Abstract
Deceased-donor kidneys with acute kidney injury (AKI) are often discarded due to fear of poor outcomes. We performed a multicenter study to determine associations of AKI (increasing admission-to-terminal serum creatinine by AKI Network stages) with kidney discard, delayed graft function (DGF) and 6-month estimated glomerular filtration rate (eGFR). In 1632 donors, kidney discard risk increased for AKI stages 1, 2 and 3 (compared to no AKI) with adjusted relative risks of 1.28 (1.08–1.52), 1.82 (1.45–2.30) and 2.74 (2.0–3.75), respectively. Adjusted relative risk for DGF also increased by donor AKI stage: 1.27 (1.09–1.49), 1.70 (1.37–2.12) and 2.25 (1.74–2.91), respectively. Six-month eGFR, however, was similar across AKI categories but was lower for recipients with DGF (48 [interquartile range: 31–61] vs. 58 [45–75] ml/min/1.73m2 for no DGF, P<0.001). There was significant favorable interaction between donor AKI and DGF such that 6-month eGFR was progressively better for DGF kidneys with increasing donor AKI (46 [29–60], 49 [32–64], 52 [36–59] and 58 [39–71] ml/min/1.73m2 for no AKI, stage 1, 2 and 3, respectively; interaction P=0.05). Donor AKI is associated with kidney discard and DGF, but given acceptable 6-month allograft function, clinicians should consider cautious expansion into this donor pool.
Keywords: Kidney transplantation, deceased donor, acute kidney injury, organ discard, delayed graft function, allograft function
Introduction
The growing disparity between organ supply and demand for kidney transplantation necessitates ongoing efforts to both expand the donor pool and maximize the use of good quality organs. While there is an undeniable organ shortage for transplantation, there is also clear regional heterogeneity in deceased-donor organ acceptance and discard patterns (1). Such inconsistencies may be partially explained by differences in organ availability and need due to regional population variation in the number of potential donors and transplant candidates, which may be driven by variations in population density, socioeconomics, race/ethnicity and disease burden (2, 3). In addition to supply and demand differences, however, variance in clinician and/or center-level aggressiveness likely account for some of the regional variability in organ discard rates (4). In particular, acute kidney injury (AKI) in the donor can profoundly complicate an otherwise straightforward organ offer. For these cases, on-call clinicians often scrutinize donor renal function beyond the single "terminal" serum creatinine (SCr) value and consider changes in SCr throughout the hospitalization relative to light-microscopic biopsy findings and urine output (UOP) at the time of procurement.
Donor AKI could plausibly lead to adverse outcomes in the transplant recipient. Severe ischemic AKI often results in the need for renal replacement therapy in the recipient until the kidney can repair itself. These “delays” in functional recovery of injured allografts lead to prolonged hospital stays and increased costs due to dialysis, or even primary non-function of the allograft in the most severe cases. With regard to longer-term function, preclinical studies have linked episodes of AKI with progressive renal fibrosis and the development of chronic kidney disease (5). Human studies have also shown associations between clinically-defined AKI and poor long-term renal outcomes (6).
Our group recently reported a lack of association between pre-implant (procurement) biopsy-reported acute tubular necrosis (ATN) and the development of delayed graft function (DGF) or early graft failure (7). Given evidence of the limited value of pre-implant biopsy reported ATN, we performed the current multicenter cohort study to evaluate potential associations between clinically-defined donor AKI (categorized by changes in SCr from admission to the terminal value) and important transplant outcomes, namely kidney discard, DGF and 6-month allograft function. We hypothesized that the severity of donor AKI would be independently associated with increasing rates of organ discard and DGF as well as worse 6-month allograft function.
Materials and Methods
This is a prospective, multicenter, observational cohort study involving clinical data from deceased kidney donors from five organ procurement organizations (OPOs) between May 2010 and December 2013. Donor characteristics and clinical variables were abstracted from OPO donor charts. Clinical data for all recipients of the kidneys from these donors were obtained from the United Network for Organ Sharing (UNOS) database. Personnel at each OPO followed institutional protocols for managing deceased donors and obtaining consent for research from donor surrogates. The scientific review committees for participating OPOs as well as the institutional review boards for the participating investigators approved the study. Donors aged 16 years or older were included if at least one kidney was procured for potential transplantation, resulting transplants were for separate recipients (i.e., dual kidney transplants into the same recipient were excluded), and both admission and terminal SCr values were available for analysis.
As the primary exposure variable, donor AKI was defined according to AKI Network criteria based on admission to terminal SCr (irrespective of time between measurements and UOP cut-offs) as follows: stage 1, increase in SCr by ≥0.3 mg/dl or 1.5 to <2-fold increase; stage 2, 2 to <3-fold increase; and stage 3, ≥3-fold increase, or terminal SCr ≥4.0 mg/dl after a rise of at least 0.5 mg/dl (no donors were dialyzed) (8). We calculated the kidney donor risk index (KDRI) as described by Rao et al. (9, 10). We converted the KDRI score for each donor, as per convention (9), to obtain the kidney donor profile index (KDPI). We also reviewed available pathology reports from procurement kidney biopsies with regard to both glomerulosclerosis and ATN, which we defined as present if any histologic evidence of acute tubular injury was mentioned in the report (without regard to severity). Reports that specified the absence of ATN or had no mention of tubular injury were categorized as no ATN. The following outcomes were analyzed as recorded in the UNOS database: discard (kidney procured for transplant but not transplanted), DGF (any dialysis in the first week of transplantation), 6-month estimated glomerular filtration rate (eGFR, calculated from 6-month SCr via the Chronic Kidney Disease Epidemiology Collaboration equation) (11) and death-censored graft failure (return to chronic dialysis or retransplantation).
