Abstract
Context:
Spinal anesthesia is preferred choice of anesthesia in lower abdominal surgeries since long time. However problem with this is limited duration of action, so for long duration surgeries alternative are required. Dexmedetomidine is a highly selective alpha-2-adrenergic agonist has property to potentiate the action of local anesthetic used in spinal anesthesia. Fentanyl is an opioid and it has also the same property.
Aims:
To compare the efficacy, analgesic effects, and side-effects of dexmedetomidine and fentanyl as adjuvant to bupivacaine for lower abdominal surgery.
Settings and Design:
The type of this study was double-blind randomized trial.
Subjects and Methods:
A total of 80 patients were randomly allocated in two Group D and Group F. Group D were injected injection bupivacaine 0.5% heavy × 3.0 ml + 0.5 ml of preservative free normal saline containing 5 μg dexmedetomidine. Group F were received injection bupivacaine 0.5% heavy × 3.0 ml + 0.5 ml fentanyl equivalent to 25 μg.
Statistical Analysis Used:
The statistical analysis was performed using SPSS (Statistical Package for Social Sciences) version 15.0 statistical analysis software.
Results:
The results show that highest sensitivity level of T6 and T8 was achieved by higher proportion of subjects from Group D when compared to Group F and sensitivity level T7 was achieved by higher proportion of subjects of Group F when compared to Group D. Duration of analgesic properties was significantly higher in Group D when compared to Group F.
Conclusion:
The findings in the present study suggested that intrathecal adjuvant use of dexmedetomidine as compared to fentanyl provides a longer sensory and motor blockade and also prolongs the postoperative analgesic effect.
Keywords: Analgesia, dexmedetomidine, fentanyl, motor block, sensory block, spinal anesthesia
INTRODUCTION
Lower abdominal and lower limb surgeries may be performed under local, regional (spinal or epidural) or general anesthesia, but neuraxial blockade is the preferred mode of anesthesia. Spinal block is still the first choice because of its rapid onset, superior blockade, low risk of infection as from catheter in situ, less failure rates and cost-effectiveness. However, postoperative pain control is a major problem because spinal anesthesia using only local anesthetics is associated with relatively short duration of action, and thus early analgesic intervention is needed in the postoperative period. A number of adjuvants have been studied to prolong the effect of spinal anesthesia.[1,2]
Dexmedetomidine is a highly selective alpha-2-adrenergic agonist, which has been used as a premedication and as an adjuvant to general anesthesia. Dexmedetomidine have several beneficial actions during perioperative period.
It reduces opioids and inhalational anesthetic requirement and have been widely used for Intensive Care Unit sedation with hemodynamic stability.[3]
Fentanyl is an opioid and some studies suggest that addition of fentanyl to hyperbaric bupivacaine improves the quality of subarachnoid block but addition of opioids to local anesthetic solution have disadvantages, such as pruritus and respiratory depression.
In the present study, an attempt has been made to evaluate the use of intrathecal combination of dexmedetomidine with bupivacaine in lower abdominal surgeries when compared to bupivacaine with fentanyl in terms of achievement and maintenance of block and postoperative analgesia among patients undergoing lower abdominal surgeries.
SUBJECTS AND METHODS
After obtaining clearance from ethical committee, the enrolled patients were randomized in two groups of 40 patients each (n = 40) using random number table.
Group D: Patients receiving bupivacaine with dexmedetomidine
Group F: Patients receiving bupivacaine with fentanyl.
All the patients fulfilling the inclusion criteria were invited to participate in the study. After obtaining informed consent and ascertaining that they do not fall into exclusion criteria, the patients were randomly allocated to one of the two study groups as indicated.
Demographic data of all the patients was noted.
All the patients in both the groups were premedicated with tablet diazepam 5 mg, tablet rantac 150 mg, night prior to surgery.
On the day of surgery, the patients were wheeled into the operation theatre and connected to all noninvasive monitors. Baseline parameters including pulse rate, arterial blood pressure (BP) (noninvasive BP [NIBP]) and oxygen saturation were noted. Electrocardiography monitoring was enabled.
