Abstract
Objective
To determine if intracranial pressure (ICP) monitor placement in acute liver failure (ALF) patients is associated with significant clinical outcomes.
Design
Retrospective multicenter cohort study.
Setting
Academic liver transplant centers comprising the US ALF Study Group.
Patients and participants
Adult critically ill ALF patients presenting with Grade III/IV hepatic encephalopathy (n=629) prospectively enrolled between 3/2004 to 8/2011.
Intervention
ICP monitored (ICPM; n=140) vs. non-monitored controls (n=489).
Measurements and Results
ICPM patients were younger than controls (35 vs. 43 years, p<0.001) and more likely to be on renal replacement therapy (52% vs. 38%, p=0.003). Of 87 ICPM patients with detailed information, 44 (51%) had evidence of intracranial hypertension (ICH ~ ICP >25 mmHg) and overall 21-day mortality was higher in patients with ICH (43% vs. 23%, p=0.05). During the first 7 days, ICPM patients received more ICH-directed therapies (mannitol: 56% vs. 21%; hypertonic saline: 14% VS. 7%; hypothermia: 24% vs. 10%; p< 0.03 for each). 41% of ICPM patients received LT (vs. 18% controls; p<0.001). Overall 21-day mortality was similar (ICPM 33% vs. controls 38%, p=0.24). Where data were available, hemorrhagic complications in ICPM patients were rare (4/56 (7%); 3 died). When stratifying by acetaminophen (APAP) status and adjusting for confounders, ICPM placement did not impact 21-day mortality in APAP patients (p=0.89). However, ICPM was associated with increased 21-day mortality in non-APAP patients (OR ~ 3.04, p=0.014).
Conclusions
In ICP monitored ALF patients, ICH is commonly observed. The use of ICPM in APAP ALF did not confer a significant 21-day mortality benefit while in non-APAP ALF it may be associated with worse outcomes. Hemorrhagic complications from ICPM placement were uncommon and cannot account for mortality trends. While our results cannot conclusively confirm or refute the utility of ICP monitoring in ALF patients, patient selection and ancillary assessments of cerebral blood flow likely have a significant role. Prospective studies would be required to conclusively account for confounding by illness severity and transplant.
Keywords: Acetaminophen, Acute liver failure, cerebral edema, fulminant hepatic failure, intracranial hypertension, intracranial pressure monitoring
Introduction
Acute liver failure (ALF) is defined by hepatic encephalopathy (HE) and synthetic dysfunction within 26 weeks of the first symptoms of liver disease (1). Severe coagulopathy, HE and hemodynamic instability contribute to a picture of multi-organ failure. Currently the most common cause of ALF in North America is acetaminophen (APAP) (2, 3). Particularly in APAP-induced ALF, cerebral edema and intracranial hypertension (ICH) are major causes of morbidity and mortality (4, 5). Currently the role of intracranial pressure (ICP) monitoring is still an area of controversy (6).
ICP monitoring (ICPM) has generally been used primarily to exclude patients from liver transplant (LT) and to manage ICH intra-operatively when transplantation is performed. The presence of clinically significant ICH prior to emergency LT portends a higher likelihood of ischemic brain injury post LT (7, 8). Furthermore increased use of ICP related interventions (including osmotic agents, propofol, hyperventilation) are associated with ICPM (9). This likely reflects an increase in detection of clinically silent ICH (10). However, despite this rationale, a mortality benefit in ICPM ALF patients has not been previously demonstrated and no prospective, controlled trials exist (9).
The major concern previously with placement of such monitors is intracranial hemorrhage. An initial survey across medical centers in the United States described a complication rate of roughly 10% (27/263) with significantly higher rates in subdural or parenchymal compared with epidural transducers (11). More recent studies from the US (2005) and the United Kingdom (2010) have published rates of intracranial hemorrhage between 2.5 and 10% (9, 12).
The US ALFSG has collected clinical and laboratory data on > 2,300 patients with ALF over the past 15 years. In this study, we reviewed all (n=140) ALF patients with Grade III or IV HE who received an ICPM between March 2004 and August 2011. For control purposes, we examined all (n=489) ALF patients during the same time period with grade III or IV HE that did not receive an ICPM. We hypothesized that patients with ALF who underwent ICPM insertion would have significantly more manipulations to treat ICP and that this would beneficially impact 21-day survival. We also hypothesized that more patients undergoing LT would have ICP monitoring and the rate of complications with ICPM insertion would be low.
Accordingly our objectives were:
To determine whether placement of an ICPM in ALF patients is significantly associated with 21-day mortality.
To determine the association between ICP monitoring and management; specifically need for ICP therapies and listing/receipt of LT.
To determine the current safety profile of ICP monitoring.
MATERIALS AND METHODS
The reporting of this study followed the STROBE guideline (13).
Design and Setting
We performed a retrospective cohort study of a total of 629 ALF patients (all patients with Grade III or IV HE) enrolled by the US ALFSG registry between March 2004 and August 2011. All enrolling centers are tertiary academic liver transplant centers. The Institutional Review Board at each participating center approved all protocols. One hundred and forty (22%) received ICP monitors (ICPM) and 489 did not (controls). Because of HE in all patients, next of kin were asked to provide consent for study participation. Each center implemented monitoring and therapeutic interventions according to institutional standards of care. While there were no study-wide protocols for indications for ICPM insertion or management of ICP across the USALFSG sites, based on previously published US and UK literature some indications for ICPM insertion may include hyperammonemia (ammonia > 150 μmol/L), listing for LT, meeting King’s College criteria, or presence of multisystem organ failure (14, 15).
Participants
Inclusion criteria were: 1) evidence of ALF according to the enrollment criteria for the ALFSG (see operational definitions) AND 2) age ≥18 years; and 3) Grade III or IV HE during the first seven days of study admission (West Haven Criteria) (16, 17).
Exclusion criteria were: 1) Cirrhosis/acute on chronic liver failure; and 2) patients < 18 years.
Operational Definitions
For the purposes of this study, ALF is defined as INR ≥ 1.5 and HE within the first 26 weeks of liver disease in a patient with an acute hepatic insult (18). HE grade is defined by the West Haven Criteria (simplified) as follows; grade 1 ~ any alteration in mentation, grade 2 being somnolent or obtunded but easily rousable or presence of asterixis, grade 3 being rousable with difficulty and, grade 4: unresponsive to deep pain (16, 17).