We evaluated 6-month eGFR as the primary outcome of interest and considered kidney discard and DGF as secondary outcomes. Understanding there would be relatively low event rates and an unavoidable delay in UNOS reporting at this point, we evaluated available 12-month eGFR and death-censored graft failure as currently recorded for study transparency and to explore possible trends for the cohort. We used exclusion to accommodate for missing data, with the notable exception for missing 6-month eGFR values related to graft failure or recipient death before 6 months. For the few recipients with UNOS-reported death-censored graft failure prior to 6 months, eGFR was imputed as 10 ml/min/1.73m2. We carried forward the last available SCr value to calculate 6-month eGFR in the rare event of recipient death before 6 months.
Descriptive statistics were reported as mean (standard deviation) or median [interquartile range] for continuous variables and as frequency (percentage) for categorical variables. Donor and recipient characteristics and outcomes as well as procurement kidney biopsy reports for ATN and glomerulosclerosis ≥20% were compared between donor AKI stages using the Kruskall-Wallis test for continuous variables and Pearson's Chi-Square test for categorical variables. We also compared the rates of biopsy-reported ATN with donor AKI in the subset of donors that had at least one procurement kidney biopsy. We then compared donor characteristics and biopsy results between donors for which neither, one, or both kidneys were discarded. Using modified Poisson regression as described by Zou (12), we modeled the relative risks (RRs) of kidney discard and DGF as a function of donor AKI. Donor AKI was operationalized as a dichotomous exposure (any AKI compared with no AKI) as well as a multilevel categorical exposure according to stage (no AKI as reference), adjusting for all donor characteristics that comprise the KDRI except for terminal SCr (already considered for the AKI covariate). We performed further adjustment of the kidney discard model with the addition of procurement kidney biopsy performed (dichotomous) and machine pump perfusion used (dichotomous) as well as both of these covariates plus cold ischemia time in hours for the DGF model. We accounted for the cluster effect of paired kidneys from the same donor via generalized estimating equations for the Poisson models (13).
Similarly, we utilized multivariable linear regression to test for associations between donor AKI and 6-month eGFR. Given its well-described effect on subsequent outcomes, we performed a priori stratified analyses according to DGF status and formally tested for interaction between DGF and donor AKI stage on 6-month eGFR. We fit Cox proportional hazards models to evaluate the effect of donor AKI on death-censored graft failure. We used SAS 9.3 statistical software for Windows (SAS Institute, Cary, NC), and all statistical tests and confidence intervals were two-sided with a significance level of 0.05.
Results
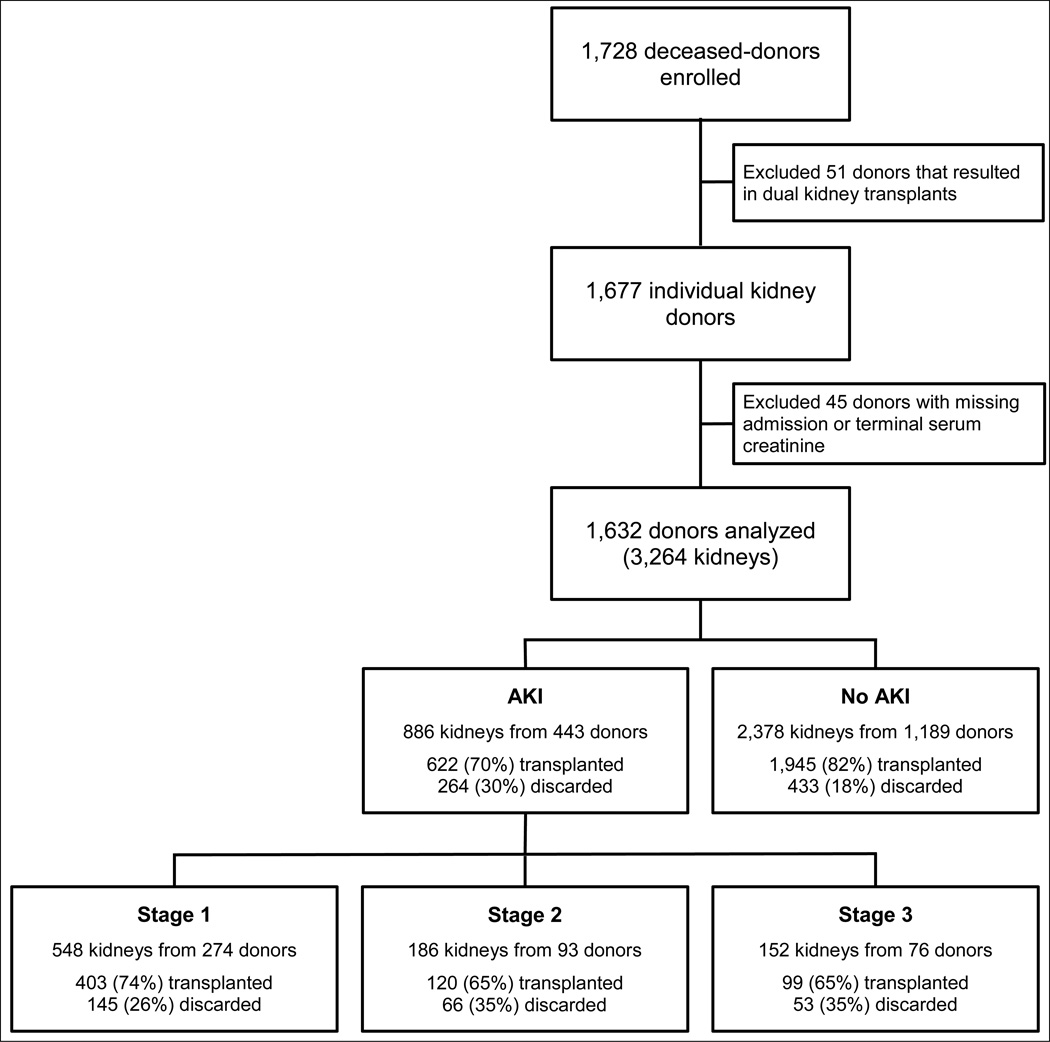
After exclusions, a total of 1632 deceased donors were available for analysis, of which 443 (27%) had some degree of AKI. A flowchart for donor enrollment, exclusions and AKI stages along with the numbers of kidney transplants and discards is shown in Figure 1. There were 697 kidney discards (21% of all potential transplants), and 800 (31%) recipients experienced DGF. Median follow-up time for the entire cohort was 625 [345, 856] days, and 185 (7%) death-censored graft failures and 180 (7%) recipient deaths have been reported.
