality of postoperative pain management. Pain in the postanesthesia care unit (PACU) may be considered a failure to adequately include an analgesic plan as part of the anesthetic plan. Pain in the PACU or resulting side effects from opioids given for analgesia are significant drivers for prolonged PACU length of stay.[5,6] Prevention is clearly the best medicine when it comes to pain in the PACU.
Poorly managed acute pain that might occur following surgery can produce pathophysiologic processes in both the peripheral and the central nervous systems (CNS) that have detrimental acute and chronic effects.[7] The attenuation of perioperative pathophysiology that occurs during surgery through reduction of nociceptive inputs to the CNS and optimization of perioperative analgesia may decrease complications and facilitate recovery during the immediate postoperative period.[8]
Multitudes of medications are available for the treatment of postoperative pain. Neuraxial blocks and regional analgesic techniques stay apart from these. Opioids the most commonly used analgesic in the perioperative setting are prone for multiple untoward side effects. Recent guidelines of the American Society of Anesthesiologists (ASA) Task Force 2012 recommend maximizing nonopioid agents and using opioids as adjuncts when needed.[9]
This study investigates if administration of newer gabapentanoid, pregabalin is effective in decreasing acute postoperative pain in postthyroidectomy patients. Pregabalin, over gabapentin, has the advantage of high oral bioavailability that results in predictable dose-dependent responses, and has a short titration period that is better tolerated by patients.[10] Considering the contradicting nature of results regarding the effectiveness of oral pregabalin in controlling acute postoperative pain as obtained from the literature,[11,12,13,14,15] it is clear that the strategy needs further investigations in a variety of surgeries.
MATERIALS AND METHODS
We designed a prospective cohort study based on observation of two different, but common, perioperative practices at our institution. The study was conducted during the 6-month period from February to July 2013. After obtaining institutional ethics committee approval and written patient consent; study subjects included ASA physical status I and II patients of either sex, aged 20–55 years, weight between 40 and 80 kg, undergoing thyroidectomy surgery. Patients who refused to consent and with a history of allergy to any of the drugs observed in the study were excluded. 60 patients undergoing thyroidectomy surgery were observed as two groups of thirty each. The two groups were Group PM – receiving oral pregabalin 300 mg 1 h prior to surgery and morphine for postoperative rescue analgesia; and Group M - who undergoes surgery and receives rescue analgesia with morphine.
American Society of Anesthesiologists standard monitors were used for intraoperative monitoring. All patients were receiving 0.25 mg of oral alprazolam the previous night of surgery and morphine (0.1 mg/kg) at induction. Patients were induced with thiopentone (5 mg/kg) and vecuronium (0.1 mg/kg). Anesthesia was maintained with N2O, O2 and isoflurane. On completion of the surgery, patient was reversed with neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg) and extubated.
The outcome measures studied include mean postoperative pain scores, mean duration to request for rescue analgesia and number of boluses of morphine required in the 6 h postoperative period. The patient's postoperative pain was noted using Verbal Numeric Score (VNS) - patients indicate how intense their pain is on a scale from 0 to 10, on which 0 typically represents “no pain at all” and 10 “the worst pain imaginable” - at hourly intervals till the patient complains of pain or 6 h postoperatively whichever is earlier. Patients with postoperative VNS of 4 or more of either group was given a bolus of morphine (0.1 mg/kg) intravenously. The time duration from the end of surgery to the first analgesic bolus dose was recorded and compared. The total opioid consumption during a 6 h postoperative period was also measured. Any adverse effects to the drugs given were also observed. The observations made were tabulated and analyzed using Statistical Package for Social Sciences (SPSS) software Version 21.0.0. For all statistical evaluations, a two-tailed P < 0.05 was considered as significant.