They were preloaded with 20 ml/kg Ringer's lactate.
Under strict aseptic precaution, 25 gauge spinal needle was inserted in L3-L4 interspinal space with patient in sitting position using a midline approach. After confirmation with free flow of cerebrospinal fluid, patients allocated to Group D were injected injection bupivacaine 0.5% heavy × 3.0 ml + 0.5 ml of preservative free normal saline containing 5 μg dexmedetomidine. Patients allocated to Group F received injection bupivacaine 0.5% heavy × 3.0 ml + 0.5 ml fentanyl equivalent to 25 μg.
The anesthesiologist performing the block recorded the baseline value of vital signs (BP, heart rate [HR], oxygen saturation [SpO2]) before performing the spinal anesthesia, after administering the study drug intrathecally and thereafter once in every 5 min till the surgery is concluded.
The sensory dermatome level was assessed by pin prick sensation using 23 gauge hypodermic needle along the mid clavicular line bilaterally. The sensory level and Bromage scale were recorded every 2 min after the spinal injection up to the 10 min and after that every 10 min until the highest dermatome was reached. In the postanesthesia care unit (PACU), the sensory level and Bromage scale were recorded every 10 min until the patient was discharged from the PACU. All durations were calculated considering the time of spinal injection as time 0. When sensory levels of anesthesia were not equal bilaterally, the higher level was used for the statistical analysis. Recovery time for sensory blockade was defined as two dermatome regression of anesthesia from maximum level.
Adverse effects such as nausea, vomiting, pruritus, respiratory depression and shivering were also documented and managed symptomatically.
Heart rate, NIBP, respiratory rate (RR) and peripheral SpO2 was recorded at:
T1 = Immediately before dural puncture for spinal anesthesia
T2 = Immediately after dural puncture for spinal anesthesia
T3 to T26 = Every 5 min thereafter for 120 min.
Hypotension was defined by decrease in mean arterial pressure (MAP) below 20% of baseline or systolic BP (SBP) <90 mm Hg and was treated with injection mephentermin 6 mg/ml.
Respiratory depression was defined as RR <8 breaths/min or SpO2 <95% and was treated with oxygen supplementation and respiratory support, as and when required.
Statistical tools employed
The statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 15.0 statistical analysis software. The values were represented in number (%) and mean ± standard deviation (SD).
The following statistical formulas were used:
Mean
The individual observation is denoted by the sign X, number of observation denoted by n, and the mean by X̄.
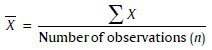
Standard deviation
It is denoted by the Greek letter σ. If a sample is more than 30 then.
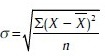
When sample in <30 then.
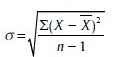
Chi-square test
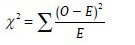
Where, O = Observed frequency
E = Expected frequency
Student's t-test
To test the significance of two means the Student's t-test was used
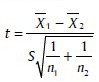
Where, 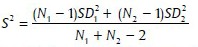
Where, X1, X2 are means of group 1 and group 2
N1, N2 are number of observation group 1 and group 2 SD1, SD2 are SD in group 1 and group 2.
Paired t-test
To compare the change in a parameter at two different time intervals paired t-test was used.
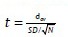
Where, dav is the mean difference, that is, the sum of the differences of all the data points (set 1 point 1 - set 2 point 2,…) divided by the number of pairs SD is the SD of the differences between all the pairs N is the number of pairs.
Level of significance
P is level of significance
P > 0.05: Not significant
P < 0.05: Significant
P < 0.01: Highly significant
P < 0.001: Very highly significant.
RESULTS
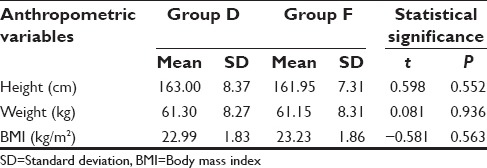
Patients were comparable to each other in terms of demographic characteristics and anthropometric data [Tables 1 and 2]. At baseline, that is, before dural puncture all the hemodynamic parameters were matched and did not show a significant difference between two groups (P > 0.05).