Figure 1.
Flowchart showing distribution of acute kidney injury (AKI) among deceased organ donors
Donor and recipient characteristics by donor AKI stage are shown in Table 1. Donors with higher AKI stages were less likely to have both kidneys transplanted, and more procurement kidney biopsies were performed for donors with higher AKI stages. Compared to donors without AKI, donors with stage 3 AKI tended to be younger but had similar mean KDRI and higher mean admission eGFR. The kidneys from donors with AKI were more often transported via machine pump perfusion, had longer cold ischemia times and were transplanted into older recipients.
Table 1.
| A: Donor characteristics by donor AKI stage | |||||||
|---|---|---|---|---|---|---|---|
| Variable | ALL (N=1369) |
No AKI (N=1027) |
Stage 1 (N=218) |
Stage 2 (N=68) |
Stage 3 (N=56) |
P-value 1 | |
| Age, years | 41 (15) | 41 (16) | 41 (14) | 40 (14) | 35 (13) | 0.040 | |
| Male | 829 (61%) | 613 (60%) | 139 (64%) | 39 (57%) | 38 (68%) | 0.423 | |
| Black race | 220 (16%) | 138 (13%) | 58 (27%) | 13 (19%) | 11 (20%) | <.001 | |
| Weight, kg | 82 (23) | 81 (22) | 88 (24) | 87 (32) | 84 (23) | 0.001 | |
| Hypertension | 406 (30%) | 287 (28%) | 83 (38%) | 24 (35%) | 12 (21%) | 0.008 | |
| Diabetes | 136 (10%) | 95 (9%) | 30 (14%) | 6 (9%) | 5 (9%) | 0.234 | |
| Cause of death | Head trauma | 412 (31%) | 314 (31%) | 67 (31%) | 16 (24%) | 15 (27%) | 0.030 |
| Anoxia | 467 (35%) | 342 (34%) | 69 (32%) | 24 (36%) | 32 (58%) | ||
| Stroke | 436 (33%) | 329 (33%) | 74 (34%) | 25 (38%) | 8 (15%) | ||
| Other | 21 (2%) | 15 (2%) | 5 (2%) | 1 (2%) | |||
| Hepatitis C seropositive | 48 (4%) | 37 (4%) | 6 (3%) | 3 (4%) | 2 (4%) | 0.906 | |
| ECD | 250 (18%) | 180 (18%) | 50 (23%) | 13 (19%) | 7 (13%) | 0.184 | |
| DCD | 228 (17%) | 184 (18%) | 27 (12%) | 12 (18%) | 5 (9%) | 0.090 | |
| KDRI | 1.28 (0.41) | 1.25 (0.41) | 1.4 (0.41) | 1.4 (0.34) | 1.26 (0.37) | <.001 | |
| KDPI, % | 66 (24) | 64 (24) | 73 (21) | 76 (16) | 67 (19) | <.001 | |
| Admission to procurement, days | 4 (7) | 4 (8) | 3 (3) | 4 (5) | 2 (2) | 0.097 | |
| Admission SCr, mg/dL | 1.09 (0.6) | 1.1 (0.64) | 1.05 (0.35) | 0.89 (0.32) | 1.2 (0.84) | 0.008 | |
| Admission eGFR, ml/min/1.73m2 | 85 (29) | 84 (28) | 87 (27) | 99 (33) | 90 (43) | 0.004 | |
| Terminal SCr, mg/dL | 1.17 (0.88) | 0.87 (0.35) | 1.58 (0.52) | 2.08 (0.81) | 4.05 (1.81) | <.001 | |
| Admission SCr > Terminal SCr | 655 (48%) | 655 (64%) | <.001 | ||||
| Terminal urine output, ml/hr | 187 (148) | 190 (150) | 204 (147) | 156 (116) | 116 (126) | <.001 | |
| Machine perfusion used 2 | 554 (40%) | 387 (38%) | 90 (41%) | 41 (60%) | 36 (64%) | <.001 | |
| Procurement biopsy performed 2 | 672 (49%) | 452 (44%) | 121 (56%) | 50 (74%) | 49 (88%) | <.001 | |
| Biopsy report describing acute tubular necrosis in at least 1 kidney | 108 (8%) | 62 (6%) | 22 (10%) | 11 (16%) | 13 (23%) | <.001 | |
| Biopsy report describing ≥20% glomerulosclersosis in at least 1 kidney | 39 (3%) | 26 (3%) | 8 (4%) | 3 (4%) | 2 (4%) | 0.657 | |
| Kidneys transplanted | 1 | 171 (12%) | 109 (11%) | 33 (15%) | 16 (24%) | 13 (23%) | <.001 |
| 2 | 1198 (88%) | 918 (89%) | 185 (85%) | 52 (76%) | 43 (77%) | ||
| B: Recipient and transplant characteristics by donor AKI stage | |||||||
|---|---|---|---|---|---|---|---|
| Variable | ALL (N=2567) |
No AKI (N=1945) |
Stage 1 (N=403) |
Stage 2 (N=120) |
Stage 3 (N=99) |
P-value 1 | |
| Age, years | 53 (15) | 52 (15) | 55 (14) | 52 (13) | 55 (14) | 0.008 | |
| Male | 1576 (61%) | 1180 (61%) | 249 (62%) | 75 (63%) | 72 (73%) | 0.117 | |
| Black race | 1012 (39%) | 759 (39%) | 156 (39%) | 57 (48%) | 40 (40%) | 0.316 | |
| Hispanic ethnicity | 289 (11%) | 221 (11%) | 40 (10%) | 20 (17%) | 8 (8%) | 0.154 | |
| Cause of ESRD | Diabetes | 750 (29%) | 564 (29%) | 125 (31%) | 28 (23%) | 33 (33%) | 0.680 |
| Hypertension | 695 (27%) | 515 (26%) | 111 (28%) | 42 (35%) | 27 (27%) | ||
| Glomerulonephritis | 422 (16%) | 324 (17%) | 65 (16%) | 18 (15%) | 15 (15%) | ||
| Graft failure | 162 (6%) | 129 (7%) | 18 (4%) | 9 (8%) | 6 (6%) | ||
| Other or unknown | 538 (21%) | 413 (21%) | 84 (21%) | 23 (19%) | 18 (18%) | ||
| Dialysis duration, months | 52 (36) | 51 (36) | 52 (37) | 56 (37) | 48 (35) | 0.256 | |
| Preemptive transplant | 290 (11%) | 228 (12%) | 44 (11%) | 13 (11%) | 5 (5%) | 0.227 | |
