Abstract
Aims and Objectives:
The aim of our study is to compare the efficacy and side-effects of Ketamine and Midazolam administered nasally for the pediatric premedication.
Materials and Methods:
We studied 100 American Society of Anesthesiology I and II children aged from 1 to 10 years undergoing various surgical procedures. Totally, 50 children were evaluated for nasal ketamine (using 50 mg/ml vials) at the dose of 5 mg/kg and the other 50 received nasal midazolam 0.2 mg/kg, before induction in operation theater each patient was observed for onset of sedation, degree of sedation, emotional status being recorded with a five point sedation scale, response to venipuncture and acceptance of mask, whether readily, with persuasion or refuse.
Results:
The two groups were homogenous. Midazolam showed a statistically significant early onset of sedation (10.76 ± 2.0352 vs. 16.42 ± 2.0696 min). There were no significant differences in venipuncture score, sedation scale at 20 min, acceptance of mask and oxygen saturation throughout the study. Significant tachycardia and ‘secretions were observed in the ketamine group intra operatively. Postoperatively emergence (8% vs. 0%) and secretions (28% vs. 4%) were significant in the ketamine group. Nausea and vomiting occurred in l6% versus 10% for midazolam and ketamine group.
Conclusions:
Both midazolam and ketamine nasally are an effective pediatric premedication. Midazolam has an early onset of sedation and is associated with fewer side-effects.
Keywords: Nasal, pediatric, premedication
INTRODUCTION
Premedication in pediatric age group presents a challenging situation. The young children are not fully able to understand the necessity for their surgery nor are they likely to be amenable for a reasoned explanation. Fear of operation theater, injections, and separation from parents prior to anesthesia produces traumatic experiences in tender mind of young children.[1] In the past, psychological preparation was used before surgery. Many drugs like morphine, paraldehyde meperedine, diazepam, trimeprazine, promethazine lorazepam and barbiturates have been used. Various routes of administration-oral’ intramuscular (IM), rectal and naial have been tried. There is still no ideal premedication or route of administration.
An ideal premedicant should act rapidly with adequate sedation and analgesia, cause less respiratory depression, no postoperative sickness and no hypersensitivity reaction likewise the ideal route should be atraumatic, less unpleasant and should require little co-operation.[2,3]
Intranasal premedication provides good conditions for induction of anesthesia in preschool children.[4] Intranasal midazolam for premedication in preschool children was first described by Wilton et al. and later studied by García-Velasco et al.[5,6] In our study, we comparatively evaluate the efficacy and side-effects of ketamine and midazolam administered nasally for paediatric patients aged 1–10 years.
MATERIALS AND METHODS
After approval by the Ethical Committee and obtaining informed parental consent, we studied one hundred American Society of Anesthesiology I and II children aged from 1 to 10 years undergoing various surgical procedures. Children were assigned randomly to receive either ketamine 5 mg or midazolam 0.2 mg/kg nasaly. All the children underwent a general assessment for mental status, weight, pulse, blood pressure and every child was investigated hemoglobin and urine analysis.
Demographic data including age, weight and sedation scale before premedication were recorded. Totally, 50-children were evaluated for nasal ketamine (using 50 mg/ml vials) at the dose of 5 mg/kg and the other 50 received nasal midazolam 0.2 mg/kg, (using 5 mg/ml ampules). The calculated dose for each patient was administered in each nostril divided equally 30 min before induction of anesthesia containing respective drugs were administered drop by drop slowly over 3–4 min and children were asked to put their tongue out and instructed not to swallow. For the next 30 min patients were asked to maintain supine position with slight head low.
Before induction in operation theater, each patient was observed for onset of sedation, degree of sedation recorded with a five point sedation scale, response to venipuncture and acceptance of mask, whether readily, with persuasion or refuse.
General anesthesia was standardized for all 100 patients to minimize confounding factors. After intravenous glycopyrrolate 0.004 mg/kg, induction was done with thiopentone 5–7 mg/kg. Intubation was facilitated by suxamethoniuin 2 mg/kg. All patients were maintained on oxygen, nitrous-oxide, halothane and pancuronium 0.08 mg/kg. The lungs were ventilated mechanically. At the conclusion of surgery, reversal was done with atropine 0.02 mg/kg and neostigmine 0.05 mg/kg and trachea was extubated.
