Abstract
Context:
Elevation of intraocular pressure (IOP) is an inherent and inadvertent association with the use of succinylcholine and alpha2 agonists can be used to obtund this effect.
Aims:
The study was aimed to assess the efficacy of intravenous dexmedetomidine and clonidine premedication in attenuating rise in IOP during laryngoscopy and intubation following administration of succinylcholine.
Settings and Design:
This prospective, observational study was conducted in 40 patients aged 20–60 years undergoing non ophthalmic surgical procedures.
Subjects and Methods:
For patients in Group D, dexmedetomidine 0.4 mcg/kg and in Group C clonidine 1 μg/kg over 10 min was administered before induction. All patients were induced with propofol. Laryngoscopy and intubation were performed 1 min after administration of succinylcholine 2 mg/kg.
Statistical Analysis Used:
Mann–Whitney, Chi-square and Wilcoxon tests.
Results:
Mean baseline IOP of both groups were comparable (15.4 ± 2.6 vs. 14.7 ± 2.3). Following premedication and induction, IOP decreased in both groups and the reduction was significantly more in Group D. Following administration of succinylcholine and 1 min after intubation IOP raised and exceeded the baseline value in Group C (16.0 ± 1.6 and 18.6 ± 2.2). Though there was an increase in IOP in Group D (12.0 ± 1.9 and 14.0 ± 2.1), it did not reach up to baseline values. Then there was a gradual reduction in IOP in both groups at 3, 5, and 10 min and Group D continued to have a significantly low IOP than Group C up to 10 min.
Conclusions:
Dexmedetomidine 0.4 μg/kg resulted in a reduction of IOP and blunted the increase in IOP, which followed administration of succinylcholine, laryngoscopy, and intubation. Though clonidine 1 μg/kg reduced IOP, it did not prevent rise in IOP following succinylcholine, laryngoscopy, and intubation.
Keywords: Clonidine, dexmedetomidine, intraocular pressure, intubation, laryngoscopy, succinylcholine
INTRODUCTION
Elevation of intraocular pressure (IOP) is an inherent and inadvertent association with the use of succinylcholine. The hemodynamic stress response of laryngoscopy and intubation results in an additional insult. Though many newer short-acting muscle relaxants have been introduced into the anesthetic practice, none of these agents could be projected as a replacement to succinylcholine in emergency situations. Hence, premedication with alpha2 agonists such as dexmedetomidine and clonidine which are known to reduce IOP[1,2] could help us to combat the ocular adverse effects of succinylcholine.
Aim of study
The aim was to assess the efficacy of intravenous (IV) dexmedetomidine and clonidine premedication in attenuating rise in IOP during laryngoscopy and intubation following administration of succinylcholine.
SUBJECTS AND METHODS
This prospective, observational study was conducted from January 2012 to December 2014. After obtaining Ethics Committee approval and patients’ consent, 40 patients of both sexes, aged 20–60 years of American Society of Anesthesiologists (ASA) physical status I-II undergoing elective non-ophthalmic surgical procedures under general anesthesia requiring endotracheal intubation were recruited.
Patients with glaucoma, uncontrolled hypertension, congestive heart failure, renal or hepatic impairment, hyperthyroidism, difficult airway, those on antipsychotics and those with a history of hypersensitivity to the test drugs were excluded.
Following a detailed preanesthetic evaluation, patients were kept fasting for 6 h for solids and 2 h for clear fluids. Consecutive patients were allocated to either Group D or Group C. On day of surgery, in the operation theater, a 18 gauge IV cannula was put under local anesthesia and Ringer lactate solution was started for all the patients. Pre induction monitors like a pulse oximeter, non-invasive blood pressure monitor, and electrocardiogram were attached. Eyes of the patients were anesthetized with 4% topical lignocaine. For patients allocated in Group D, a bolus of dexmedetomidine 0.4 μg/kg body weight over 10 min was given. Patients in Group C received clonidine 1 μg/kg body weight over 10 min before induction.
All patients received a standardized anesthesia management and were induced with glycopyrrolate 0.2 mg, midazolam 2 mg, fentanyl 2 μg/kg, and propofol IV till loss response to verbal commands, following preoxygenation with 100% oxygen for 3 min. Laryngoscopy and intubation were attempted 1 min after administration of IV succinylcholine 2 mg/kg, meanwhile the patients were mask ventilated with 1% isoflurane in oxygen. After securing the airway with an appropriate sized endotracheal tube and after confirming the correct placement with end tidal carbon dioxide waveform and auscultation, vecuronium 0.1 mg/kg was given IV. Anesthesia was maintained with oxygen-nitrous oxide mixture (1:2) with isoflurane (1–1.5%) and intermittent muscle relaxants.