Table 1.
Demographic profile of study population
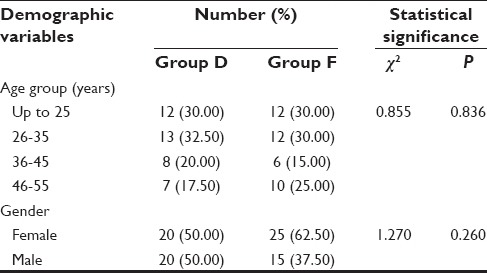
Table 2.
Anthropometric profile of study population
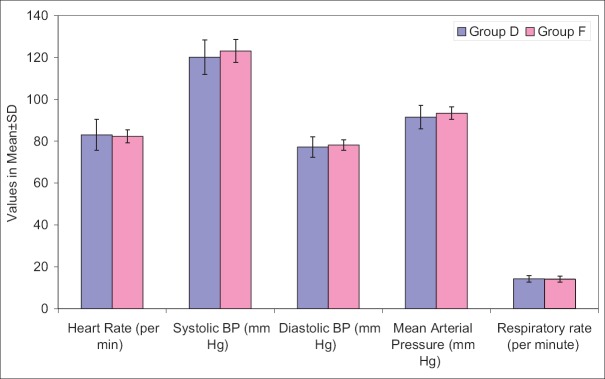
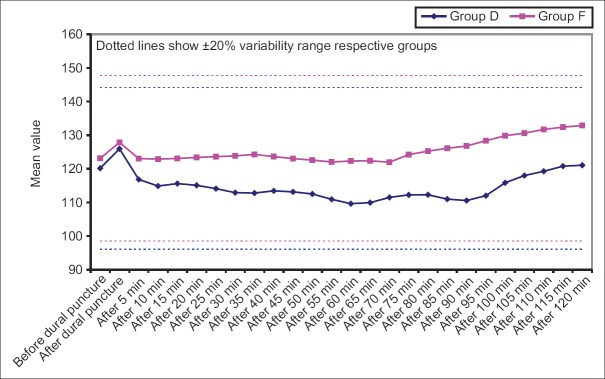
Heart rate, SBP, mean diastolic BP (DBP) at all the above intervals was lower in Group D when compared to Group F. Difference of HR was statistically significant at all the above intervals except at before dural puncture, 35 min, 40 min and 120 min after dural puncture, whereas difference of SBP was statistically significant at all the above intervals except at baseline, just after dural puncture and 5 min after dural puncture and mean DBP did not show a statistically significant difference at baseline, after dural puncture and 5 min after dural puncture, however, at all the subsequent intervals, except after 70 min interval, the difference between two groups was significant statistically [Figures 1-4].
Figure 1.
Baseline hemodynamic variables in study population
Figure 4.
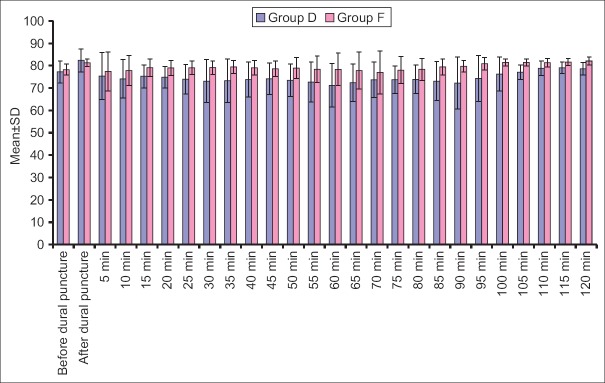
Diastolic blood pressure at different time intervals
Figure 2.