Variables
The exposure of interest was placement of an ICPM in patients with ALF (both APAP and non-APAP). The primary outcome of interest was 21-day mortality. Secondary outcomes included differences in management for ICH (ICP-related therapies ~ hypertonic saline, mannitol, hypothermia, barbiturates, sedation (propofol)), and complications of ICPM insertion. Confounding factors, which may impact the primary outcome (mortality), included age, gender, etiology of ALF (APAP vs. non-APAP), requirement for organ support (vasopressors, mechanical ventilation [MV]), renal replacement therapy (RRT), and LT. Confounding factors that may affect the secondary outcome (complications of ICPM insertion) included type of ICPM and blood products used for placement.
Data Sources and Collection
Data were collected prospectively as part of the US ALFSG and retrospectively analyzed. Prior to February 2010, each individual site prospectively sent case report forms to the University of Texas Southwestern for entry into a central database. Following this date, individual sites entered data electronically into a central database housed at the ALFSG Data Coordinating Center at the Medical University of South Carolina (Charleston, USA). Registry data assessed in this study included demographics (age, race, sex), etiology of ALF, biochemistry (admission, day of ICPM insertion or development of Grade III or IV HE), requirement for organ support on day of admission or during first 7 days, ICP measurement (mm Hg), requirement for ICP related therapies and outcomes (receipt of LT, 21-day mortality). Data regarding the use of surrogate markers of ICP (reverse jugular bulb saturations, cerebral blood flow velocity) were not collected in the registry forms. Where available, the type of ICP transducer used (epidural, subdural, intra-parenchymal, intra-ventricular, lumbar) and hemostatic preparation used for ICPM placement (fresh frozen plasma, cryoprecipitate, recombinant factor VIIa) were examined. To acquire missing data (not initially captured) from US ALFSG sites on ICPM techniques and complications, data queries were sent and source documentation was reviewed for missing data at all active recruiting sites of the US ALFSG (n=13/28).
Statistical Analysis
Statistical analysis was performed using IBM SPSS version 19 (2010) and SAS version 9.2 (SAS Institute, North Carolina, USA). In the event of missing values, data were not replaced or estimated. Data were analyzed using descriptive statistics to characterize demographics and other clinical variables. Categorical variables were compared using the Chi-square test or Fisher’s exact test (< 5 subjects). For continuous variables, normally distributed variables were reported as means with standard deviations (SD) and compared by Student’s t-test. Non-normally distributed continuous data were reported as medians with inter-quartile ranges (IQR) and compared by Wilcoxon rank sum test. Survival was defined as the dichotomous outcome, alive at 21-days (transplant and transplant free) after enrollment into the Registry. Transplant and transplant free refer to whether patients received LT during the first 21-days of follow-up. A two-sided significance level of <0.05 was used for all comparisons.
Multivariable analysis
In order to control for variables that may confound the effect of ICP monitoring on 21-day mortality, a multivariable logistic regression analysis was performed. The pre-specified prognostic variables included age at admission into the registry, study enrollment year, high enrolling site (> 6 vs. ≤ 6 patients enrolled per year; 6 was the median for all sites), gender, race (white vs. non-white), lactate (admission), MELD score (admission), receipt of LT during 21 days after enrollment, and receipt during the 7-days post study admission of MV, vasopressors, RRT and ICPM. Model performance was assessed using the c-statistic and the Hosmer-Lemeshow test for goodness of fit. Multivariate associations are reported as odds ratios (OR) with 95% confidence limits.
RESULTS
General characteristics of 629 ALF patients with Grade III/IV hepatic coma
ICP monitoring was used in 140 (22%) of ALF patients with Grade III–IV HE included in this analysis. Demographic, biochemical and therapeutic characteristics of ICPM patients (n=140) and controls (n=489) are shown in Table 1. There were no significant differences in gender (71% female) and race (74% Caucasian) between groups. The most common etiology of ALF was APAP (ICPM 49%, Controls 52%, p= 0.05). Overall, ICPM patients were significantly younger (35 vs. 43 years; p < 0.001). On study admission (Table 1), hematological indicators were similar apart from median platelet count (ICPM 133 vs. controls 122; p=0.02). ICPM patients had significantly higher bilirubin (7.5 vs. 6.5 mg/dL; p=0.03), and lower phosphate levels (mg/dL) (2.7 vs. 3.3, p<0.001).
Table 1.