Intraocular pressure was measured with a Schiotz tonometer by an ophthalmologist just before and after test drug administration, immediately after induction, 45 s following administration of succinylcholine, then at 1 min, 3 min, 5 min, and 10 min after intubation. Heart rate (HR) and mean arterial blood pressure (MAP) were also documented at these time points. 30% reduction in systolic blood pressure was initially treated with an IV fluid bolus of 200 ml. If hypotension persisted, phenylephrine 50 μg was given IV, which was repeated if necessary.
Mann–Whitney test was used to compare the age, weight, HR, MAP, and IOP among groups, while Chi-square test was used to compare gender and ASA physical status. Wilcoxon test was used to compare the average IOP at different time points from the baseline in each group (intragroup analysis). The level of significance was taken as P < 0.05.
RESULTS
Comparison of the distribution of age, sex, weight, and ASA physical status of patients in both the groups were comparable [P ≤ 0.05, Table 1]. Baseline HR and that following premedication were comparable between the groups. However, comparison of HRs following induction, administration of succinylcholine, 1 min, 3 min, 5 min, and 10 min following intubation showed that the HR was significantly high in the Group C P ≤ 0.05, Table 2 and Figure 1].
Table 1.
Distribution of demographics and ASA status
Table 2.
Comparison of heart rate
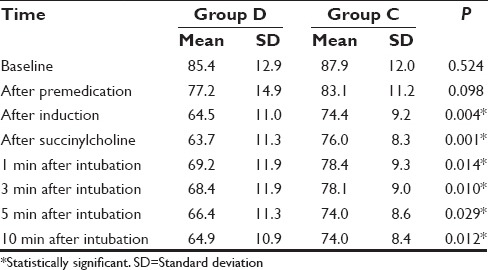
Figure 1.
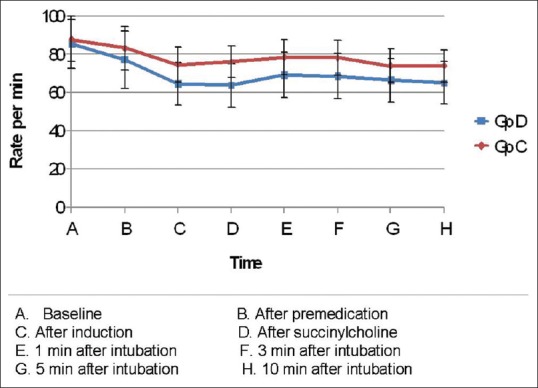
Changes in mean heart rate
Baseline MAP was comparable in both groups. After premedication, there was a reduction in MAP in both the groups, which was significantly low in Group D (P = 0.021). However, following induction and succinylcholine both the groups had comparable MAP. At 1 min following intubation, there was an increase in MAP in both groups from the previous values, and Group C had a significantly higher MAP compared to Group D (P ≤ 0.001). At 3, 5, and 10 min, MAP started to decrease in both groups with Group C having significantly higher MAP at 3 and 5 min (P ≤ 0.001). At 10 min, MAP of both groups were comparable [P = 0.050, Table 3 and Figure 2].
Table 3.
Comparison of mean arterial pressures
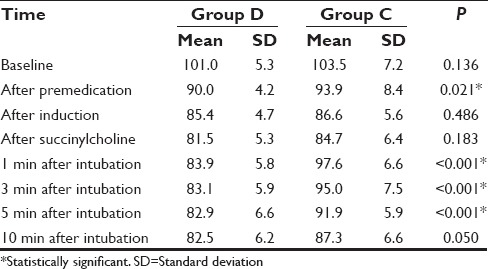
Figure 2.