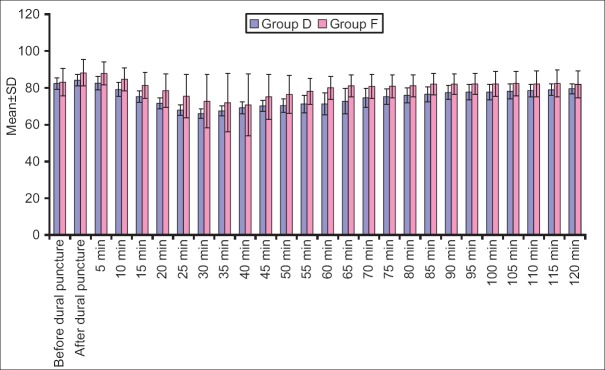
Heart rate at different time intervals
Figure 3.
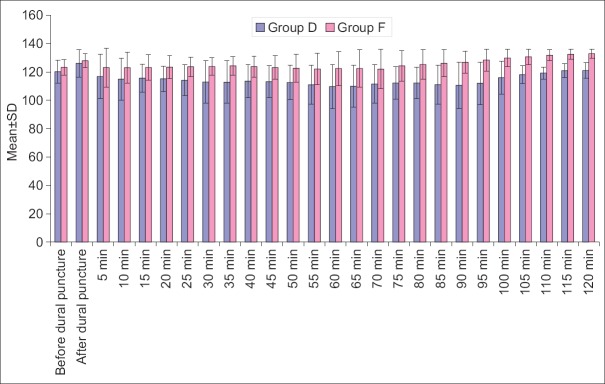
Systolic blood pressure at different time intervals
Results indicate that MAP at all the intervals was lower in Group D when compared to Group F. This difference was statistically significant at all the above intervals except at baseline, at just after dural puncture and 5 min and 55 min after dural puncture [Figure 5].
Figure 5.
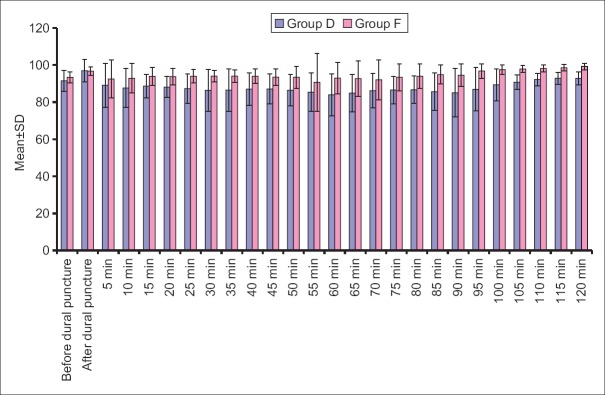
Mean arterial pressure at different time intervals
In Group D, mean change in HR from baseline was significant statistically at all-time intervals except after 5 min of dural puncture and from after 95 to 120 min intervals whereas In Group F, significant differences from baseline were observed at all-time intervals except after 15 min interval and from after 85 min interval till the end of study period [Figure 6].
Figure 6.
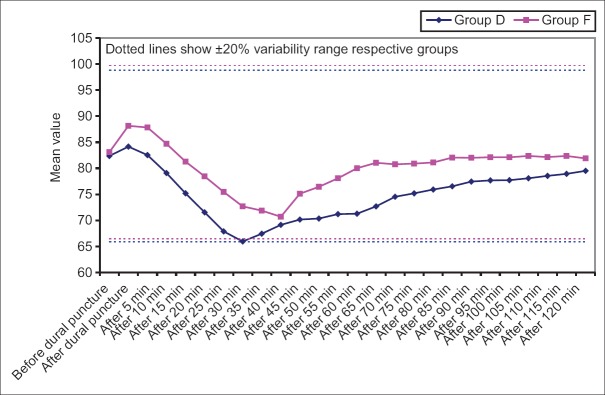
Change in heart rate within group at different time intervals from baseline (before dural puncture)
In Group D except at immediately after dural puncture, at all the time intervals mean SBP was lower than baseline. The difference from baseline was also significant statistically immediately after dural puncture interval and from 10 min after puncture interval till 95 min after puncture interval (P < 0.05). Though mean SBP was lower than baseline from 100 min till the end of study too yet the difference from baseline was not significant statistically (P > 0.05).