Demographic, Clinical and Biochemical Parameters in patients with ICP monitoring (ICPM group, n=140) and without (Control group, n=489)
| No ICP monitoring (control) group (N=489) | ICP monitoring (ICPM) group (N=140) | ||||
|---|---|---|---|---|---|
| N | Number (%) or median (IQR) | N | Number (%) or median (IQR) | P value | |
| Age | 489 | 43.0 (31.0–54.0) | 140 | 35.0 (26.0–45.0) | <0.001 |
| Sex (female) | 489 | 344 (70.4%) | 140 | 101 (72.1%) | 0.68 |
| Race | 489 | 140 | 0.24 | ||
| White | 375 (76.7%) | 98 (70.0%) | |||
| African-American | 74 (15.1%) | 29 (20.7%) | |||
| Other | 40 (8.2%) | 13 (9.3%) | |||
| Etiology | 488 | 140 | 0.046 | ||
| Acetaminophen | 252 (51.6%) | 69 (49.3%) | |||
| Viral hepatitis | 41 (8.4%) | 18 (12.9%) | |||
| Drug Induced | 42 (8.6%) | 11 (7.9%) | |||
| Indeterminate | 49 (10.0%) | 23 (16.4%) | |||
| Other | 104 (21.3%) | 19 (13.6%) | |||
| Admission biochemistry | |||||
| Hemoglobin (g/dl) | 488 | 10.4 (9.3–12.0) | 139 | 10.5 (9.2–12.2) | 0.80 |
| White Blood count (×109/L) | 487 | 10.7(7.0–15.1) | 140 | 10.8 (7.0–14.9) | 0.98 |
| Platelet count (×109/L) | 487 | 122.0 (71.0–181.0) | 139 | 133.0 (84.0–206.0) | 0.02 |
| INR | 481 | 2.8 (2.0–4.3) | 133 | 2.7 (1.9–3.8) | 0.22 |
| AST (IU/L) | 483 | 1623.0 (421.0–5924.0) | 139 | 1885.0 (536.0–3278.0) | 0.40 |
| ALT (IU/L) | 479 | 1773.0 (602.0–4305.0) | 139 | 2154.0 (878.0–3880.0) | 0.26 |
| Bilirubin (mg/dl) | 483 | 6.5 (3.5–17.5) | 139 | 7.5 (4.6–17.4) | 0.03 |
| pH | 418 | 7.4 (7.3–7.5) | 136 | 7.4 (7.4–7.5) | 0.001 |
| Ammonia (venous; μmol/L) | 245 | 106.0 (67.0–166.0) | 82 | 113.5 (84.0–177.0) | 0.07 |
| Creatinine (mg/dL) | 489 | 2.0 (1.0–3.3) | 139 | 1.8 (1.0–3.5) | 0.84 |
| Lactate (mmol/L) | 308 | 4.9 (2.9–10.0) | 93 | 5.0 (2.9–9.0) | 0.82 |
| Phosphate (mg/dL) | 440 | 3.3 (2.2–4.9) | 131 | 2.7 (2.0–4.1) | <0.001 |
| PO2/FiO2 ratio | 363 | 3.4 (2.1–4.5) | 122 | 3.4 (2.4–4.4) | 0.98 |
| MELD (admission) | 475 | 33.1 (27.3–39.6) | 131 | 33.0 (25.4–39.7) | 0.73 |
| Organ support (7-days)* | |||||
| Mechanical ventilation | 489 | 403 (82.4%) | 140 | 137 (97.9%) | <0.001 |
| Vasopressors | 489 | 189 (38.7%) | 140 | 77 (55.0%) | <0.001 |
| Renal Replacement therapy | 489 | 186 (38.0%) | 140 | 73 (52.1%) | 0.003 |
| ICP therapies (7-days) | |||||
| Mannitol | 489 | 100 (20.5%) | 139 | 78 (55.7%) | <0.001 |
| Hypertonic saline | 489 | 36 (7.4%) | 139 | 19 (13.7%) | 0.02 |
| Barbiturates | 489 | 26 (5.3%) | 140 | 35 (25.0%) | <0.001 |
| Hypothermia | 489 | 49 (10.0%) | 139 | 33 (23.7%) | <0.001 |
| Sedatives | 489 | 365 (74.6%) | 140 | 95 (67.9%) | 0.11 |
| Blood products (7-days) | |||||
| Fresh Frozen Plasma | 489 | 297 (60.7%) | 140 | 118 (84.3%) | <0.001 |
| Recombinant VIIA | 489 | 10 (2.0%) | 139 | 3 (2.2%) | 1.0 |
| Platelets | 489 | 120 (24.5%) | 140 | 60 (42.9%) | <0.001 |
- Abbreviations: INR, international normalized ratio;
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; pO2, partial pressure of oxygen, FiO2, fraction of inspired oxygen.
- *7-days: Values refer to therapies at any time during the 7-days of study
During the first 7 study days, ICPM patients were more likely to be on RRT (52% vs. 38%), MV (98% vs. 82%) and vasopressors (55% vs. 39%, p < 0.004 for all). More ICPM patients received intracranial hypertension (ICH) directed therapies; including mannitol (56% vs. 21%), hypertonic saline (14% vs. 7%), barbiturates (25% vs. 5%) and hypothermia (24% vs. 10%); p < 0.03 for all (see Figure 1). More ICPM patients also received fresh frozen plasma (84% vs. 61%) and platelets (43% vs. 25%) than controls (p < 0.001 for both).
Figure 1.
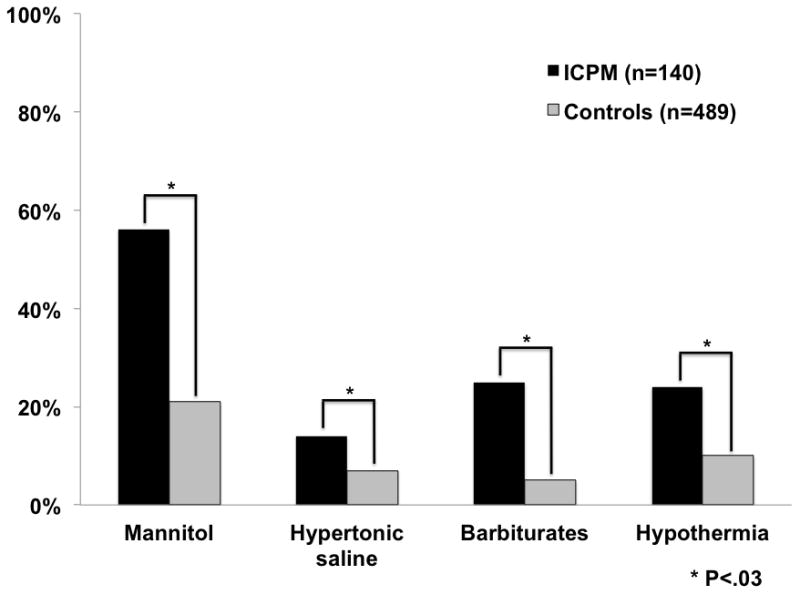
- Types of ICP therapies received during first 7 days
- Therapies: mannitol, hypertonic saline, barbiturates, hypothermia
- Missing data listed in Table 1
Outcomes in 629 ALF patients with Grade III–IV HE
Complications and outcomes are shown in Table 2. There were no significant differences in complications (seizures, arrhythmias, GI bleeding, bacteremia, tracheal aspirate infection, abnormal CXR or CT; p >0.1 for all comparisons). ICPM use was strongly associated with listing for LT (79% vs. 27%, p <0.001) and receipt of LT (41% vs. 18%, p< 0.001). Overall 21-day mortality was similar between ICPM patients and controls when all (33% and 38%, p= 0. 24) or only non-LT patients (46% vs. 45%, p= 0.92) were considered. There were no differences in rates of ICPM use comparing APAP to non-APAP patients (21% vs. 23%, p=0.48).
Table 2.