| Pre-transplant blood transfusion | 443 (17%) | 335 (17%) | 67 (17%) | 22 (18%) | 19 (19%) | 0.925 | |
| Kidney pumped | 1019 (40%) | 718 (37%) | 165 (41%) | 72 (60%) | 64 (65%) | <.001 | |
| Cold ischemia time, hours | 15.4 (7.2) | 15 (7) | 15.5 (7.5) | 18.6 (7.2) | 18.8 (8.7) | <.001 | |
| HLA mismatch level | 0 | 160 (6%) | 130 (7%) | 20 (5%) | 6 (5%) | 4 (4%) | 0.599 |
| 1 | 20 (1%) | 18 (1%) | 2 (0%) | ||||
| 2 | 87 (3%) | 66 (3%) | 14 (3%) | 2 (2%) | 5 (5%) | ||
| 3 | 312 (12%) | 232 (12%) | 52 (13%) | 15 (13%) | 13 (13%) | ||
| 4 | 676 (26%) | 520 (27%) | 101 (25%) | 33 (28%) | 22 (22%) | ||
| 5 | 876 (34%) | 670 (35%) | 135 (34%) | 35 (29%) | 36 (37%) | ||
| 6 | 430 (17%) | 306 (16%) | 78 (19%) | 28 (24%) | 18 (18%) | ||
Values are mean (SD) or n (%). AKI, acute kidney injury; ECD, expanded-criteria donor; DCD, donation after cardiac death; KDRI, kidney donor risk index; KDPI, kidney donor profile index; SCr serum creatinine; eGFR, estimated glomerular filtration rate.
Kruskal-Wallis test for continuous variables and Chi-Square test for categorical variables
Variable considered at the level of the donor (i.e., information available for at least one of the kidneys from a particular donor)
Values are mean (SD) or n (%). AKI, acute kidney injury; ESRD, end-stage renal disease; HLA, human leukocyte antigen.
Kruskal-Wallis test for continuous variables and Chi-Square test for categorical variables
As shown in Table 1, the proportion of donors with biopsy-reported ATN significantly increased according to AKI stage. However, within the subset of 909 donors that had at least one procurement biopsy report (which included donors resulting in kidney discards), there was disagreement between ATN and AKI (Table S1). The majority (59%) of the donors with biopsy-reported ATN did not have clinically-defined AKI based on changes in SCr values.
A total of 171 (10%) donors had a single kidney discard, and both kidneys were discarded from 263 (16%) donors (Table 2). The proportion of donors with AKI differed significantly by kidney discard status (23%, 36% and 38% for none, one or both kidneys discarded, respectively; P<0.001), as did nearly all other donor characteristics. Table 2 also shows the reported reasons for discard, of which ‘biopsy’ was most common. From the individual kidney perspective, the rate of discard was higher for kidneys from donors with AKI (30% vs. 18% for kidneys from donors without AKI, P<0.001) (Table 3). Donor AKI was independently associated with kidney discard with an adjusted RR of 1.55 (95% confidence interval 1.34–1.79). In addition, a dose-response relationship was apparent for increasing donor AKI stage on the risk of discard with adjusted RRs of 1.28 (1.08–1.52), 1.82 (1.45–2.30) and 2.74 (2.0–3.75), respectively.
Table 2.
Donor characteristics by number of kidneys discarded
| Variable | ALL (N=1632) |
None Discarded (N=1198) |
One Discarded (N=171) |
Both Discarded (N=263) |
P-value 1 | |
|---|---|---|---|---|---|---|
| AKI stage | Any AKI | 443 (27%) | 280 (23%) | 63 (36%) | 101 (38%) | <.001 |
| Stage 1 | 274 (17%) | 185 (15%) | 33 (19%) | 56 (21%) | ||
| Stage 2 | 93 (6%) | 52 (4%) | 16 (9%) | 25 (10%) | ||
| Stage 3 | 76 (5%) | 43 (4%) | 13 (8%) | 20 (8%) | ||
| Age, years | 43 (16) | 39 (15) | 50 (12) | 57 (15) | <.001 | |
| Male | 974 (60%) | 737 (62%) | 92 (54%) | 145 (55%) | 0.041 | |
| Black race | 269 (16%) | 195 (16%) | 25 (15%) | 49 (19%) | 0.509 | |
| Weight, kg | 83 (24) | 82 (23) | 88 (26) | 86 (27) | 0.004 | |
| Hypertension | 587 (36%) | 317 (26%) | 89 (52%) | 181 (69%) | <.001 | |
| Diabetes | 136 (8%) | 102 (9%) | 34 (20%) | <.001 | ||
| Cause of death | Head trauma | 446 (28%) | 385 (33%) | 27 (16%) | 34 (13%) | <.001 |
| Anoxia | 538 (34%) | 401 (34%) | 66 (40%) | 71 (27%) | ||
| Stroke | 589 (37%) | 365 (31%) | 71 (43%) | 153 (59%) | ||
| Other | 23 (1%) | 18 (2%) | 3 (2%) | 2 (1%) | ||
| Hepatitis C seropositive | 69 (4%) | 26 (2%) | 22 (13%) | 21 (8%) | <.001 | |
| ECD | 418 (26%) | 186 (16%) | 64 (37%) | 168 (64%) | <.001 | |
| DCD | 255 (16%) | 198 (17%) | 30 (18%) | 27 (10%) | 0.031 | |
| KDRI | 1.38 (0.49) | 1.24 (0.39) | 1.59 (0.42) | 1.91 (0.52) | <.001 | |
| KDPI, % | 70 (24) | 63 (24) | 83 (16) | 91 (13) | <.001 | |