Postoperatively patients were observed for restlessness, nausea and vomiting, secretions as well as pulse rate and respiratory status. Occurrence of emergence reactions was noted.
Five point sedation scale
Agitated - Clinging to parent/crying
Alert - Awake, not clinging to parent/no cry
Calm - Sitting or lying comfortably with eyes spontaneously open eyes spontaneously closed but responds
Drowsy - Comfortable with minor stimuli.
Asleep - Eyes closed, arousable, does not respond to minor stimuli.
Acceptance of mask
Refuses
Accepts with persuasion
Accepts readily.
Venipuncture score
Crying, uncooperative not able to start IV line
Withdrawal for painful stimuli but allows to crying
Calm no quantity, no-withdrawal, for painful stimuli and IV cannulation
Asleep - No response to painful stimuli and IV cannulation’.
Grades of salivation: Grade
Copious - 3
Moderate - 2
Mild - 1
None - 0.
Statistical analysis
Parametric data were reported as arithmetic mean ± standard deviation. Demographic data-age and weight distribution and quantitative data-pulse, respiratory rate, oxygen saturation and onset of sedation were analyzed using Z-test. Qualitative parameters degree of sedation on five point sedation scale, response to painful stimuli and venipuncture score acceptance of mask and intra operative secretion grading were analyzed using Chi-square test. Nominal data of postoperative observations were analyzed using Z proportionate test. P < 0.05 was accepted as significant’. Significance tests were performed using online GraphPad Software, Inc., La Jolla, CA, USA.
RESULTS
The average age in the ketamine group was 7.22 ± 2.4830 and in the midazolam group was 6.61± 2.6557. P value for age and weight distribution was >0.050 (0.2698). Both the groups belonged to a homogenous population [Figure 1].
Figure 1.
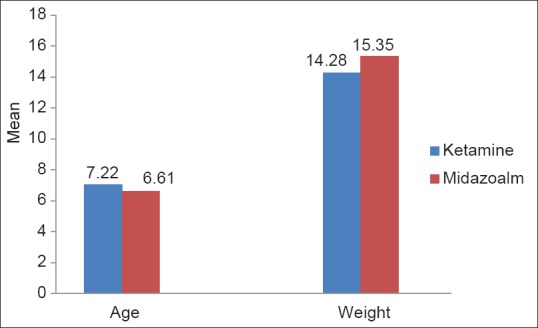
Demography of the two groups
The heart rates for the ketamine and midazolam groups pulse rates were preoperatively 103.82 ± 8.4633 and 101.22 ± 10.2884, after premedication 112.84 ± 9.1397 and 101.88 ± 13.47, intra operatively 121.64 ± 12.8557 and 108.2 ± 7.5683 and postoperatively 114 ± 10.5917 and 110.2 ± 8.9397 respectively [Figure 2].
Figure 2.
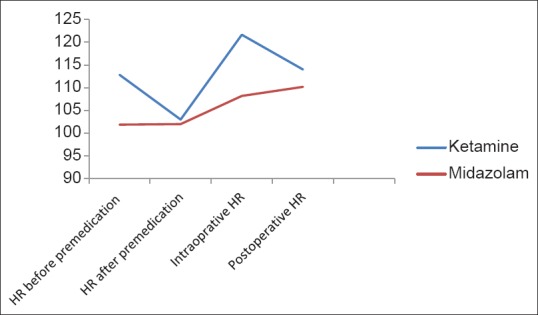
Heart rate trends for the two groups
Significant tachycardia in ketamine group P <0.001.
The respiratory rates before premedication in the ketamine and midazolam groups was 22.5 ± 3.3541 and 22.76 ± 3.1019 while after premedication 22.02 ± 2.7312 and 21.82 ± 7977 respectively. The postoperative respiratory rates in the two groups were 23.86± 3.1812 and 24.82 ± 4.1696. There were no significant differences in pulse rate and respiratory rate between two groups before premedication. Tachycardia was statistically highly significant in the ketamine group after premedication (P < 0.001 P = 0.0019). Five patients in the midazolam group showed heart rate <70. This was not statistically significant P = 0.718.