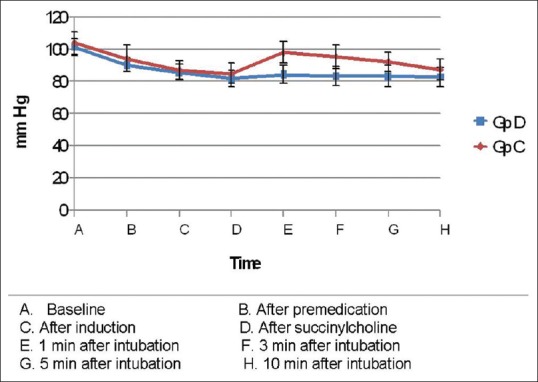
Changes in mean arterial pressure
Intergroup analysis had shown that the mean baseline IOP of both Groups D and C were comparable (15.4 ± 2.6 vs. 14.7 ± 2.3, P = 0.277). Following premedication and induction, IOP decreased in both the groups and the reduction was significantly more in Group D (P ≤ 0.05). Following administration of succinylcholine and 1 min after intubation though there was an increase in IOP in both groups, the IOP still remained significantly low in Group D compared to Group C. There was gradual reduction in IOP in both groups at 3, 5, and 10 min and Group D continued to have a significantly low IOP than Group C up to 10 min [Table 4 and Figure 3].
Table 4.
Comparison of IOPs
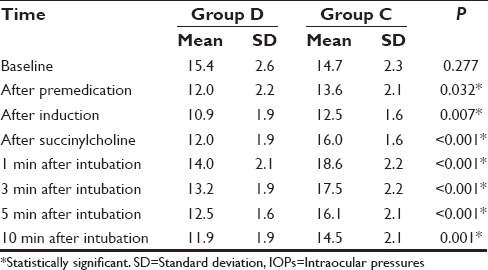
Figure 3.
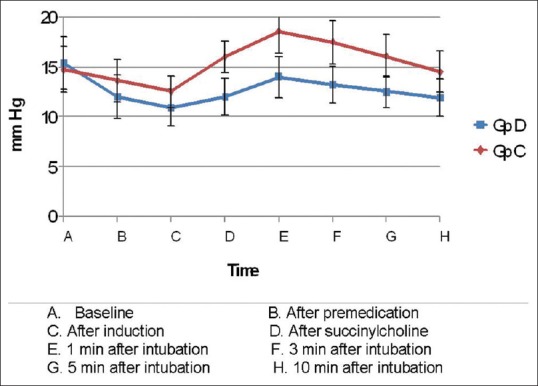
Changes in mean intraocular pressure
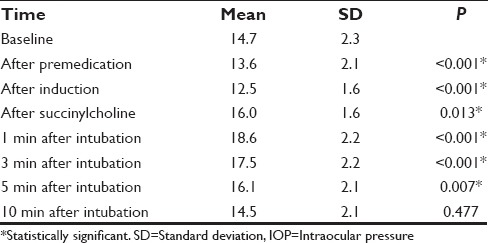
Intragroup analysis was done to find out the changes in IOP in each group from baseline values. Following premedication, Group D showed a significant reduction in IOP from baseline (15.4 ± 2.6) throughout the study period (P ≤ 0.05). Though there was an increase following succinylcholine and intubation, IOP never reached baseline values [Table 5]. Group C also showed a reduction in IOP following premedication and induction. However, following succinylcholine administration and intubation there was significant rise in IOP (16.0 ± 1.6 and 18.6 ± 2.2, respectively), which exceeded the baseline value (14.7 ± 2.3). By 10 min, IOP normalized and was comparable with the baseline value [Table 6].
Table 5.
Comparison of baseline IOP with subsequent IOP in Group D
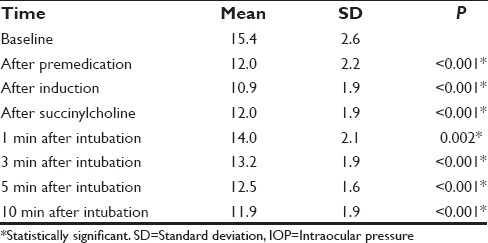
Table 6.
Comparison of baseline IOP with subsequent IOP in Group C
DISCUSSION
The IOP reflects the tissue pressure of the intraocular contents and the normal range is 10–20 mm Hg. An acute increase in IOP may cause expulsion of the global contents through an open globe injury or surgical incision. It can also lead to retinal artery occlusion and retinal ischemia. Chronic increase in IOP ultimately results in ischemic optic neuropathy either due to a direct effect or because of chronic underperfusion of the nerve.[3] Perioperative visual loss is a catastrophic event which can follow non ophthalmic surgery, especially cardiac and spine surgery, and the most common causes are retinal vascular occlusion and ischemic optic neuropathy.[4]
Rise in IOP during anesthesia is often overlooked during non ophthalmic surgery. A certain degree of increase in IOP is inevitable during the regular conduct of general anesthesia. The common causes of rise in IOP under anesthesia include hypertension, hypercarbia, hypoxia, head low position, expansion of the intravascular volume, undue flexion of neck leading to venous congestion, external pressure on eyeball, use of atropine, in patients with narrow angle glaucoma, and succinylcholine.