Outcomes of 629 Acute Liver Failure Patients with (n=140) and without (n=489) ICP monitoring
| Control Group (n=489) | ICPM group (n=140) | ||||
|---|---|---|---|---|---|
| N | Number (%) | N | Number (%) | P value | |
| ICU Complications (7-days) | |||||
| Seizures | 489 | 33 (6.8%) | 140 | 13 (9.3%) | 0.31 |
| Arrythmias | 489 | 126 (25.8%) | 140 | 43 (30.7%) | 0.24 |
| Gastrointestinal bleeding | 489 | 67 (13.7%) | 140 | 13 (9.3%) | 0.17 |
| Acute Respiratory Distress Syndrome | 489 | 8 (1.6%) | 139 | 2 (1.4%) | 0.69 |
| Abnormal CT of head | 196 | 89 (45.4%) | 66 | 35 (53.0%) | 0.28 |
| Abnormal Chest X-ray | 466 | 373 (80.0%) | 137 | 106 (77.4%) | 0.50 |
| Bacteremia/Blood stream infection | 489 | 68 (13.9%) | 140 | 19 (13.6%) | 0.92 |
| Tracheal aspirate infection | 489 | 75 (15.3%) | 140 | 21 (15.0%) | 0.92 |
| Listed for LT | 486 | 134 (27.5%) | 140 | 110 (78.6%) | <0.001 |
| Received LT | 488 | 87 (17.8%)* | 140 | 58 (41.4%)* | <0.001 |
| 21-day mortality | |||||
| Overall Deaths | 489 | 187 (38.2%) | 140 | 46 (32.9%) | 0.24 |
| Transplant-free (at 21 days) | 404 | 183 (45.3%) | 85 | 39 (45.9%) | 0.92 |
| Acetaminophen | |||||
| Transplanted | 251 | 22 (8.8%) | 69 | 18 (26.1%) | <0.001 |
| 21 day mortality | 252 | 77 (30.6%) | 69 | 19 (27.5%) | 0.63 |
| Non-acetaminophen | |||||
| Transplanted | 237 | 65 (27.4%) | 71 | 40 (56.3%) | <0.001 |
| 21 day mortality | 237 | 110 (46.4%) | 71 | 27 (38.0%) | 0.21 |
- *Of 145 patients who underwent LT, transplant date unavailable for five subjects (ICP: n=3/58, control: n=2/87)
Patient characteristics of 244 ALF patients listed for LT
In comparing ICPM patients OLT (n=110) compared with controls (n=134) listed for OLT (see Supplementary File 1), there was a trend towards an increased association between listed controls and receipt of OLT (controls 65% vs. ICPM 53 %, p=0.05). However in patients listed for LT, there were no associations between ICP monitoring and overall (controls 20% vs. ICPM 29%; p=0.10) or transplant free mortality at 21 days (47% vs. 46%, p=0.88).
Patient characteristics of 145 transplanted ALF patients with Grade III–IV hepatic coma
Clinical and biochemical information on 55 ICPM and 85 control patients who underwent LT are presented in Table 3 (date of transplant was unavailable for 5 subjects). The median time to LT was similar in both groups (~ 2 days). Apart from ICPM patients having a significantly lower median INR (2.1 vs. 3.0, p=0.003), there were no other significant hematological or biochemical differences within 24 hours of LT (p ≥ 0.05 for all). During the inpatient phase prior to LT (up to 7 days) ICPM patients who underwent LT received vasopressors (55% vs. 22%), MV (100% vs. 75%) and RRT (56% vs. 34%, p<0.01 for all) more frequently than controls. ICPM patients received more mannitol (22% vs. 7%) and barbiturates (15% vs. 2%, p < 0.03 for both) than controls undergoing LT.
Table 3.
Clinical and biochemical information of patients with (n=55) and without (n=85) ICP monitoring who underwent liver transplantation (data within 24 hours of transplant)
| Control group (n=85) | ICPM group (n=55) | p-value | |||
|---|---|---|---|---|---|
| Time to transplant (days) | 85 | 2.0 (1.0–3.0) | 55 | 2.0 (1.0–4.0) | 0.71 |
| Biochemistry | |||||
| Hemoglobin (g/dl) | 54 | 9.8 (8.3–10.8) | 23 | 9.6 (8.6–11.2) | 0.92 |
| White Blood count (×109/L) | 54 | 9.3 (6.7–12.8) | 24 | 8.8 (5.6–13.3) | 0.996 |
| Platelet count (×109/L) | 53 | 78.0 (54.0–145.0) | 23 | 114.0 (69.0–180.0) | 0.07 |
| INR | 52 | 3.0 (2.3–3.8) | 24 | 2.1 (1.6–2.7) | 0.003 |
| AST (IU/L) | 49 | 633.0 (264.0–1951.0) | 24 | 629.5 (141.5–1325.0) | 0.70 |
| ALT (IU/L) | 49 | 701.0 (324.0–2371.0) | 23 | 652.0 (230.0–1817.0) | 0.54 |
| Bilirubin (mg/dl) | 49 | 15.0 (6.6–24.6) | 24 | 16.4 (6.2–22.3) | 0.80 |
| Creatinine (mg/dL) | 53 | 1.7 (1.0–3.3) | 23 | 1.8 (0.8–2.7) | 0.94 |
| Phosphate (mg/dL) | 35 | 3.3 (2.5–4.7) | 13 | 3.6 (2.8–3.9) | 0.88 |
| Lactate (mmol/L) | 19 | 4.8 (3.7–15.0) | 13 | 3.8 (2.8–8.2) | 0.19 |
| pH | 47 | 7.4 (7.4–7.5) | 24 | 7.5 (7.4–7.5) | 0.05 |
| pO2 (mmHg) | 48 | 147.0 (93.0–207.0) | 23 | 111.0 (95.0–137.0) | 0.06 |
| Fio2 (%) | 40 | 40.0 (30.0–60.0) | 21 | 40.0 (30.0–50.0) | 0.44 |
| Ammonia (venous; μmol/L) | 11 | 122.0 (60.0–319.0) | 14 | 127.0 (75.0–180.0) | 0.85 |
| Physiology - During Inpatient Phase (up to 7 days) | |||||
| Vasopressors | 85 | 19 (22.4%) | 55 | 30 (54.6%) | <0.001 |
| Mechanical ventilation | 85 | 64 (75.3%) | 55 | 55 (100.0%) | <0.001 |
| Renal replacement therapy | 85 | 29 (34.1%) | 55 | 31 (56.4%) | 0.009 |
| ICP therapies (within 24 hr of LT) | |||||
| Mannitol | 75 | 5 (6.7%) | 46 | 10 (21.7%) | 0.02 |
| Hypertonic saline | 75 | 3 (4.0%) | 46 | 2 (4.4%) | 0.63 |
| Barbiturates | 75 | 2 (2.7%) | 46 | 7 (15.2%) | 0.02 |
| Hypothermia | 75 | 2 (2.7) | 46 | 3 (6.5%) | 0.28 |
| Sedation | 75 | 29 (38.7%) | 46 | 13 (28.3%) | 0.24 |
| Transfusions (within 24 hr of LT) | |||||
| FFP | 75 | 30 (40.0%) | 46 | 18 (39.1%) | 0.92 |
| Platelets | 75 | 15 (20.0%) | 46 | 11 (23.9%) | 0.61 |
| VIIa | 75 | 3 (4.0%) | 46 | 0 (0.0%) | 0.29 |
- *Of 145 patients who underwent LT, transplant date unavailable for five subjects (ICP: n=3/58, control: n=2/87)
- Abbreviations: INR, international normalized ratio;
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; pO2, partial pressure of oxygen, FiO2, fraction of inspired oxygen.