Tachycardia was highly significant in the ketamine group P < 0.001. Tachycardia persisted in ketamine group P = 0.0440. There were no significant differences in respiratory rate in both groups postoperatively (P = 0.1986). One patient in midazolam group had respiratory rate <15. This was not statistically significant.
There were no significant changes in the oxygen saturation throughout the study (P = 0.5246) [Figure 3].
Figure 3.
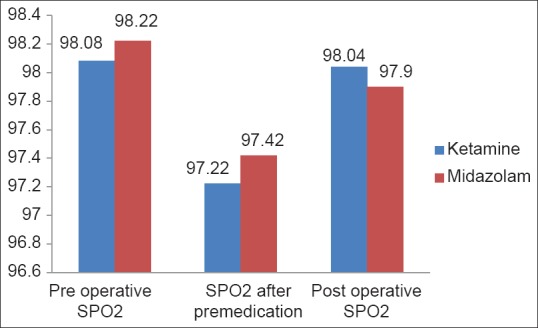
Oxygen saturations of two the groups
A total of 18 patients (36%) were calm in ketamine group while 23 patients (46%) were calm in the midazolam group. However, number of patients were asleep were more in the ketamine group than in the midazolam (7 vs. 3, 14% vs. 6%). For the calm group, comparison was done for both the drugs with baseline values using McNemars test [Tables 1-3]. Significant proportion of children were calm compare d to baseline sedation scale (P < 0.05).
Table 1.
Baseline sedation scale
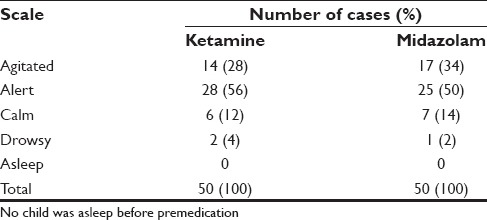
Table 3.
Five point sedation scale at 20 min
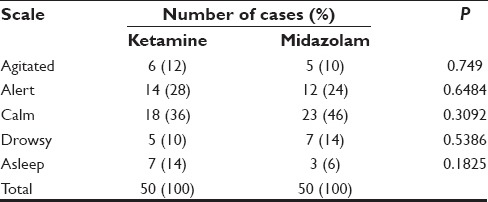
Table 2.
Onset of sedation

Forty percent in midazolam group and 32% in ketamine group belonged to grade III. Not possible to take IV line was observed in 12% in ketamine group and 6% in midazolam group. Overall there was no statistically significant difference in venipuncture score in both the groups. Calculations based on the z-ratio for the significance of the difference between two independent proportions [Table 4].
Table 4.
Venipuncture score

More number of patients in midazolam group accepted mask readily (29 vs. 23, 58% vs. 46%). Less number of patients refused face mask in midazolam group (3 vs. 6, 6% vs. 12%). However, more number of patients were able to accept to face mask in the ketamine group with persuasion [Table 5].
Table 5.
Acceptance of mask before induction
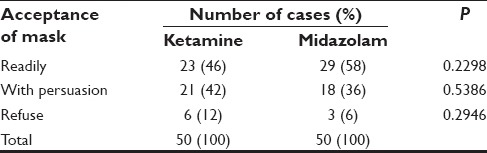
These differences were statistically insignificant (P > 0.05). Thus, comparable results were obtained for acceptance of mask in both the groups. Calculations based on the z-ratio for the significance of the difference between two independent proportions.
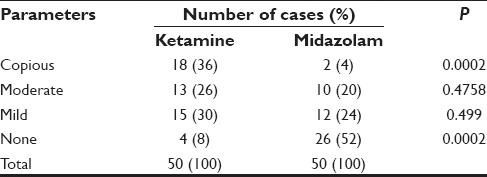
Copious secretions were observed in 36% in ketamine group versus only 4% in the midazolam group 52% in midazolam group showed no secretions. These were statistically highly significant (P < 0.001) [Table 6].
Table 6.