In contrast, in Group F, at most of the time intervals, mean SBP was higher than the baseline but the difference was not significant statistically. In this group, statistically significant difference from baseline was observed immediately after puncture and after 95 min of puncture and subsequent intervals. At all these time intervals, mean SBP was higher than baseline [Figure 7].
Figure 7.
Change in systolic blood pressure within group at different time intervals from baseline (before dural puncture)
In Group D, as compared to baseline, mean DBP was significantly higher at immediately after dural puncture interval, however, thereafter at all the subsequent intervals, mean value was lower than baseline till 105 min after dural puncture interval. The difference from baseline was significant statistically too immediately after puncture and from 10 min after puncture interval to 80 min after puncture interval. At subsequent intervals, irrespective of the direction of change from baseline, the difference was not significant statistically. However, in Group F, most of the time mean DBP was above baseline but the difference from baseline was significant statistically only immediately after dural puncture interval and then from 90 min after puncture till the end of follow-up period [Figure 8].
Figure 8.
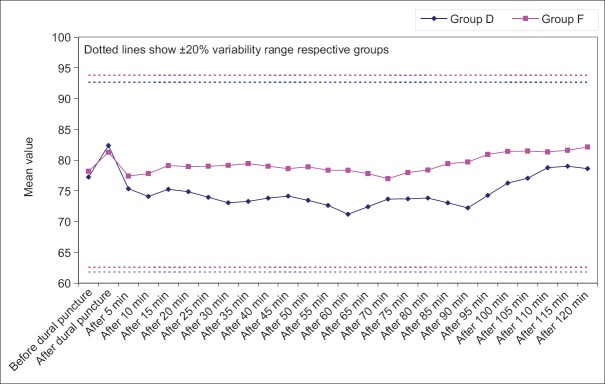
Change in diastolic blood pressure at different time intervals from baseline (before dural puncture)
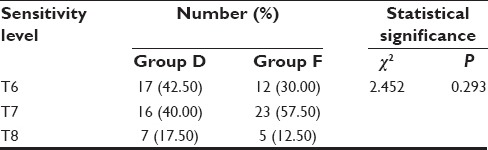
Results shows that highest sensitivity level of T6 and T8 was achieved by higher proportion of subjects from Group D when compared to Group F and sensitivity level T7 was achieved by higher proportion of subjects of Group F when compared to Group D. Difference in highest sensitivity level achieved by both the groups was statistically nonsignificant [Table 3].
Table 3.
Highest level of sensory block in study population
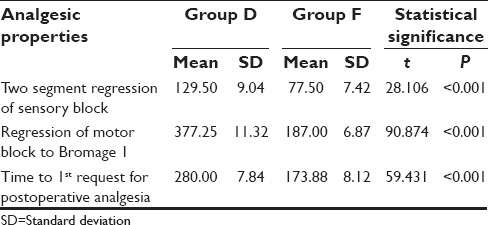
Duration of analgesic properties was significantly higher in Group D when compared to Group F [Table 4].
Table 4.
Duration (in min) of analgesic properties
Except for pruritus and nausea, all the other side effects/complications were higher in Group D as compared to Group F. However, a significant difference between two groups was not observed for any of the side-effects [Figure 9].
Figure 9.