| Admission SCr, mg/dL | 1.11 (0.67) | 1.08 (0.56) | 1.12 (0.84) | 1.25 (0.93) | 0.002 | |
| Admission eGFR, ml/min/1.73m2 | 83 (30) | 86 (29) | 81 (30) | 70 (26) | <.001 | |
| Terminal SCr, mg/dL | 1.24 (0.95) | 1.14 (0.85) | 1.4 (1.03) | 1.57 (1.2) | <.001 | |
| Admission SCr > Terminal SCr | 745 (46%) | 593 (49%) | 62 (36%) | 90 (34%) | <.001 | |
| Terminal urine output, ml/hr | 184 (147) | 189 (145) | 174 (172) | 165 (141) | <.001 | |
| Machine perfusion used 2 | 665 (41%) | 476 (40%) | 78 (46%) | 111 (43%) | 0.217 | |
| Procurement biopsy performed 2 | 909 (56%) | 540 (45%) | 137 (80%) | 232 (88%) | <.001 | |
| Biopsy report describing acute tubular necrosis in at least 1 kidney | 155 (17%) | 81 (15%) | 27 (20%) | 47 (20%) | 0.137 | |
| Biopsy report describing ≥20% glomerulosclersosis in at least 1 kidney | 136 (15%) | 18 (3%) | 21 (15%) | 97 (42%) | <.001 | |
| Discard reason3 | Biopsy | 154 (35%) | 38 (22%) | 116 (44%) | <.001 | |
| Poor quality | 128 (29%) | 53 (31%) | 75 (29%) | 0.063 | ||
| Anatomical abnormalities | 75 (17%) | 45 (26%) | 30 (11%) | 0.105 | ||
| Vascular damage | 17 (4%) | 15 (9%) | 2 (1%) | 0.002 | ||
| Other | 29 (7%) | 14 (8%) | 15 (6%) | 1.00 | ||
| Not reported | 15 (3%) | 6 (4%) | 9 (3%) | 1.00 | ||
| Discordant reasons | 16 (4%) | 16 (6%) | ||||
Values are mean (SD) or n (%). AKI, acute kidney injury; ECD, expanded-criteria donor; DCD, donation after cardiac death; KDRI, kidney donor risk index; KDPI, kidney donor profile index; SCr serum creatinine; eGFR, estimated glomerular filtration rate.
Kruskal-Wallis test for continuous variables and Chi-Square test for categorical variables
Variable considered at the level of the donor (i.e., information available for at least one of the kidneys from a particular donor)
The same reason was reported for both discarded kidneys in all but 16 donors as shown. For these discordant reasons, ‘biopsy’ was most common–reported for one but not the other discarded kidney in 12 of these donors.
Table 3.
Risk of kidney discard by donor AKI status
| AKI Status | Number of Discards (%) |
Relative Risk (95% Confidence Interval) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1 | Adjusted 2 | ||
| No AKI | 433 (18%) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Any AKI | 264 (30%) | 1.64 (1.38–1.94) | 1.60 (1.38–1.85) | 1.55 (1.34–1.79) |
| No AKI | 433 (18%) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Stage 1 AKI | 145 (26%) | 1.45 (1.17–1.80) | 1.28 (1.07–1.52) | 1.28 (1.08–1.52) |
| Stage 2 AKI | 66 (35%) | 1.95 (1.49–2.55) | 1.95 (1.54–2.46) | 1.82 (1.45–2.30) |
| Stage 3 AKI | 53 (35%) | 1.91 (1.42–2.58) | 3.03 (2.23–4.13) | 2.74 (2.00–3.75) |
AKI, acute kidney injury.
Adjusted for donor variables that comprise the kidney donor risk index (KDRI), with the exception of terminal serum creatinine (i.e., age, height, weight, Black race, death from stroke, donation after cardiac death, and history of hypertension, diabetes, and hepatitis C seropositivity)
Includes donor variables listed above plus procurement biopsy performed and use of machine pump perfusion
Results for DGF are shown in Table 4. The DGF rate progressively increased from 28% for kidneys from donors without AKI to 34%, 52% and 57% for donor AKI stage 1, 2 and 3, respectively (trend test P<0.001). The adjusted RR of DGF for any donor AKI was 1.48 (1.30–1.68), and a dose response was again noted for increasing AKI stage with adjusted RRs for the development of DGF of 1.27 (1.09–1.49), 1.70 (1.37–2.12) and 2.25 (1.74–2.91), respectively.
Table 4.
Risk of delayed graft function by donor AKI status
| AKI Status | Number with DGF (%) |
Relative Risk (95% Confidence Interval) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1 | Adjusted 2 | ||
| No AKI | 543 (28%) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Any AKI | 257 (41%) | 1.50 (1.31–1.70) | 1.56 (1.37–1.77) | 1.48 (1.30–1.68) |
| No AKI | 543 (28%) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Stage 1 AKI | 139 (34%) | 1.25 (1.06–1.47) | 1.28 (1.09–1.49) | 1.27 (1.09–1.49) |
| Stage 2 AKI | 62 (52%) | 1.88 (1.53–2.30) | 1.82 (1.47–2.25) | 1.70 (1.37–2.12) |
| Stage 3 AKI | 56 (57%) | 2.03 (1.65–2.48) | 2.55 (2.04–3.21) | 2.25 (1.74–2.91) |
AKI, acute kidney injury; DGF, delayed graft function.