In emergency situations and during difficult intubation, succinylcholine is considered as the most useful muscle relaxant to aid laryngoscopy and intubation because of its faster onset of action and excellent skeletal muscle relaxation properties. Use of succinylcholine in ocular trauma is controversial as it leads to an increase in IOP of up to 10 mm Hg which lasts for 10 min.[3] Hence, in the presence of an open globe injury succinylcholine can result in expulsion of the contents of the eyeball. Though in normal patients the increase in IOP following succinylcholine may not increase IOP sufficient enough to cause permanent damage, in those with an already high IOP the outcome can be devastating.
Securing the airway with a cuffed endotracheal tube necessitates instrumentation of the airway. The hemodynamic stress response that accompany laryngoscopy and intubation can increase IOP by 10–20 mm Hg.[3] Among the various pharmacological measures adopted to blunt the hemodynamic stress response that accompany laryngoscopy and intubation, administration of dexmedetomidine prior to induction of general anesthesia has shown promising results.[5,6,7] This alpha2 agonist has shown to produce anxiolysis, sedation, sympatholysis, and analgesia. Secondary to the alpha2 mediated decrease in sympathetic tone, it blunts the hemodynamic responses of intubation, surgery, and extubation. It has approximately 7–8 times alpha2 selectivity than that of clonidine.[8]
Another advantage of dexmedetomidine is its ability to reduce IOP.[9] There are many mechanisms by which dexmedetomidine reduces IOP. By its direct vasoconstrictor effect on the afferent blood vessels of the ciliary body, it reduces aqueous humor production. It facilitates the drainage of aqueous humor by reducing sympathetically mediated vasomotor tone of the ocular drainage system.[10] In addition, the hypotension that follow dexmedetomidine administration can also result in a reduced IOP.
Dexmedetomidine in doses like 0.4 μg/kg body weight and 0.6 μg/kg body weight given IV as premedication were found to be effective in preventing the rise of IOP associated with administration of succinylcholine and endotracheal intubation. But dexmedetomidine 0.4 μg/kg was recommended for this purpose as this dose did not result in significant hypotension.[11] But another study has shown that even 0.6 μg/kg given IV over 10 min, 10 min before induction, effectively prevented the rise in IOP associated with suxamethonium, laryngoscopy, and intubation with stable hemodynamics.[12]
Following succinylcholine and intubation, even if the patient is premedicated with dexmedetomidine 0.6 μg/kg, IOP increases, but the IOP rise is not significantly different from the baseline value. Based on these observations, dexmedetomidine is now recommended as a beneficial premedication in open globe injuries.[13] The drug was found to reduce the IOP by 34% after a single IV dose of 0.6 μg/kg[14] Higher doses of dexmedetomidine >0.6 μg/kg is associated with an additional reduction in the arterial pressure and HR without any further decrease in the IOP.[15,16]
Clonidine, another alpha2 agonist, also decreases IOP following systemic[1,17] as well as topical administration.[18] It causes an increase in vascular resistance and thereby reduces retinal blood flow. Oral clonidine 300 μg premedication can prevent the increase in IOP following succinylcholine administration.[19] However, conflicting results were reported following its IV use. Tripathi et al., reported that clonidine given at a dose of 1 μg/kg IV did not attenuate hemodynamic stress response to intubation and extubation. However, clonidine 2 μg/kg prevented hemodynamic stress response to intubation and extubation.[20]
Another study showed that the IV clonidine in doses of 1 μg/kg and 2 μg/kg administered 10 min prior to induction significantly attenuated the stress response to laryngoscopy and intubation. While clonidine 2 μg/kg resulted in hypotension at the time of induction and postoperative sedation, it was not observed with clonidine 1 μg/kg. Hence, a single IV low-dose clonidine 1 μg/kg combined with fentanyl 2 μg/kg was suggested as a safe and effective method with minimal side effects to attenuate the hemodynamic stress response to laryngoscopy and intubation.[21] However, clonidine even at a dose of 2 μg/kg IV did not seem to have any effect in IOP just before and just after intubation.[22]
A recently published study comparing the effect of the IV clonidine and dexmedetomidine on IOP had shown that clonidine 2 μg/kg attenuated rise in IOP following succinylcholine, but not that following laryngoscopy and intubation. But dexmedetomidine 0.5 μg/kg was effective in this regard.[23] The main differences of this study from ours being in the dose of dexmedetomidine and clonidine used. We used dexmedetomidine 0.4 μg/kg and obtained similar results. A reduction in the dose may ensure hemodynamic stability and an improved margin of safety. In our study, clonidine 1 μg/kg only was used, which did not prevent the rise in IOP after succinylcholine, laryngoscopy or intubation. We did not use a higher dose for fear of causing prolonged intraoperative hypotension.