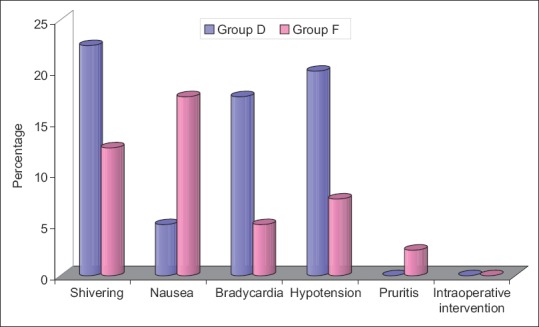
Side effects in study population
DISCUSSION
Spinal anesthesia results from injecting local anesthetic directly into the intrathecal space. To improve the spinal anesthetic efficacy, adjuvants from different pharmacological classes of drugs are used to enhance and prolong analgesia, to lower dose requirements, and to reduce dose-dependent side-effects. Studies using dexamethasone for postoperative pain relief have produced positive results mainly in surgery involving large amounts of tissue trauma. Following the successful use of intrathecal dexmedetomidine in animal studies in a dose range of 2.5–100 μg,[4,5,6,7] its use in human studies has also shown promising results in terms of early sensory and motor blocks and enhanced postoperative analgesic effect.[8,9,10,11] On the other hand fentanyl, a lipophilic μ-receptor agonist opioid, is being used as an adjuvant for a long time with no major complications.[12] In the present study, an attempt was made to compare the analgesic effects and side-effects of dexmedetomidine and fentanyl for lower abdominal surgery when used as an adjuvant with bupivacaine in patients undergoing lower abdominal surgery.
For this a comparative study was carried out in which a total of 80 patients undergoing abdominal limb surgery under spinal anesthesia using bupivacaine were enrolled. Of these 80 patients, 40 (50%) each were randomly allocated to two study groups – Group D, patients managed with spinal bupivacaine with dexmedetomidine as adjuvant and Group F, patients managed with bupivacaine and fentanyl as adjuvant. The two groups were matched demographically, hemodynamically, biochemically and for surgical complexity at baseline, to rule out any confounding effect.
In both groups, a significant rise in mean HR was observed immediately after dural puncture. Increase in the HR immediately after dural puncture could be attributed to the patient anxiety as none of the drugs used in the study had such quick onset time. The transitory nature of this effect was evident from the fact that in very next interval a decline in HR was observed in both the groups, though it was quicker, continuous and sustained for longer duration of time in dexmedetomidine supplemented group as compared to fentanyl supplemented group. Dexmedetomidine has a known regressive effect on HR.[13]
While evaluating the analgesic efficacy of intrathecally administered dexmedetomidine also found a suppressive effect on HR in the dexmedetomidine supplemented group. In a similar study Singh and Shukla[14] also reported similar findings as compared to control group. With respect to comparative evaluation of HR in dexmedetomidine and fentanyl supplemented groups, our results were similar to those observed by Gupta et al.[15] and Bajwa et al.[16] who also reported similar differences between dexmedetomidine and fentanyl supplemented groups. They also reported a relatively lower HR in dexmedetomidine supplemented group as compared to the fentanyl supplemented group. As a consequence, in the present study relatively higher proportion of patients in dexmedetomidine group showed bradycardia (17.5%) as compared to fentanyl supplemented group (5%). Bupivacaine itself has a bradycardic effect.[17,18,19]
Dexmedetomidine is an alpha-2 agonist drug, which affects the alpha-2 receptors that are found in peripheral and central nervous systems, platelets, and many other organs, including the liver, pancreas, kidney, and eye. Stimulation of the receptors in the brain and spinal cord inhibits neuronal firing, causing hypotension, bradycardia, sedation, and analgesia.[20] Although, hypotensive effect is reported to last for brief duration after bolus dose of the drug[21] yet in the present study such effect was noticed even while administering the drug through spinal route. Although bradycardia due to dexmedetomidine is reported in literature, yet two studies reporting their use in spinal anesthesia[16] did not report a significant difference in bradycardiac effect as compared to fentanyl. Contrary to results in the present study, where 8 cases of bradycardia were reported in dexmedetomidine supplemented group.
Similar to trends observed for HR, for BP too, a suppressive effect of dexmedetomidine was observed. In contrast, in fentanyl supplemented group most of the times mean values remained close to baseline and did not show a significant difference.
Dexmedetomidine, a highly selective alpha-2 agonist drug, is approved as an intravenous sedative and co-analgesic drug. Its use is often associated with a decrease in HR and BP.[13] Intrathecal and epidural characteristics of dexmedetomidine have been studied in animals.[7,22]
In the present study, no effect of adjuvant administration of either of two drugs resulted in any respiratory complication such as respiratory depression.