Adjusted for donor variables that comprise the kidney donor risk index (KDRI), with the exception of terminal serum creatinine (i.e., age, height, weight, Black race, death from stroke, donation after cardiac death, and history of hypertension, diabetes, and hepatitis C seropositivity)
Includes donor variables listed above plus procurement biopsy performed, use of machine pump perfusion, and cold ischemia time in hours
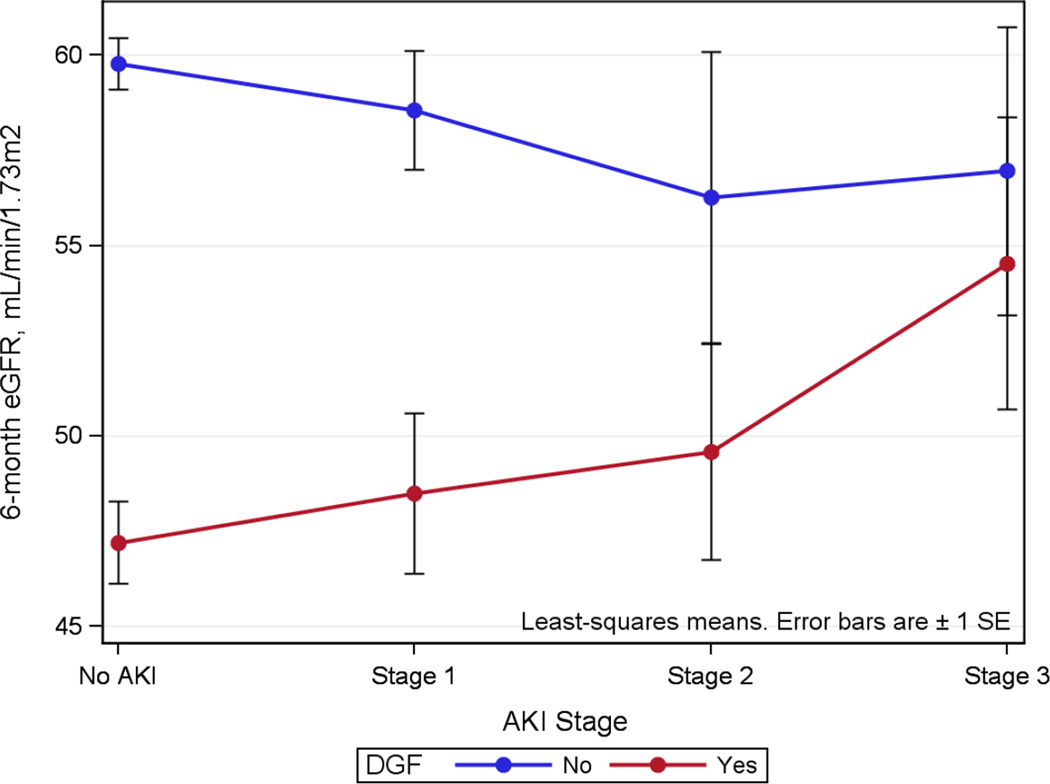
Allograft function at 6 months was not statistically different by donor AKI on its own (Table 5). Median 6-month eGFR for all recipients was 55 [40, 71] ml/min/1.73m2. Following stratification by DGF status, however, some differences were observed between donor AKI stages. For recipients that did not experience DGF, allograft function at 6 months was well-preserved and did not vary significantly by donor AKI stage with an overall 6-month eGFR of 58 [45, 75] ml/min/1.73m2. Overall 6-month eGFR for recipients with DGF was lower than those without DGF at 48 [31, 61] ml/min/1.73m2 (P<0.001), but allograft function was progressively better for the recipients with DGF that received kidneys from donors with increasing stages of AKI (Table 5 and Figure 2). Formal interaction testing via linear regression demonstrated a statistically significant 5 ml/min/1.73m2 average increase in 6-month eGFR for the combination of any donor AKI and the development of DGF (P=0.05 for the interaction term).
Table 5.
Allograft function at 6 months by donor AKI and stratified by DGF status
| 6-month SCr, mg/dl | 6-month eGFR, ml/min/1.73m2 | |||||
|---|---|---|---|---|---|---|
| All recipients (n=2567) |
Recipients with DGF (n=800) |
Recipients without DGF (n=1767) |
All recipients (n=2567) |
Recipients with DGF (n=800) |
Recipients without DGF (n=1767) |
|
| No Donor AKI (N=1945) | 1.34 [1.07, 1.7] | 1.52 [1.27, 2.1] | 1.3 [1.0, 1.6] | 55 [41, 72] | 46 [29, 60] | 59 [45, 75] |
| Stage 1 AKI (N=403) | 1.35 [1.1, 1.8] | 1.5 [1.2, 1.95] | 1.3 [1.1, 1.6] | 54 [40, 68] | 49 [32, 64] | 56 [46, 72] |
| Stage 2 AKI (N=120) | 1.5 [1.15, 1.75] | 1.54 [1.3, 1.85] | 1.3 [1.0, 1.65] | 53 [38, 67] | 52 [36, 59] | 55 [40, 74] |
| Stage 3 AKI (N=99) | 1.3 [1.1, 1.68] | 1.3 [1.1, 1.75] | 1.3 [0.97, 1.6] | 59 [40, 73] | 58 [39, 71] | 59 [41, 78] |
| P-value 1 | 0.276 | 0.049 | 0.497 | 0.377 | 0.060 | 0.537 |
Values are median [interquartile range]. AKI, acute kidney injury; DGF, delayed graft function; eGFR, estimated glomerular filtration rate; SCr, serum creatinine
Kruskal-Wallis test.
Figure 2.
Linear regression interaction plot for donor acute kidney injury (AKI) and delayed graft function (DGF) on predicted 6-month estimated glomerular filtration rate (eGFR). P=0.05 for the interaction term (any AKI*DGF).
While follow-up data beyond 6 months are not yet complete for the cohort, an interaction was again observed between donor AKI and DGF on subsequent allograft function for recipients with available 12-month eGFR values (Table S2). Compared with no donor AKI, the risk of death-censored graft failure as currently reported was not significantly different for any donor AKI [adjusted hazard ratio (HR) of 1.23 (0.89–1.71)], nor by donor AKI stage [adjusted HRs of 1.28 (0.88–1.87), 0.98 (0.48–2.0) and 1.35 (0.66–2.77), respectively for AKI stage 1, 2 and 3]. Furthermore, no associations between donor AKI and death-censored graft failure were noted after stratifying by DGF status (not shown); however, DGF itself had an adjusted HR for death-censored graft failure of 3.09 (2.30–4.17).