Preparation and complications of ICP monitoring in 140 ICPM ALF patients
Of ICPM patients (n=140), 85% received devices within 24 hours of study admission. The median INR (IQR) on the day of ICPM insertion was 2.2 (1.6–2.9) and platelet count 121 (87–174). On day of ICPM insertion, fresh frozen plasma was given to 74% of ICPM patients, 19% received platelets, 4% received factor VIIa and 32% received packed red blood cells. Of 75 patients with known ICPM device, 17 received epidural catheters, 20 subdural, 18 intraparenchymal, 7 intraventricular and 13 lumbar monitors (see Figure 2). Of 87 patients in the ICPM group with available detailed information, 44 (51%) had evidence of ICH (ICP >25 mmHg) during study period and overall 21-day mortality was higher in patients with evidence of ICH (43% vs. 23%, p=0.05). Where data were available (n=87), there were no differences in prevalence of ICH between APAP (57%) and non-APAP (44%) patients (p=0.24). In 56 ICPM patients where complete complication data were available, 4 (7%) reported evidence of significant neurological hemorrhage; intracranial (n=3) and spinal (n=1). One patient survived and the other 3 died due to hemorrhagic complications. Of 46 ICPM patients who died before 21 days, 21 (46%) died of neurological causes (cerebral edema n=18, intracranial hemorrhage, n=3). Comparatively, 24 of 187 (13%) deaths of non-monitored controls before 21 days were due to neurological complications (cerebral edema).
Figure 2.
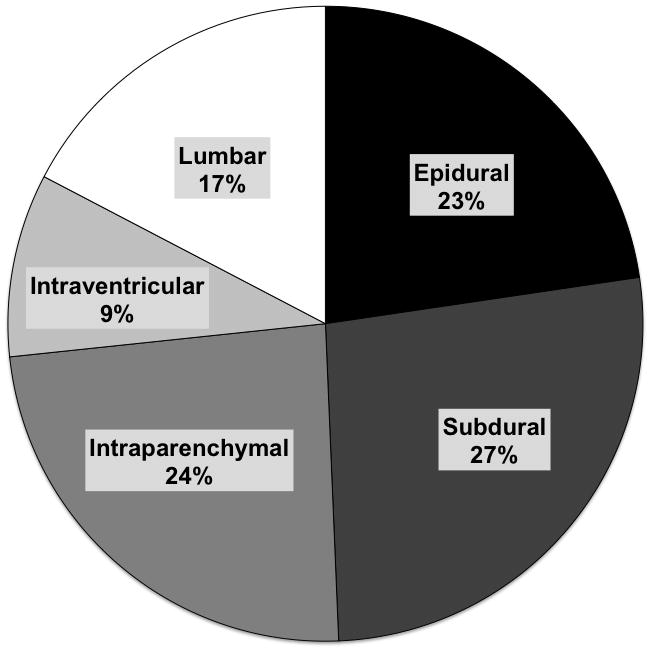
- Where data available after querying of US ALFSG sites
- Data reported as percentages
Multivariable analysis of 311 APAP-induced ALF patients
Given that LT (12.2% vs. 32.8%, p = 0.0001) and 21-day mortality rates (29.9% vs. 44.5%, p=0.0002) differed between APAP and non-APAP etiologies, the cohort was stratified by etiology (APAP and non-APAP) when assessing outcome data. The APAP-ALF model included 311 patients (10 subjects were excluded due to missing data for at least one prognostic variable). Multivariable logistic regression results are shown in Table 4. There were no significant interaction terms. The c-statistic (i.e., how well the model distinguishes between survivors and non-survivors at 21 days) was 0.84 (good predictive accuracy). Age, transplant, MELD (admission), vasopressors within 7 days, and year of enrollment were found to significantly impact death within the first 21 days of study admission. Receiving LT (OR: 0.04 [0.008, 0.21]) and successive year of enrollment (OR 0.87[0.75–1.00]) were associated with lower 21- day mortality. Increasing age (OR 1.02[1.001–1.05]), MELD (OR 1.09[1.05–1.12]) and requirement for vasopressors at any time during the 7 days (OR 5.92 [3.26, 10.74]) were associated with increased 21-day mortality. Gender, race, high enrolling center (> 6 patients per year) and presence of ICP monitor were not significant in the final model.
Table 4.