One of the drawbacks of our study was that the only option available for us to measure IOP was by using Schiotz tonometry. This is an indentation tonometer which determines IOP by measuring how much cornea is indented by a given weight. The indentation tonometry is considered less accurate as it doesn’t take into account corneal thickness and viscoelasticity. However, in patients with no significant corneal disease and in the absence of corneal edema, this technique provides IOP values with reasonable accuracy. Moreover, Schiotz tonometers are commonly available and easy to use.
The accuracy of IOP measurement depends on the circadian variation,[24] corneal thickness, curvature, and biomechanical properties.[25,26,27,28] Goldmann applanation tonometry is taken nowadays as the gold standard and is more precise in patients having an average central corneal thickness of between 500 and 525 μm. Dynamic contour tonometry and ocular response analyzer seem to be largely independent of the corneal biomechanical properties and may be particularly helpful in eyes with corneal abnormalities. However, among all tonometers, none is highly accurate when both corneal thickness and surface irregularities are present.[29]
CONCLUSION
Dexmedetomidine 0.4 μg/kg premedication resulted in a reduction of IOP and effectively blunted the increase in IOP, which followed administration of succinylcholine, laryngoscopy, and intubation. Though clonidine 1 μg/kg reduced IOP, it did not prevent rise in IOP following succinylcholine, laryngoscopy, and intubation. Both dexmedetomidine and clonidine effectively attenuated hemodynamic responses to laryngoscopy and intubation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Weigert G, Resch H, Luksch A, Reitsamer HA, Fuchsjager-Mayrl G, Schmetterer L, et al. Intravenous administration of clonidine reduces intraocular pressure and alters ocular blood flow. Br J Ophthalmol. 2007;91:1354–8. doi: 10.1136/bjo.2007.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim NY, Yoo YC, Park H, Choi YD, Kim CY, Bai SJ. The effect of dexmedetomidine on intraocular pressure increase in patients during robot-assisted laparoscopic radical prostatectomy in the steep trendelenburg position. J Endourol. 2015;29:310–6. doi: 10.1089/end.2014.0381. [DOI] [PubMed] [Google Scholar]
- 3.Murgatroyd H, Bembridge J. Intraocular pressure. Contin Educ Anaesth Crit Care Pain. 2008;8:100–3. [Google Scholar]
- 4.Roth S. Perioperative visual loss: What do we know, what can we do? Br J Anaesth. 2009;103(Suppl 1):i31–40. doi: 10.1093/bja/aep295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 6.Saraf R, Jha M, Sunil Kumar V, Damani K, Bokil S, Galante D. Dexmedetomidine, the ideal drug for attenuating the pressor response. Pediatr Anesth Crit Care J. 2013;1:78–86. [Google Scholar]
- 7.Manne GR, Upadhyay MR, Swadia V. Effects of low dose dexmedetomidine infusion on haemodynamic stress response, sedation and post-operative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Indian J Anaesth. 2014;58:726–31. doi: 10.4103/0019-5049.147164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haselman MA. Dexmedetomidine: A useful adjunct to consider in some high-risk situations. AANA J. 2008;76:335–9. [PubMed] [Google Scholar]
- 9.Eskandr AM, Elbakry AE, Elmorsy OA. Dexmedetomidine is an effective adjuvant to subtenon block in phacoemulsification cataract surgery. Egypt J Anesth. 2014;30:261–6. [Google Scholar]
- 10.Vartianinen J, MacDonald E, Urtti A, Ronhiainen H, Virtanen R. Dexmedetomidine induced ocular hypotension in rabbits with normal or elevated intraocular pressure. Invest Ophthalmol Vis Sci. 1992;33:2019–23. [PubMed] [Google Scholar]
- 11.Pal CK, Ray M, Sen A, Hajra B, Mukherjee D, Ghanta AK. Changes in intraocular pressure following administration of suxamethonium and endotracheal intubation: Influence of dexmedetomidine premedication. Indian J Anaesth. 2011;55:573–7. doi: 10.4103/0019-5049.90611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalini A, Srinivas VY, Gurudatt CL. Can dexmedetomidine premedication obtund the intraocular pressure rise after suxamethonium and endotracheal intubation. J Evol Med Dent Sci. 2014;3:7441–9. [Google Scholar]