Discussion
The expanding organ transplant shortage has naturally led to more aggressive organ procurement considerations; however, transplanting clinically injured kidneys raises logical concerns about poorer outcomes and increasing healthcare costs. To critically examine the issue of AKI in deceased donors, we performed the largest multicenter observational study of its kind to date in order to assess additional donor information that is not available in the UNOS database (i.e., changes in SCr). We found that clinically-defined AKI is common in deceased kidney donors and that the severity of AKI is, as expected, associated with increasing rates of kidney discard as well as DGF. Accounting for additional donor and transplant characteristics, the risk of discard as well as the risk of DGF was over 2-fold higher for kidneys from donors with stage 3 AKI compared with no AKI. However, our primary hypothesis about a relationship between donor AKI and poorer 6-month allograft function was not substantiated by these data. Furthermore, we found evidence for a favorable interaction between donor AKI and DGF on subsequent 6-month eGFR.
In native kidneys, the severity of AKI tends to associate with poorer clinical outcomes including the development of chronic kidney disease and end-stage renal disease (6). In kidney transplantation, DGF is the traditional surrogate exposure for severe AKI at the time of transplant and is a known risk factor for poorer allograft function and survival (14–16). Based on this prior evidence, it seemed reasonable to suppose that even earlier episodes of renal injury in the donor immediately leading up to the period of complete ischemia during organ transport would have detrimental effects. While there is credence to the argument that organ discard and dialysis decisions immediately post-transplant are subjective outcomes (i.e., influenced by the accepting clinician’s knowledge about the donor), the fact that we observed no detrimental effect of donor AKI on an objective measure of allograft function at 6 months post-transplant provides reasonably solid evidence in support of the current clinical practice for utilizing these kidneys. One can only speculate, however, about potential transplant outcomes for discarded kidneys in the current cohort, which is an unavoidable limitation of the observational study design. It is quite possible that the discarded AKI kidneys would have had worse outcomes than those that were selected for transplantation.
Data from trials that effectively reduce donor AKI at procurement and include ‘protocolized’ organ acceptance algorithms with prospective recipient follow-up would advance our understanding of the true impact of donor AKI. Such research protocols are logistically quite difficult, however, considering the level of cooperation needed between multiple stakeholders, including informed consent for donor surrogates as well as potential recipients. Nonetheless, a growing body of literature from observational studies does address the relative importance of deceased-donor AKI. Using specific cutoffs for the single terminal SCr value (rather than changes in SCr) to define donor AKI severity within the Scientific Registry of Transplant Recipients database, Kayler et al. described progressively worse long-term allograft survival for recipients of expanded-criteria donor (ECD) kidneys with increasing “AKI” severity but not for standard-criteria donor (SCD) kidneys (17). The authors also determined that the risks for kidney discard and DGF were greater for these “AKI” kidneys regardless of ECD/SCD status. More recently, Klein et al. also used the single terminal SCr value to define AKI (>1.5 mg/dl) in a single-center cohort of 1235 deceased donors and noted higher rates of DGF but no differences in 1-year patient, allograft or rejection-free survival (18). The handful of other studies conducted to address this issue appropriately utilized changes in SCr values to define donor AKI but were all single-center and had relatively small numbers (19–26). All but one of these prior studies reported higher rates of DGF and no discernable differences in intermediate or longer-term allograft function or survival. While our results corroborate these prior studies, our multicenter data also help to explain these somewhat counterintuitive findings considering the evidence for AKI’s detrimental effect on subsequent function in native kidney (non-transplant) settings.
We found that while kidneys from donors with AKI (which have been selected for transplant) are at increased risk for DGF, they appear to provide similar 6-month allograft function compared with kidneys from donors without AKI. The lack of association with this continuous outcome cannot be dismissed as a power issue, though such an argument could have been considered had we specified a dichotomous, low-event outcome like graft failure within a period for which we had complete follow-up data (i.e., 6 months). Finding a significant favorable interaction between donor AKI and DGF on 6-month eGFR was unexpected, however, and suggests that current AKI kidney utilization practices are quite reasonable for selected kidney-recipient matches. In their report of a protocol to utilize AKI kidneys, Farney et al. noted a similar interaction between donor AKI and DGF on subsequent allograft outcomes (21). They found that donor AKI nullified the deleterious effect of DGF on allograft survival. This does not mean that donor AKI directly improves long-term allograft function. Rather, it appears to modify the effects of DGF. The clinical interpretation could be that DGF resulting from donor AKI is less worrisome (in terms of long-term function) than DGF from other causes.
An epidemiological explanation for this apparent effect modification is that AKI kidneys selected for transplant predominantly come from otherwise high-quality donors. This also likely explains the association we observed between donor AKI and organ discard. Donors for which both kidneys were discarded had the highest AKI burden, but they were also biopsied more frequently and had more significant glomerulosclerosis than biopsied donors that resulted in transplants. High rates of biopsy likely reveal underlying concerns about donor quality, which may or may not be related to increasing SCr values. Thus, one could argue that clinical gestalt drives discard decisions, with histopathologic findings sealing the fate of the unrealized transplant. Nonetheless, our data indicate that among the kidneys selected for transplant, those with AKI tended to come from younger donors that were less likely to die from stroke (i.e., were likely otherwise higher quality) than the non-AKI kidneys.
Besides the obvious donor selection issue, however, other potential mechanistic explanations for the interaction between donor AKI and DGF on 6-month allograft function are worthy of further investigation. Ischemic preconditioning is a particularly intriguing possibility. The inflammatory/repair pathways initiated in kidneys from donors with AKI prior to procurement may make them more prone to reperfusion injury and the development of DGF. Those previously activated pathways, however, could potentially make AKI kidneys more capable of subsequent successful repair with better intermediate and long-term function than kidneys from donors without AKI that develop DGF from other causes.