Intra operative secretion grading
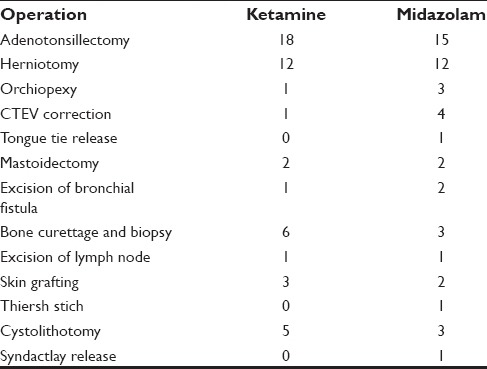
Emergence was observed in four patients of ketamine (8%) while eight patients in the midazolam group (16%) had nausea and vomiting. No emergence was seen with midazolam. These data were analyzed using Z proportionate test. The emergence and secretions postoperative side-effects were significant in ketamine group (P < 0.05) [Table 7]. Table 8 shows the types of surgery in both the groups.
Table 7.
Postoperative observations
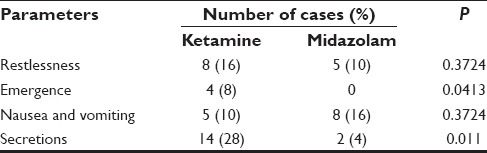
Table 8.
Types of surgery
DISCUSSION
Pediatric premedication is a challenging situation. Outcasting the psychological preparation of the child before surgery drugs have been tried by various routes for preanesthetic sedation. There is still no ideal premedication or route of administration. We studied a cohort of children who were scheduled for various surgeries because: They required general anesthesia: It was possible to use a standardized anesthetic technique with minimum confounding factors. A healthy and homogenous cohort of children could be recruited. At the conclusion of this study, we were satisfied that this population of children and this type of surgery were appropriate to undertake this comparative trial.
The design of our study may be criticized in that it was not a not a placebo controlled trial of two nasal premedications. Alderson and Lerman in their comparative study of oral ketamine and oral midazolam for pediatric ambulatory-anesthesia, have questioned the ethics of including a placebo arm in a study, where superiority of these medications has been established.[7]
Ketamine and midazolam have been tried by various routes for the pediatric premedication. Low-dose (2 mg/kg) IM ketamine has been used in young children under going brief out-patient procedures.[8] There were no unacceptable induction. The anesthetic times were shorter but discharge times were longer with ketamine. This technique was not recommended as routine induction method but deserves consideration in the management of difficult pediatric patients.
Midazolam and ketamine have been used for premedication by oral route. Gutstein et al. used ketamine 3 mg/kg and 6 mg/kg. This route was easy, predictable and satisfactory without significant side-effects.[9] However, oral premedicants are frequently rejected by children even when palatable.[10] Only 16% of ketamine is bio available by oral route and bioavailability of oral midazolam is 27%.[11,12]
Disadvantages of other routes of premedication include painful injection (IM), slow onset (oral and rectal) and delayed recovery (oral).[5] Nasal route has the advantage of rapid absorption of the drug directly into the systemic circulation from an area rich in blood supply without the disadvantage of passing through portal circulation.[13]
Intranasal midazolam has been studied in 45 preschool children between 18 months and 5 years. The absence of changes in respiratory rate, absence of clinical respiratory depression and apnea during induction suggested that this medication is safe.[5] García-Velasco et al. compared the efficacy and side-effects of midazolam 0.25 mg/kg and ketamine 5 mg/kg nasally for the pediatric premedication.[6]
In our study, the mean pulse late before and I and II premedication were 103.82 ± 8633 and 112.84 ± 9.1397 in ketamine group. This difference was statistically significant and is consistent with the known cardiovascular effect of ketamine. Ketamine produces its-sympathomimetic actions primarily by direct stimulation of central nervous system structures.[14] Certain drugs may achieve higher concentrations within brain or faster onset when administered nasally, and it is possible that these compounds are absorbed into the brain and cerebrospinal fluid directly through cribriform plate.[15] Corresponding parameters for midazolam were 101.22 ± 10.2884 and 101.88 ± 113.4724. Both the drugs did not produce any significant changes in respiration and oxygen saturation after premedication and throughout the study With lower doses used for premedication or sedation important respiratory depression does not occur. These findings are consistent with those of García-Velasco et al. and Wilton et al.[5,6] One patient in the midazolam (2%) group showed-decrease in heart rate and oxygen saturation after administration. Midazolam is known to depress both chemoreceptor response to hypoxia and ventilatory response to CO2.