RESULTS
The age distribution of the study population was as in Figure 1. Maximum numbers of patients were in the 40–50 age group. The mean age of group PM was 37.90 ± 5.98 years and that of group M was 39.20 ± 7.26 years. The gender distribution of study groups were as – group PM: Female 28, male 2; and group M: Female 27 and male 3. Age, gender and weight comparability between the two groups were assessed with Levene's test for equality of means, which showed no significant difference in age, gender and weight characteristics among the two groups (P = 0.46, 0.60, 0.20 respectively [all > 0.05]). The mean weight of Group PM was 54.57 ± 7.71 kg and that of Group M was 56.87 ± 6.12 kg and mean duration of surgery among the two groups was 114.8 ± 28.012 min in Group PM, and 116.83 ± 27.275 min in the Group M, hence comparable. The mean pain scores of the two groups during the 6 h postoperative period were as in Tables 1, 2 and Figure 2. The mean duration to request for rescue analgesia with morphine (i.e. VNS ≥ 4) was compared between the two groups. The mean duration to the request of rescue analgesia was more in Group PM (322.07 ± 69.106) min when compared to Group M (256.33 ± 111.978) min. Further analysis was done to see if the difference in duration to request for rescue analgesia was statistically significant using independent samples t-test. The correlation was found to be statistically significant with a P = 0.021 (<0.05). The number of boluses of rescue analgesic morphine consumed in a 6 h period was analyzed. Among the 30 patients in Group PM, 19 patients required no analgesic boluses, 8 patients required 1 bolus of morphine and 3 patients required 2 boluses of morphine. In Group M, among the 30 patients, 13 patients needed no analgesic boluses, 11 patients requested for 1 bolus and 6 patients requested for 2 boluses of rescue analgesic [Figure 3]. The median number of doses requested for in Group M was 1 additional bolus dose in 6 h whereas the median number of rescue analgesic bolus in Group PM was 0 in 6 h. The decrease in the number of boluses of rescue analgesic morphine required by the group PM was analyzed for significance using Levene's test for equality of variances. The reduction in number of boluses was not significant (P = 0.31) statistically.
Figure 1.
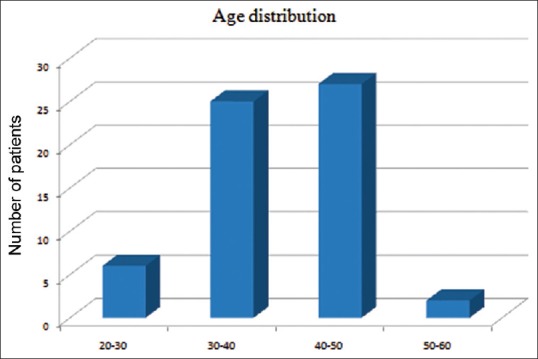
Age distribution of study population
Table 1.
Pain scores over 6 h in group M
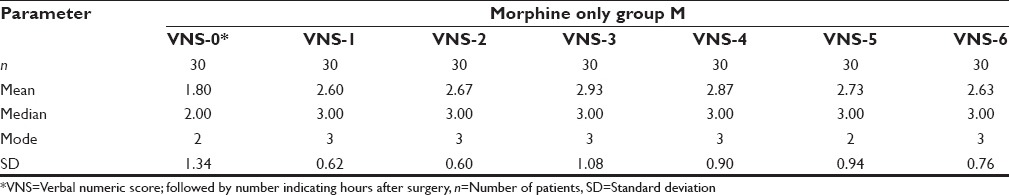
Table 2.
Pain scores over 6 h in group PM
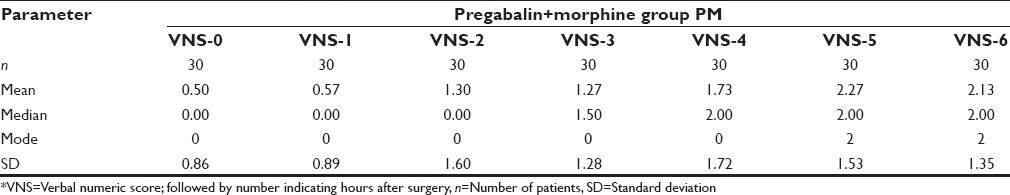
Figure 2.
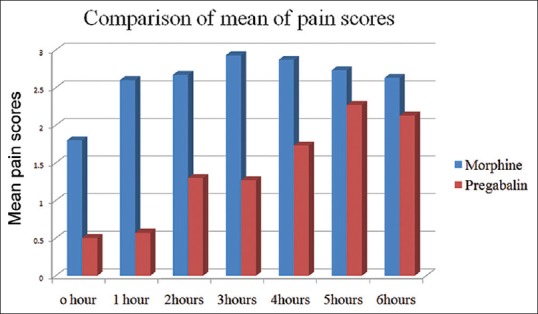
Pain scores over 6 h in both groups
Figure 3.