Overall, the hemodynamic stability of dexmedetomidine supplemented combination was alerting and indicated the need for exercising caution while using the same.
Although, no significant difference was observed between two groups with respect to highest level of sensory block achieved yet the number of patients achieving T6 level of block was higher in dexmedetomidine supplemented group (42.50%) as compared to fentanyl supplemented group (30%). However, a significant difference between two groups was observed with respect to duration of sensory block was 129.50 min for Group D whereas 77.50 min for Group F and motor block for Group D 377.25 min and for Group F 187.00 min which is significant. And analgesic effect of two drugs with dexmedetomidine supplemented group showing the significantly longer duration of blocks and analgesic effect as compared to fentanyl supplemented group.
Short duration of action and prolongation of the block and analgesic effect are the main characteristics of adjuvants for their use during spinal anesthesia.[1,2] Although, one of the limitations of present study was absence of a control group, owing to which, it is difficult to comment whether both the adjuvants were able to fulfill this objective for the use of spinal anesthesia yet in relative terms, the present study found that dexmedetomidine supplemented group shows a superiority over fentanyl supplemented group.
Local anesthetic agents such as bupivacaine act by blocking sodium channels while alpha-2 adrenoceptor agonist such as dexmedetomidine act by binding to presynaptic C-fibers and postsynaptic dorsal horn neurons. The prolongation of effect may result from synergism between local anesthetic and alpha-2-adrenoceptor agonist while the prolongation of the motor block of spinal anesthetics may result from the binding of alpha-2-adrenoceptor agonists to motor neurons in the dorsal horn.[8] Intrathecal alpha-2-receptor agonists have been found to have antinociceptive action for both somatic and visceral pain.[23] Fentanyl is a lipophilic μ-receptor agonist opioid. Intrathecally, fentanyl exerts its effect by combining with opioid receptors in the dorsal horn of spinal cord and may have a supraspinal spread and action.[7]
Thus the present study showed that the intrathecal adjuvant administration of dexmedetomidine as compared to fentanyl provided a longer duration of sensory and motor blockade apart from providing a longer postoperative analgesic effect, however, a point of concern was a higher prevalence of side effects, which was though not significant statistically yet has to be reported specifically to keep the operative room preparations to meet any such eventuality. Moreover, the nonexistence of a statistically significant difference does not undermine the seriousness of any such event.
Thus, based on the findings of the present study, we recommend a cautious use of dexmedetomidine after properly trading off between the potential benefits and risks.
CONCLUSION
On the basis of observations made, the following conclusions have been drawn:
As compared to fentanyl group, in dexmedetomidine group, mean HR and BP (SBP, DBP and MAP) was significantly lower for most of the perioperative intervals
As compared to baseline, fentanyl group did not show a significant difference in mean HR and BP (SBP, DBP and MAP) throughout the perioperative period
Mean duration of sensory and motor block and analgesic effect (time till first postoperative dose of analgesic) was significantly longer in dexmedetomidine group as compared to fentanyl group
Incidence of bradycardia and hypotension was higher in dexmedetomidine group as compared to fentanyl group yet the difference between two groups was not significant statistically.