- 13.Mowafi HA, Aldossary N, Ismail SA, Alqahtani J. Effect of dexmedetomidine premedication on the intraocular pressure changes after succinylcholine and intubation. Br J Anaesth. 2008;100:485–9. doi: 10.1093/bja/aen020. [DOI] [PubMed] [Google Scholar]
- 14.Abdalla MI, Al Mansouri F, Bener A. Dexmedetomidine during local anesthesia. J Anesth. 2006;20:54–6. doi: 10.1007/s00540-005-0351-z. [DOI] [PubMed] [Google Scholar]
- 15.Virkkilä M, Ali-Melkkilä T, Kanto J, Turunen J, Scheinin H. Dexmedetomidine as intramuscular premedication for day-case cataract surgery. A comparative study of dexmedetomidine, midazolam and placebo. Anaesthesia. 1994;49:853–8. doi: 10.1111/j.1365-2044.1994.tb04257.x. [DOI] [PubMed] [Google Scholar]
- 16.Virkkilä M, Ali-Melkkilä T, Kanto J, Turunen J, Scheinin H. Dexmedetomidine as intramuscular premedication in outpatient cataract surgery. A placebo-controlled dose-ranging study. Anaesthesia. 1993;48:482–7. doi: 10.1111/j.1365-2044.1993.tb07066.x. [DOI] [PubMed] [Google Scholar]
- 17.Santiago AE, Issy AM, Sakata RK. Effects of preoperative intravenous clonidine in patients undergoing cataract surgery: A double-blind, randomized trial. J Ophthalmol 2014. 2014 doi: 10.1155/2014/346549. 346549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigert G, Resch H, Garhofer G, Fuchsjäger-Mayrl G, Schmetterer L. Effects of topical clonidine versus brimonidine on choroidal blood flow and intraocular pressure during squatting. Invest Ophthalmol Vis Sci. 2007;48:4220–5. doi: 10.1167/iovs.07-0178. [DOI] [PubMed] [Google Scholar]
- 19.Polarz H, Böhrer H, Martin E, Wolfrum J, Völcker HE. Oral clonidine premedication prevents the rise in intraocular pressure following succinylcholine administration. Ger J Ophthalmol. 1993;2:97–9. [PubMed] [Google Scholar]
- 20.Tripathi DC, Shah KS, Dubey SR, Doshi SM, Raval PV. Hemodynamic stress response during laparoscopic cholecystectomy: Effect of two different doses of intravenous clonidine premedication. J Anaesthesiol Clin Pharmacol. 2011;27:475–80. doi: 10.4103/0970-9185.86586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora S, Kulkarni A, Bhargava AK. Attenuation of hemodynamic response to laryngoscopy and orotracheal intubation using intravenous clonidine. J Anaesthesiol Clin Pharmacol. 2015;31:110–4. doi: 10.4103/0970-9185.150559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiou M, Parlapani A, Argiriadou H, Papagiannopoulou P, Katsikis G, Kaprini E. Sufentanil or clonidine for blunting the increase in intraocular pressure during rapid-sequence induction. Eur J Anaesthesiol. 2002;19:819–22. doi: 10.1017/s0265021502001321. [DOI] [PubMed] [Google Scholar]
- 23.Banga PK, Singh DK, Dadu S, Singh M. A comparative evaluation of the effect of intravenous dexmedetomidine and clonidine on intraocular pressure after suxamethonium and intubation. Saudi J Anaesth. 2015;9:179–83. doi: 10.4103/1658-354X.152878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu JH. Diurnal measurement of intra-ocular pressure. J Glaucoma. 2001;10:39–41. doi: 10.1097/00061198-200110001-00015. [DOI] [PubMed] [Google Scholar]
- 25.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 26.Shimmyo M, Ross AJ, Moy A, Mostafavi R. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasians, Asians, Hispanics, and African Americans. Am J Ophthalmol. 2003;136:603–13. doi: 10.1016/s0002-9394(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J Cataract Refract Surg. 2005;31:146–55. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma. 2006;15:364–70. doi: 10.1097/01.ijg.0000212268.42606.97. [DOI] [PubMed] [Google Scholar]
- 29.Moraes CG, Prata TS, Liebmann J, Ritch R. Modalities of tonometry and their accuracy with respect to corneal thickness and irregularities. J Optom. 2008;1:43–9. [Google Scholar]