Multivariable Analysis: Predictors of 21-day mortality in 311 patients with acetaminophen-induced Acute Liver Failure.
| Univariate (N=321) | Multivariate (N=311) | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | |
| Age | 1.024 | 1.005 – 1.042 | 0.011 | 1.023 | 1.001 – 1.046 | 0.041 |
| Male | 1.005 | 0.545 – 1.855 | 0.986 | 0.538 | 0.242 – 1.199 | 0.129 |
| White | 0.718 | 0.381 – 1.355 | 0.307 | 0.848 | 0.383 – 1.877 | 0.684 |
| Transplant | 0.108 | 0.026 – 0.458 | 0.003 | 0.040 | 0.008 – 0.210 | <0.001 |
| Lactate | 1.000 | 0.998 – 1.001 | 0.623 | |||
| MELD | 1.069 | 1.040 – 1.099 | <0.001 | 1.086 | 1.049 – 1.124 | <0.001 |
| Mechanical ventilation* | 3.872 | 1.141 – 13.146 | 0.030 | |||
| Vasopressors | 5.566 | 3.303 – 9.378 | <0.001 | 5.915 | 3.259 – 10.735 | <0.001 |
| Hemodialysis* | 1.368 | 0.844 – 2.217 | 0.204 | |||
| Enrollment Year | 0.840 | 0.751 – 0.940 | 0.002 | 0.867 | 0.753 – 1.000 | 0.049 |
| High Enrolling site** | 0.742 | 0.445 – 1.239 | 0.254 | 0.638 | 0.326 – 1.246 | 0.188 |
| ICP Monitor | 0.864 | 0.478 – 1.562 | 0.628 | 0.945 | 0.441 – 2.028 | 0.885 |
- 10 patients excluded for missing data
- Final model was adjusted for site of age, gender, race, transplant status, enrollment year, high enrolling site** and presence of ICP monitor
- Variables included in final model if significant on univariable logistic regression included MELD on admission and vasopressors during 7 days in study
- Lactate (admission) was not significant on univariable analysis
- *Requirement for mechanical ventilation and hemodialysis were not statistically significant in multivariable model. (Not included in final model)
- **High enrolling site was defined as a site that enrolled more than 6 subjects per year on average while open from 1998 – 2012 (median for all sites).
- The multivariable model had a c-statistic of 0.84, Hosmer-Lemeshow p=0.62
Multivariable analysis of 295 Non-Acetaminophen-induced ALF patients
Multivariable logistic regression results of 295 non-APAP ALF patients are shown in Table 5 (13 patients had missing data). The model c-statistic was 0.86. Increasing age (OR 1.02 [1.002, 1.04]), requirement for vasopressors (OR 2.13 [1.16, 3.92]), and placement of ICP monitor (OR 3.04 [1.26, 7.34]) were associated with increased 21-day mortality. A qualitative interaction effect was found between LT status at 21 days post admission and MELD at admission. For subjects who did not receive LT within the first 21 days, the likelihood of death increased as admission MELD increased. In patients who underwent LT, transplant is more protective with increasing admission MELD.
Table 5.
Multivariable Analysis: Predictors of 21-day mortality in 295 patients with non-acetaminophen-induced Acute Liver Failure.
| Univariate (N=308) | Multivariate (N=295) | |||||
|---|---|---|---|---|---|---|
| Measures | Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value |
| Age | 1.017 | 1.002 – 1.032 | 0.027 | 1.022 | 1.002 – 1.043 | 0.029 |
| Male | 1.303 | 0.824 – 2.060 | 0.258 | 0.770 | 0.410 – 1.446 | 0.417 |
| White | 0.952 | 0.593 – 1.527 | 0.837 | 0.783 | 0.412 – 1.489 | 0.455 |
| Transplant** | 0.060 | 0.029 – 0.127 | <0.001 | |||
| Lactate | 1.017 | 0.994 – 1.040 | 0.145 | |||
| MELD** | 1.050 | 1.022 – 1.079 | <0.001 | |||
| Mechanical ventilation | 1.098 | 0.624 – 1.934 | 0.745 | |||
| Vasopressors | 2.635 | 1.656 – 4.194 | <0.001 | 2.133 | 1.160 – 3.923 | 0.015 |
| Hemodialysis | 1.417 | 0.896 – 2.240 | 0.136 | |||
| Enrolment Year | 0.957 | 0.860 – 1.066 | 0.428 | |||
| High Enrolling site* | 0.976 | 0.602 – 1.580 | 0.920 | 1.316 | 0.673 – 2.571 | 0.423 |
| MELD*Transplant** | 0.004 | |||||
| ICP Monitor | 0.708 | 0.412 – 1.219 | 0.213 | 3.040 | 1.258 – 7.344 | 0.014 |
- 13 patients excluded for missing data
- *High enrolling site defined as a site that enrolled more than 6 subjects per year on average while open from 1998 – 2012 (median for all sites).
- Final model was adjusted for site, age, gender, race, transplant status, high vs. low enrolling site and presence of ICP monitor
- Other variables were included in final model if significant on univariable logistic regression (MELD on admission and vasopressors during 7 days in study)
-
**There was a significant interaction MELD and transplant status; an interaction term was included in the final model which was statistically significant (p=0.003)
- In non-transplanted patients, increasing meld was significantly associated with worse outcomes when accounting for the interaction with LT
- The multivariable model had a c-statistic of 0.86, Hosmer-Lemeshow p=0.68
DISCUSSION
Summary of Key Results
In this retrospective study of 629 patients with high-grade (III/IV) HE, we found that clinically significant ICH is common (~51%). ICPM use was significantly associated with listing for and receipt of LT. Patients with ICPM received significantly more ICH-directed therapies. However, when adjusting for confounding variables reflecting severity of illness, ICPM use in APAP-ALF was not significantly associated with mortality while in non-APAP ALF patients it was associated with significantly increased 21-day mortality. In ICPM patients where complication data were available, 5% (n=3) died directly of hemorrhagic complications related to ICPM insertion.
Comparison with previous studies
While retrospective, this is the largest multicenter study to assess the potential benefit of ICP monitoring in ALF patients recruited from US ALFSG sites. Given the heterogeneity of indications for ICPM insertion across sites, judging appropriateness of a potential candidate for an ICPM is important to ensure generalizability of results. Bernal and colleagues previously reported that patients who had the highest risk of clinically significant ICH had high serum ammonia levels, required RRT, were hemodynamically unstable (required vasopressors) and were younger (14). In this cohort, patients who underwent ICPM insertion were significantly younger, had higher serum ammonia levels on admission (non-significant trend, p=0.07) and had an increased requirement for RRT during the first 7 days. Thus the patients undergoing ICPM use were appropriately selected.
Our study confirms results of prior studies and also expands on these data. In this study, ICPM use was not associated with an improvement in 21-day survival in APAP-ALF patients, similar to an earlier study from our group (1998–2004) and other older studies (9, 10, 19). In this cohort, ICPM use once again was associated with more ICP-related interventions, possibly related to recognition of ICH (9). ICP readings were not available in the earlier study; thus, we were somewhat surprised at the high rate of ICH (ICP > 25 mm Hg), with 51% showing ICH in this cohort.