Hence, continuous monitoring is mandatory whenever midazolam is administered, irrespective of the route of administration. Minor respiratory depression with 0.2 mg/kg of nasal midazolam and severe respiratory depression has been was noted midazolam 0.3 mg/kg dose.[16] Overall there were no significant changes in pulse rate and oxygen saturation. These findings are consistent with other studies.[17]
Intra operatively significant tachycardia (l21.64 ± 12.8557 vs. 108.2 ± 7.5683) was observed in the ketamine group.
Onset of sedation was 16.42 ± 2.0696 versus 10.76 ± 2.0352 for ketamine and midazolam. García-Velasco et al. in their comparison found that with both the drugs significant sedation occurred in l0 min. However, the mean onset time is not mentioned in their study. Wilton et al. found that significant sedation developed from 5 min with 0.2 mg/kg to 10 min with 0.3 mg/kg midazolam nasally. Otsuka et al. reported onset of sedation of 4 min with 0.2 mg/kg.[17] Malinovsky et al. found that adequate sedation with midazolam developed in 7.7 ± 2.4 min with nasal and 12.5 ± 4.9 by rectal routes.[18] In a recent study, the onset time of sedation with midazolam was 10.27 ± 3.35 min.[20] In our study, midazolam showed a significantly early onset of sedation compared to ketamine. This is consistent with the studies of plasma concentrations of both the drugs used nasally. l00 ng/ml (sedative) levels occurred within 6 min and maximum concentration at about 12 min with midazolam 0.2 mg/kg in the other study by Malinovsky et al.[18] Mean plasma concentration of ketamine peaked at 496 ng/ml at 20 min with 3 mg/kg and 2104 ng/ml at 21 min with 9 mg/kg nasally.[19] A slightly delayed onset of sedation with both the drugs in our study might be due to a part of nasal dose being swallowed and unnoticed in spite of patients being instructed not to swallow after nasal administration of the drug.
On the five point sedation scale, both the drugs were equally effective without any statistically significant differences. The response to painful stimuli and venipuncture score did not show any statistically significant differences between both the groups. The same was true for acceptance of mask. These findings are consistent with those of García-Velasco et al.
Intra operative secretion grading was highly significant in the ketamine group. This finding is not consistent with that reported by Weksler et al.[21] where no increase in airway secretion were noted, but are comparable with the findings of García-Velasco et al. Use or withhold of atropine is not mentioned in Weskler's study. We have replaced atropine with glycopyrrolate in premedication to avoid excessive tachycardia.
Postoperatively 62% of ketamine group showed one or the other side-effect while with midazolam group it was 30%. Emergence was observed in four patients (8%) with ketamine. Hollister and Burn have reported a 33% incidence of post operative vomiting with ketamine.[22] A clustering of older children in the other end of the age spectrum in our study might have resulted in a slightly higher incidence of emergence. 28% of ketamine group showed mild to moderate secretions in the postoperative period. In the midazolam goup, 10% had restlessness, nausea and vomiting 16% and secretions 4%. Wilton et al. reported an incidence of nausea and vomiting in l7% of their series. The other findings of increased secretions, and emergence reactions are consistant with those of García-Velasco.