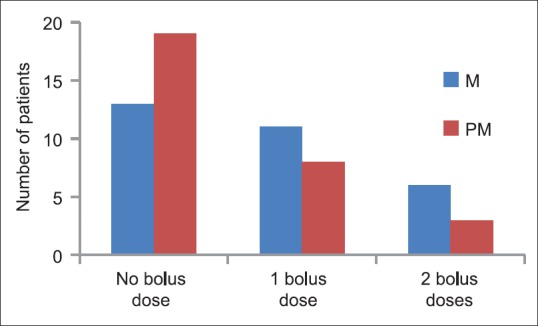
Distribution of number of boluses of rescue analgesic morphine in both groups
DISCUSSION
Nociceptive pain perceived after surgery mandates accurate assessment to direct rational drug choices while minimizing side-effects, which along with careful planning, multimodal analgesic techniques instituted preoperatively, help in the reduction of postoperative pain. Pain generates a neuroendocrine stress response mediated by a combination of local and systemic inflammatory substances, which results in increased catecholamine and catabolic hormone secretion that mediates sodium and water retention and increased levels of blood glucose, free fatty acids, ketone bodies, and lactate. Hyperglycemia from the stress response may contribute to poor wound healing and immunosuppression. The extent of the stress response is proportional to the degree of surgical trauma.[16] The detrimental physiologic effects of stress response include inhibition of fibrinolysis, hypercoagulability and related events like deep venous thrombosis, vascular graft failure, and myocardial ischemia.[17] Apart from the neuroendocrine stress response it generates, postoperative pain is known to decrease gastrointestinal motility resulting in paralytic ileus, decrease postoperative pulmonary function and result in pulmonary complications.
Transduction, transmission, perception and modulation are classically described in the processing of nociception.[18] Multimodal analgesic technique, an approach for balanced analgesia aims reduction in postoperative pain by targeting one or more of these pathways of nociception, thereby reducing the side effects of either. Better management of pain in the PACU setting will likely improve patient satisfaction and facilitate shorter PACU stays.
We investigated the efficacy of a single preoperative oral dose of pregabalin as an adjuvant to morphine in providing postoperative analgesia after thyroidectomy. Thyroid surgeries were chosen in our study as thyroidectomy elicits pain through multiple mechanisms like cervicotomy, intraoperative cervical hyperextension that causes postoperative muscular cervicalgia, laryngeal discomfort caused by frequent tracheal stimulation and movements of the endotracheal tube during surgical manipulation.[19] In addition, pain can also be caused by cervical drains, which are kept in place usually for 24 h. Mean pain scores in the range of 6.9 on a visual analog scale from 0 to 10 has been reported following thyroidectomy in previous studies.[20]
Another gabapentinoid, gabapentin also reduces pain intensity, opioid consumption, and opioid-related adverse effects after surgery.[21,22] However, compared with gabapentin; pregabalin has greater lipid solubility, better pharmacokinetic properties, and fewer drug interactions due to an absence of hepatic metabolism. Pregabalin is more potent than gabapentin and achieves its efficacy at lower doses, and, therefore, may be associated with fewer side-effects. Animal studies demonstrate pregabalin to be more potent than gabapentin as an antiepileptic[23] and as an analgesic in the treatment of chronic neuropathic pain.[24] In our study, pregabalin was administered prior to surgery based on pharmacokinetic considerations and findings in laboratory animals that pretreatment with pregabalin is more effective and longer lasting than posttreatment. Oral administration of pregabalin approximately an hour before surgery appears rational in order to attain maximal plasma concentration at the time of surgical stimuli. Pregabalin rapidly crosses the blood-brain barrier and consequently, its concentration in brain tissue, is nearly as high as in blood.[25] The dose we studied (300 mg) is within the prescribed limits of a single dose in the treatment of neuropathic pain. Administration of higher doses of pregabalin was associated with documented adverse effects such as somnolence, dizziness, ataxia and blurred vision.