Besides an absence of previous studies demonstrating a survival benefit, another significant barrier to routine ICPM use is concern about intracranial hemorrhage. In our earlier study, the overall rate of hemorrhage was similar (10.3% experienced bleeding complications), half of these were incidental radiographic findings and not clinically significant (9). In a more recent study of 117 ALF patients from King’s College Hospital, 3 patients (2.5%) developed intracranial hemorrhage after ICPM placement (12). Current conventional parameters (INR, prothrombin time, fibrinogen) do not reliably estimate hemostatic potential and bleeding risk in ALF (20, 21). Prophylactic factor VIIa use was low in this cohort (~ 4%) while fresh frozen plasma was used commonly on the day of ICPM insertion (74%) (22, 23). The indiscriminant use of fresh frozen plasma in patients with ALF provides large fluid volumes that may exacerbate ICH, particularly in patients who are not on RRT. Stravitz and colleagues demonstrated that despite elevated INR, many patients with ALF appear to maintain normal hemostasis by thromboelastography (21). A number of pro-coagulant pathways are activated that result in an increase in clot strength with increasing severity of liver injury (21). Our bleeding rate of 5–7% is similar to previous studies (9, 12) and lower than the reports from the 1990s (11). Similar rates have been reported by Kamat and colleagues recently (2012) in the pediatric literature as well (24). Perhaps thromboelastography will guide the use of blood products in the future to further minimize the risk of clinically significant hemorrhage.
After controlling for confounding on multivariable analysis, ICP monitoring in non-APAP patients was associated with increased 21-day mortality. As a retrospective study, we cannot comment directly on causation, but this raises a few possibilities. Firstly, while there is risk of complications with ICPM insertion, the low bleeding rates in this cohort suggest that ICPM insertion per se likely did not contribute to the increased mortality (similar to Vaquero(9)). Given the fact that surrogates of cerebral blood flow (e.g. transcranial Doppler, jugular bulb saturations) were not routinely used across most centers that manage ALF, it is possible that despite increased use of therapies for ICPM patients, they may not necessarily be appropriate (e.g. hyperventilation for cerebral hyperemia, mannitol for ICH). More likely, despite attempting to control for confounding by severity of illness in multivariable analysis, it is possible that sicker patients were more likely to undergo ICPM insertion in a non-randomized fashion and that there are other unknown confounding variables (see limitations). This likely explains in part why a greater proportion of ICPM patients were listed for LT. This is also evident in the traumatic brain injury literature where several studies have failed to show a mortality benefit for extraventricular drainage devices despite their widespread acceptance (25, 26). Finally, hyperacute liver failure (i.e., acetaminophen) patients typically present with rapid development of hepatic encephalopathy, multi-organ failure and cerebral edema compared to other etiologies and may be more likely to benefit from neuroprotective strategies and ICP monitoring (14, 18, 27).
In light of these issues, while our results cannot conclusively confirm or refute the utility of ICP monitoring in ALF patients, patient selection and ancillary assessments of cerebral blood flow likely have a significant role.
Study limitations
Our study has limitations that warrant consideration. First, it is a retrospective analysis of prospectively collected data, thereby potentially predisposed to selection bias and residual confounding. Individual centers vary in volume and make independent decisions about listing for LT, insertion of an ICPM, type of device and what prophylactic blood products to give. While common indications for ICPM insertion described in the methods are based on recommendations from data from King’s College Hospital (14) and the US ALFSG (15), both of these studies were published after this cohort began. Despite several reviews of source data we did not have complete intracranial pressure, monitor and complication data. Patients were not assigned randomly to ICPM and given that listing for LT was associated with increased use of ICPM, it is possible that monitoring in LT candidates was undertaken in sicker patients, though epidemiological and clinical variables were similar on admission and prior to the procedure. A prospective randomized study with standardized ICP management protocols would address these issues of bias, however no prospective studies have been performed to date. We attempted to control for confounding by performing multivariable analysis accounting for severity of liver dysfunction (MELD) and multi-organ failure (requirement for vasopressors, RRT and MV). SOFA and APACHE II could not be accurately calculated due to lack of detailed information on vasopressor doses (SOFA) and GCS (APACHE II; only hepatic coma grade available).
CONCLUSIONS
When ICP monitoring is performed in ALF patients, ICH is commonly observed. The use of ICPM in APAP ALF did not confer a significant 21-day mortality benefit while in non-APAP ALF it may be associated with worse outcomes. Significant hemorrhagic complications from ICPM placement were uncommon and cannot account for trends in mortality. While our results cannot conclusively confirm or refute the utility of ICP monitoring in ALF patients, patient selection and ancillary assessments of cerebral blood flow likely have a significant role. Prospective studies would be required to conclusively account for confounding by illness severity and transplant.
Acknowledgments
Financial support: The study was sponsored by NIH grant U-01 58369 (from NIDDK).
This study was funded by National Institutes of Health grant (DK U-01 58369) for the United States Acute Liver Failure Study Group provided by the National Institute of Diabetes and Digestive and Kidney Diseases. Members and institutions participating in the Acute Liver Failure Study Group 1998–2011 are as follows: W.M. Lee, M.D. (Principal Investigator); Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Timothy Davern, M.D., University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco, CA), Oren Fix, M.D., University of California, San Francisco; Michael Schilsky, M.D., Mount Sinai School of Medicine, New York, NY (current address: Yale University, New Haven, CT); Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased), Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., Nahid Attar, Linda S. Hynan, Ph.D., and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Catherine Dillon, Holly Battenhouse and Tomoko Goddard
Abbreviations
- ALF
Acute Liver Failure
- APAP
Acetaminophen
- CRRT
Continuous renal replacement therapy
- HBV
Hepatitis B
- HE
Hepatic Encephalopathy
- ICH
Intracranial hypertension
- ICP
Intracranial Pressure
- ICPM
Intracranial Pressure monitor/monitoring
- ICU
Intensive Care Unit
- LT
Liver transplant
- MELD
Model for End-stage Liver Disease
- MV
Mechanical ventilation
- RRT
Renal replacement therapy
- US ALFSG
United States Acute Liver Failure Study Group
Footnotes
Reprints will NOT be ordered.
Conflict of interest: none
Format: This paper followed the STROBE guideline for reporting retrospective studies (BMJ 2007).