CONCLUSION
Both midazolam and ketamine nasally are an effective pediatric premedication. Midazolam has an early onset of sedation and is associated with fewer side-effects.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Beeby DG, Hughes JO. Behaviour of unsedated children in the anaesthetic room. Br J Anaesth. 1980;52:279–81. doi: 10.1093/bja/52.3.279. [DOI] [PubMed] [Google Scholar]
- 2.Louon A, Reddy VG. Nasal midazolam and ketamine for paediatric sedation during computerised tomography. Acta Anaesthesiol Scand. 1994;38:259–61. doi: 10.1111/j.1399-6576.1994.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 3.Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: A comparison of four routes of administration. Paediatr Anaesth. 2002;12:685–9. doi: 10.1046/j.1460-9592.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 4.Weber F, Wulf H, el Saeidi G. Premedication with nasal s-ketamine and midazolam provides good conditions for induction of anesthesia in preschool children. Can J Anaesth. 2003;50:470–5. doi: 10.1007/BF03021058. [DOI] [PubMed] [Google Scholar]
- 5.Wilton NC, Leigh J, Rosen DR, Pandit UA. Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiology. 1988;69:972–5. doi: 10.1097/00000542-198812000-00032. [DOI] [PubMed] [Google Scholar]
- 6.García-Velasco P, Román J, Beltrán de Heredia B, Metje T, Villalonga A, Vilaplana J. Article in Spanish Nasal ketamine compared with nasal midazolam in premedication in pediatrics. Rev Esp Anestesiol Reanim. 1998;45:122–5. [PubMed] [Google Scholar]
- 7.Alderson PJ, Lerman J. Oral premedication for paediatric ambulatory anaesthesia: A comparison of midazolam and ketamine. Can J Anaesth. 1994;41:221–6. doi: 10.1007/BF03009834. [DOI] [PubMed] [Google Scholar]
- 8.Hannallah RS, Patel RI. Low-dose intramuscular ketamine for anesthesia pre-induction in young children undergoing brief outpatient procedures. Anesthesiology. 1989;70:598–600. doi: 10.1097/00000542-198904000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Gutstein HB, Johnson KL, Heard MB, Gregory GA. Oral ketamine preanesthetic medication in children. Anesthesiology. 1992;76:28–33. doi: 10.1097/00000542-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Madej TH, Paasuke RT. Anaesthetic premedication: Aims, assessment and methods. Can J Anaesth. 1987;34:259–73. doi: 10.1007/BF03015163. [DOI] [PubMed] [Google Scholar]
- 11.Grant IS, Nimmo WS, Clements JA. Pharmacokinetics and analgesic effects of i.m. and oral ketamine. Br J Anaesth. 1981;53:805–10. doi: 10.1093/bja/53.8.805. [DOI] [PubMed] [Google Scholar]
- 12.Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37:267–72. doi: 10.1007/BF00679782. [DOI] [PubMed] [Google Scholar]
- 13.De Boer AG, De Leede LG, Breimer DD. Drug absorption by sublingual and rectal routes. Br J Anaesth. 1984;56:69–82. doi: 10.1093/bja/56.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Chodoff P. Evidence for central adrenergic action of ketamine: Report of a case. Anesth Analg. 1972;51:247–50. [PubMed] [Google Scholar]
- 15.Hussain AA. Mechanism of nasal absorption of drugs. Prog Clin Biol Res. 1989;292:261–72. [PubMed] [Google Scholar]
- 16.Fukuta O, Braham RL, Yanase H, Kurosu K. Intranasal administration of midazolam: Pharmacokinetic and pharmacodynamic properties and sedative potential. ASDC J Dent Child. 1997;64:89–98. [PubMed] [Google Scholar]
- 17.Otsuka Y, Yusa T, Higa M, Terada T. Intranasal midazolam for sedation before anesthesia in pediatric patients. Masui. 1994;43:106–10. [PubMed] [Google Scholar]
- 18.Malinovsky JM, Populaire C, Cozian A, Lepage JY, Lejus C, Pinaud M. Premedication with midazolam in children. Effect of intranasal, rectal and oral routes on plasma midazolam concentrations. Anaesthesia. 1995;50:351–4. doi: 10.1111/j.1365-2044.1995.tb04616.x. [DOI] [PubMed] [Google Scholar]
- 19.Malinovsky JM, Lejus C, Servin F, Lepage JY, Le Normand Y, Testa S, et al. Plasma concentrations of midazolam after i.v. nasal or rectal administration in children. Br J Anaesth. 1993;70:617–20. doi: 10.1093/bja/70.6.617. [DOI] [PubMed] [Google Scholar]
- 20.Khatavkar SS, Bakhshi RG. Comparison of nasal midazolam with ketamine versus nasal midazolam as a premedication in children. Saudi J Anaesth. 2014;8:17–21. doi: 10.4103/1658-354X.125904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weksler N, Ovadia L, Muati G, Stav A. Nasal ketamine for paediatric premedication. Can J Anaesth. 1993;40:119–21. doi: 10.1007/BF03011307. [DOI] [PubMed] [Google Scholar]
- 22.Hollister GR, Burn JM. Side effects of ketamine in pediatric anesthesia. Anesth Analg. 1974;53:264–7. [PubMed] [Google Scholar]