The two groups in our study were comparable with respect to the age, weight, gender characteristics and mean duration of surgery. The mean postoperative pain scores were lower in patients receiving pregabalin preoperatively [Tables 1, 2 and Figure 2]. The mean duration to request for rescue analgesia was more in patients receiving pregabalin; group PM (322.07 ± 69.106 min) and group M (256.33 ± 111.978 min) (P = 0.02).
Among the patients receiving preoperative pregabalin lesser number requested for postoperative rescue analgesia with morphine. Among the 30 patients who received pregabalin; during the 6 h postoperative period, 19 patients required no analgesic boluses, 8 patients required 1 bolus of morphine and 3 patients required 2 boluses of morphine. Whereas, among the other group, 13 patients needed no analgesic boluses, 11 patients requested for 1 bolus and 6 patients requested for 2 boluses of rescue analgesic. Mean number of rescue analgesic boluses during the 6 h postoperative period in group M was 0.73 ± 0.78 and group PM was 0.53 ± 0.73. However, this decrease in number of additional boluses of rescue analgesics requested for was not statistically significant (P = 0.30). These findings suggest that though there was a decrease in opioid consumption in patients receiving pregabalin during the immediate 6 h postoperative period the decrease could not be proved statistically significant.
Previous clinical studies with pregabalin for postoperative analgesia provide promising results. Zhang et al., in their meta-analysis found statistically significant opioid-sparing effect of pregabalin with reduced incidence of postoperative vomiting as compared to morphine; however the authors could not find a significant difference in pain scores in early and late postoperative periods which they attributed to the heterogeneity among studies analyzed. Moreover, adverse events like visual disturbances postsurgery were reported with higher doses of pregabalin.[11]
Futher long-term follow-up study by Gianesello et al., in a more severe pain model found a significant reduction in opioid consumption and pain scores at rest and mobility in pregabalin group compared to placebo. However, the long-term advantage of perioperative pregabalin in this study were confounded by the use of other analgesics in the study period.[12]
Bornemann-Cimenti et al., in their study on patients undergoing elective transperitoneal nephrectomy found 300 mg oral pregabalin 1 h prior to surgery decreased total analgesic consumption and postoperative hyperalgesia without side-effects.[13] Unfortunately, the effects of preoperative hyperalgesia and its response to varying mechanical threshold of stimuli were not considered in their study.
Mathiesen et al., studied the combined analgesic effect of preoperative pregabalin and dexamethasone in patients undergoing total hip arthroplasty. The authors found significant reduction in opioid consumption, with no significant difference in pain scores in pregabalin group[14] however, addition of dexamethasone had no added analgesic effect, which is in contrary to the evidence from other acute pain models.
CONCLUSION
Our study provides contradictory evidence compared with previous studies in the literature that suggest oral pregabalin is an effective adjuvant to morphine in reducing postoperative pain. The study observed that the number of additional boluses of rescue analgesic requested by patients receiving preoperative pregabalin was lesser; pointing to an opioid sparing effect, but a statistically significant association could not be proved, however the duration to request for rescue analgesia was significantly prolonged. Findings of this study suggest that oral pregabalin is a good option as an adjuvant medication to morphine to reduce pain scores postoperatively, thereby facilitate faster discharge from PACU and faster rehabilitation.