The findings in the present study suggested that the use of intrathecal dexmedetomidine as adjuvant to Bupivacaine provides a longer sensory and motor blockade and also prolongs the postoperative analgesic effect than the Use of fentanyl with Bupivacaine. However, the potential risk of hypotension and bradycardia should not be ignored and should be adequately taken care of in the operation room.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Elia N, Culebras X, Mazza C, Schiffer E, Tramèr MR. Clonidine as an adjuvant to intrathecal local anesthetics for surgery: Systematic review of randomized trials. Reg Anesth Pain Med. 2008;33:159–67. doi: 10.1016/j.rapm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Boussofara M, Carlès M, Raucoules-Aimé M, Sellam MR, Horn JL. Effects of intrathecal midazolam on postoperative analgesia when added to a bupivacaine-clonidine mixture. Reg Anesth Pain Med. 2006;31:501–5. doi: 10.1016/j.rapm.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Shah A, Patel I, Gandhi R. Haemodynamic effects of intrathecal dexmedetomidine added to ropivacaine intraoperatively and for postoperative analgesia. Int J Basic Clin Pharmacol. 2013;2:26–9. [Google Scholar]
- 4.Eisenach JC, Shafer SL, Bucklin BA, Jackson C, Kallio A. Pharmacokinetics and pharmacodynamics of intraspinal dexmedetomidine in sheep. Anesthesiology. 1994;80:1349–59. doi: 10.1097/00000542-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Shimode N, Fukuoka T, Tanimoto M, Tashiro C, Tokunaga A, Noguchi K. The effects of dexmedetomidine and halothane on Fos expression in the spinal dorsal horn using a rat postoperative pain model. Neurosci Lett. 2003;343:45–8. doi: 10.1016/s0304-3940(03)00309-4. [DOI] [PubMed] [Google Scholar]
- 6.Kalso EA, Pöyhiä R, Rosenberg PH. Spinal antinociception by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol Toxicol. 1991;68:140–3. doi: 10.1111/j.1600-0773.1991.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 7.Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic alpha (2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg. 2000;90:400–7. doi: 10.1097/00000539-200002000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Mystakidou K, Katsouda E, Parpa E, Tsiatas ML, Vlahos L. Oral transmucosal fentanyl citrate for the treatment of breakthrough pain in cancer patients: An overview of its pharmacological and clinical characteristics. Am J Hosp Palliat Care. 2005;22:228–32. doi: 10.1177/104990910502200313. [DOI] [PubMed] [Google Scholar]
- 9.Hanks GW, Nugent M, Higgs CM, Busch MA OTFC Multicentre Study Group. Oral transmucosal fentanyl citrate in the management of breakthrough pain in cancer: An open, multicentre, dose-titration and long-term use study. Palliat Med. 2004;18:698–704. doi: 10.1191/0269216304pm966oa. [DOI] [PubMed] [Google Scholar]
- 10.Hammargren WR, Henderson GL. Analyzing normetabolites of the fentanyls by gas chromatography/electron capture detection. J Anal Toxicol. 1988;12:183–91. doi: 10.1093/jat/12.4.183. [DOI] [PubMed] [Google Scholar]
- 11.Jansen PA. The development of new synthetic narcotics. In: Estafanous FG, editor. Opioids in Anesthesia. Boston, Mass: Butterworth Publishers; 1984. pp. 37–44. [Google Scholar]
- 12.Ben-David B, Miller G, Gavriel R, Gurevitch A. Low-dose bupivacaine-fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25:235–9. [PubMed] [Google Scholar]
- 13.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2002;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 14.Singh RP, Shukla A. Randomized, controlled study to compare the effect of intrathecal clonidine and dexmedetomidine on sensory analgesia and motor block of hyperbaric bupivacaine. Indian J Fundam Appl Life Sci. 2012;2:24–33. [Google Scholar]
- 15.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A Comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to Bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27:339–43. doi: 10.4103/0970-9185.83678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajwa SJ, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth. 2011;5:365–70. doi: 10.4103/1658-354X.87264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbaradie S, El Mahalawy FH, Solyman AH. Dexmedetomidine vs. propofol for short-term sedation of postoperative mechanically ventilated patients. J Egypt Natl Canc Inst. 2004;16:153–8. [PubMed] [Google Scholar]
- 18.Product Information. North Chicago, IL, USA: Abbott Laboratories; 1998. Actiq®, Oral Transmucosal Fentanyl Citrate. [Google Scholar]
- 19.Simpson RK, Jr, Edmondson EA, Constant CF, Collier C. Transdermal fentanyl as treatment for chronic low back pain. J Pain Symptom Manage. 1997;14:218–24. doi: 10.1016/s0885-3924(97)00183-8. [DOI] [PubMed] [Google Scholar]
- 20.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50,488H. Anesthesiology. 1995;83:344–52. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Appl Sci. 2009;6:882–7. [Google Scholar]