Contributions:
C.J.K. – Designed the study, performed statistical analysis, drafted and revised the final manuscript.
O.F. – Provided content expertise, advice on data collection and analysis, and made significant revisions to the final manuscript.
H.B.- Prepared the database, performed significant statistical analysis and made significant revisions to the final manuscript.
V.D.- Provided statistical expertise and significantly revised the final manuscript.
C.S.- Assisted in preparation of the database, queried enrolling sites for missing data and made significant revisions to the final manuscript.
W.M.L.- (Lead investigator, US ALFSG) Assisted in the design of the study, provided content expertise and made significant revisions to the final manuscript.
Copyright Form Disclosures:
Dr. Karvellas received support for article research from the National Institutes of Health (US ALFSG is funded by an NIH grant [NIDDK] [DK U-01 58369]). Dr. Fix is employed by UCSF and received support for article research from NIH. Dr. Fix’s institution received grant support from NIDDK/NIH. Dr. Battenhouse received support for article research from NIH. Dr. Battenhouse’s institution received grant support from NIH (U01-DK58369) and is employed by NIH (U01-DK58369). Dr. Durkalski is employed by Medical University of SC and received support for article research from NIH. Dr. Durkalski’s institution received grant support from the Medical University of SC (Funding provided by ALFSG grant U01DK0583691). Dr. Sanders received support for article research from NIH grant U-01 58369 (from NIDDK). Dr. Lee consulted for Lilly, Novartis, and Pfizer (drug safety review) and support for article research from NIH. Dr. Lee’s institution received grant support from UT Southwestern MCD (NIH Grant U-01 58369 to UTSW) and UTSW Medical Ctr (Anadys, BMS, BI, Gilead, Merck, Siemens, Vertex).
References
- 1.O’Grady JG, Williams R. Classification of acute liver failure. Lancet. 1993;342(8873):743. [PubMed] [Google Scholar]
- 2.Fagan E, Wannan G. Reducing paracetamol overdoses. BMJ. 1996;313(7070):1417–1418. doi: 10.1136/bmj.313.7070.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 4.Ware AJ, D’Agostino AN, Combes B. Cerebral edema: a major complication of massive hepatic necrosis. Gastroenterology. 1971;61(6):877–884. [PubMed] [Google Scholar]
- 5.Bernal W, Wendon J. Acute liver failure; clinical features and management. Eur J Gastroenterol Hepatol. 1999;11(9):977–984. doi: 10.1097/00042737-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41(5):1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 7.Lidofsky SD, Bass NM, Prager MC, et al. Intracranial pressure monitoring and liver transplantation for fulminant hepatic failure. Hepatology. 1992;16(1):1–7. doi: 10.1002/hep.1840160102. [DOI] [PubMed] [Google Scholar]
- 8.Davies MH, Mutimer D, Lowes J, et al. Recovery despite impaired cerebral perfusion in fulminant hepatic failure. Lancet. 1994;343(8909):1329–1330. doi: 10.1016/s0140-6736(94)92471-6. [DOI] [PubMed] [Google Scholar]
- 9.Vaquero J, Fontana RJ, Larson AM, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11(12):1581–1589. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 10.Keays RT, Alexander GJ, Williams R. The safety and value of extradural intracranial pressure monitors in fulminant hepatic failure. J Hepatol. 1993;18(2):205–209. doi: 10.1016/s0168-8278(05)80247-8. [DOI] [PubMed] [Google Scholar]
- 11.Blei AT, Olafsson S, Webster S, et al. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet. 1993;341(8838):157–158. doi: 10.1016/0140-6736(93)90016-a. [DOI] [PubMed] [Google Scholar]
- 12.Peck M, Wendon J, Sizer E, et al. Intracranial pressure monitoring in acute liver failure: a review of 10 years experience. Crit Care. 2010;14(Suppl 1):542. [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernal W, Hall C, Karvellas CJ, et al. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46(6):1844–1852. doi: 10.1002/hep.21838. [DOI] [PubMed] [Google Scholar]
- 15.Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Critical care medicine. 2007;35(11):2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 16.Conn HO, Lieberthal MM, editors. The hepatic coma syndromes and lactulose. Baltimore: Williams & Wilkins; 1979. [Google Scholar]
- 17.Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978;23(5):398–406. doi: 10.1007/BF01072921. [DOI] [PubMed] [Google Scholar]
- 18.O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 19.Daas M, Plevak DJ, Wijdicks EF, et al. Acute liver failure: results of a 5-year clinical protocol. Liver Transpl Surg. 1995;1(4):210–219. doi: 10.1002/lt.500010403. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal B, Wright G, Gatt A, et al. Evaluation of coagulation abnormalities in Acute Liver Failure. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Stravitz RT, Lisman T, Luketic VA, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56(1):129–136. doi: 10.1016/j.jhep.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldwell SH, Chang C, Macik BG. Recombinant activated factor VII (rFVIIa) as a hemostatic agent in liver disease: a break from convention in need of controlled trials. Hepatology. 2004;39(3):592–598. doi: 10.1002/hep.20123. [DOI] [PubMed] [Google Scholar]
- 23.Le TV, Rumbak MJ, Liu SS, et al. Insertion of intracranial pressure monitors in fulminant hepatic failure patients: early experience using recombinant factor VII. Neurosurgery. 2010;66(3):455–458. doi: 10.1227/01.NEU.0000365517.52586.A2. discussion 458. [DOI] [PubMed] [Google Scholar]
- 24.Kamat P, Kunde S, Vos M, et al. Invasive intracranial pressure monitoring is a useful adjunct in the management of severe hepatic encephalopathy associated with pediatric acute liver failure. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13(1):e33–38. doi: 10.1097/PCC.0b013e31820ac08f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griesdale DE, McEwen J, Kurth T, et al. External ventricular drains and mortality in patients with severe traumatic brain injury. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2010;37(1):43–48. doi: 10.1017/s031716710000963x. [DOI] [PubMed] [Google Scholar]
- 26.Kasotakis G, Michailidou M, Bramos A, et al. Intraparenchymal vs extracranial ventricular drain intracranial pressure monitors in traumatic brain injury: less is more? Journal of the American College of Surgeons. 2012;214(6):950–957. doi: 10.1016/j.jamcollsurg.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet. 2010;376(9736):190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]