The limitations of the study include-observational methodology, using a fixed dose 300 mg of pregabalin for a wide range of patient weights that can affect the drug effect, the extent of tissue damage caused during thyroidectomy can differ patient-wise; moreover, verbal numeric score, pain perception, and tolerance may vary from patient to patient. Further research in this field shall incorporate preoperative pain characteristics of the patients, the wider inclusion of surgical procedures and analysis of opioid adverse effects sparing potential of newer analgesics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Warfield CA, Kahn CH. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology. 1995;83:1090–4. doi: 10.1097/00000542-199511000-00023. [DOI] [PubMed] [Google Scholar]
- 2.White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F Fast-Track Surgery Study Group. The role of the anesthesiologist in fast-track surgery: From multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104:1380–96. doi: 10.1213/01.ane.0000263034.96885.e1. [DOI] [PubMed] [Google Scholar]
- 3.Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85:808–16. doi: 10.1097/00000539-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Brennan F, Carr DB, Cousins M. Pain management: A fundamental human right. Anesth Analg. 2007;105:205–21. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 5.White PF. Multimodal pain management – The future is now! Curr Opin Investig Drugs. 2007;8:517–8. [PubMed] [Google Scholar]
- 6.Schug SA, Chong C. Pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2009;22:738–43. doi: 10.1097/ACO.0b013e32833020f4. [DOI] [PubMed] [Google Scholar]
- 7.Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005;23:21–36. doi: 10.1016/j.atc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001;87:62–72. doi: 10.1093/bja/87.1.62. [DOI] [PubMed] [Google Scholar]
- 9.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–73. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 10.Sinatra RS, Jahr JS, Watkins-Pitchford JM. The Essence of Analgesia and Analgesics. New York: Cambridge University Press; 2011. Overview and use of antidepressant analgesics in pain management; pp. 338–46. [Google Scholar]
- 11.Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: A meta-analysis. Br J Anaesth. 2011;106:454–62. doi: 10.1093/bja/aer027. [DOI] [PubMed] [Google Scholar]
- 12.Gianesello L, Pavoni V, Barboni E, Galeotti I, Nella A. Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol. 2012;24:121–6. doi: 10.1097/ANA.0b013e31823a885b. [DOI] [PubMed] [Google Scholar]
- 13.Bornemann-Cimenti H, Lederer AJ, Wejbora M, Michaeli K, Kern-Pirsch C, Archan S, et al. Preoperative pregabalin administration significantly reduces postoperative opioid consumption and mechanical hyperalgesia after transperitoneal nephrectomy. Br J Anaesth. 2012;108:845–9. doi: 10.1093/bja/aes004. [DOI] [PubMed] [Google Scholar]
- 14.Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, et al. Pregabalin and dexamethasone for postoperative pain control: A randomized controlled study in hip arthroplasty. Br J Anaesth. 2008;101:535–41. doi: 10.1093/bja/aen215. [DOI] [PubMed] [Google Scholar]
- 15.Mathiesen O, Rasmussen ML, Dierking G, Lech K, Hilsted KL, Fomsgaard JS, et al. Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy. A randomized clinical trial. Acta Anaesthesiol Scand. 2009;53:227–35. doi: 10.1111/j.1399-6576.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 16.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 17.Wu CL, Fleisher LA. Outcomes research in regional anesthesia and analgesia. Anesth Analg. 2000;91:1232–42. doi: 10.1097/00000539-200011000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Robert L, Santosh P. Systemic non-opioid adjuvant analgesics: Their role in acute postoperative pain in adults. Trends Anaesth Crit Care. 2014;4:10–18. [Google Scholar]
- 19.Gozal Y, Shapira SC, Gozal D, Magora F. Bupivacaine wound infiltration in thyroid surgery reduces postoperative pain and opioid demand. Acta Anaesthesiol Scand. 1994;38:813–5. doi: 10.1111/j.1399-6576.1994.tb04010.x. [DOI] [PubMed] [Google Scholar]
- 20.Daou R. Thyroidectomy without drainage. Chirurgie. 1997;122:408–10. [PubMed] [Google Scholar]
- 21.Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain – A systematic review of randomized controlled trials. Pain. 2006;126:91–101. doi: 10.1016/j.pain.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104:1545–56. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 23.Bryans JS, Wustrow DJ. 3-substituted GABA analogs with central nervous system activity: A review. Med Res Rev. 1999;19:149–77. doi: 10.1002/(sici)1098-1128(199903)19:2<149::aid-med3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Lauria-Horner BA, Pohl RB. Pregabalin: A new anxiolytic. Expert Opin Investig Drugs. 2003;12:663–72. doi: 10.1517/13543784.12.4.663. [DOI] [PubMed] [Google Scholar]
- 25.Shneker BF, McAuley JW. Pregabalin: A new neuromodulator with broad therapeutic indications. Ann Pharmacother. 2005;39:2029–37. doi: 10.1345/aph.1G078. [DOI] [PubMed] [Google Scholar]