Limitations of the current study predominantly stem from its observational design. We adjusted for multiple donor factors using the variables that comprise the KDRI, but residual confounding is still possible given transplant centers accept or reject organ offers based on center-specific protocols and clinical judgement. While we consider the multicenter design a strength with regard to generalizability, study outcomes were limited to available UNOS database information. In addition, we do not have complete follow-up data beyond 6 months; however, we specifically chose 6-month eGFR as the primary outcome for several reasons. First, 6 months is early enough post-transplant to be considered representative of baseline allograft function and reflective of kidney (donor) quality at the time of transplant. Second, the 6-month time-point is sufficiently beyond the period of rapid fluid shifts, variable allograft recovery phases and frequent adjustments to immunosuppression. Third, prior data demonstrate the relative stability in allograft function beyond 6 months in the current transplant era (27). Lastly, we reasoned that if any donor-level characteristic were to have a large effect on long-term allograft survival, we should begin to see a trend for that effect by 6 months (28). Notwithstanding, the fact that results were relatively unchanged using available 12-month, rather than complete 6-month, eGFR data indicates the overall robustness of these findings.
In conclusion, clinical situations that involve patient death in the hospital and considerations for organ donation can be quite variable, and as the current study shows, AKI is a common occurrence in these scenarios. While organ allocation is a regimented process by design, organ acceptance or decline is a decision based on clinical judgment, which can vary in complexity depending on the situation and a large number of factors. Donor AKI is an influential factor, but as has been shown with other donor risk factors like DCD status (29, 30), our data also indicate that current acceptance practices for AKI kidneys yield (eventually) acceptable allograft function despite higher DGF rates. A separate but related question that deserves proper investigation is whether novel AKI biomarkers provide added decision-making value in this context, as limited data from our group and others would suggest (31–34). Better understanding of the relative importance of and interaction between certain traditional risk factors, like donor AKI and DGF, will likely facilitate the optimization of organ allocation, acceptance and transplantation moving forward. Nonetheless, our current findings suggest that there is room for cautious expansion of the deceased-donor kidney pool by considering more aggressive utilization of these kidneys for transplantation.
Supplementary Material
Acknowledgments
We are tremendously grateful for the study participation of the following organ procurement organizations: Gift of Life Philadelphia, the New York Organ Donor Network, the Michigan Organ and Tissue Donation Program, the New Jersey Sharing Network, and the New England Organ Bank. We also thank the study coordinators at the following transplant centers: University of Pennsylvania Transplant Institute, Barnabas Health Renal and Pancreas Transplant Division, Mount Sinai’s Recanati/Miller Transplantation Institute, Harper Hospital Transplant Program, and Yale-New Haven Transplantation Center. This work was supported by the National Institutes of Health grant RO1DK-93770, grant K24DK090203, and a Roche Organ Transplantation Research Foundation Award to Dr. Parikh, an award from the American Heart Association to Dr. Hall, and the Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation.
Abbreviations
- AKI
acute kidney injury
- AKIN
Acute Kidney Injury Network
- ATN
acute tubular necrosis
- DCD
donation after cardiac death
- DGF
delayed graft function
- ECD
expanded-criteria donor
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- KDRI
kidney donor risk index
- KDPI
kidney donor profile index
- OPO
organ procurement organization
- OPTN
Organ Procurement and Transplantation Network
- RR
relative risk
- SCD
standard criteria donor
- SCr
serum creatinine
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008;8(4):783–792. doi: 10.1111/j.1600-6143.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg DS, Halpern SD, Reese PP. Deceased organ donation consent rates among racial and ethnic minorities and older potential donors. Crit Care Med. 2013;41(2):496–505. doi: 10.1097/CCM.0b013e318271198c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathur AK, Ashby VB, Sands RL, Wolfe RA. Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant. 2010;10(4 Pt 2):1069–1080. doi: 10.1111/j.1600-6143.2010.03043.x. [DOI] [PubMed] [Google Scholar]
- 4.Garonzik-Wang JM, James NT, Weatherspoon KC, Deshpande NA, Berger JA, Hall EC, et al. The aggressive phenotype: center-level patterns in the utilization of suboptimal kidneys. Am J Transplant. 2012;12(2):400–408. doi: 10.1111/j.1600-6143.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 7.Hall IE, Reese PP, Weng FL, Schroppel B, Doshi MD, Hasz RD, et al. Preimplant histologic acute tubular necrosis and allograft outcomes. Clin J Am Soc Nephrol. 2014;9(3):573–582. doi: 10.2215/CJN.08270813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.OPTN: Organ Procurement and Transplantation Network. A Guide to Calculating and Interpreting KDPI. [cited 2013 January 17];2012 Available from: http://optn.transplant.hrsa.gov/resources/allocationcalculators.asp?index=81.
- 10.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 13.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. USA: Oxford University Press; 2002. [Google Scholar]
- 14.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 16.Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360(1):7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 17.Kayler LK, Garzon P, Magliocca J, Fujita S, Kim RD, Hemming AW, et al. Outcomes and utilization of kidneys from deceased donors with acute kidney injury. Am J Transplant. 2009;9(2):367–373. doi: 10.1111/j.1600-6143.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Galante NZ, de Sandes-Freitas TV, de Franco MF, Tedesco-Silva H, Medina-Pestana JO. Transplantation with kidneys retrieved from deceased donors with acute renal failure. Transplantation. 2013;95(4):611–616. doi: 10.1097/TP.0b013e318279153c. [DOI] [PubMed] [Google Scholar]
- 19.Kolonko A, Chudek J, Pawlik A, Wilk J, Jalowiecki P, Wiecek A. Acute kidney injury before organ procurement is associated with worse long-term kidney graft outcome. Transplant Proc. 2011;43(8):2871–2874. doi: 10.1016/j.transproceed.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Deroure B, Kamar N, Depreneuf H, Jacquet A, Francois H, Charpentier B, et al. Expanding the criteria of renal kidneys for transplantation: use of donors with acute renal failure. Nephrol Dial Transplant. 2010;25(6):1980–1986. doi: 10.1093/ndt/gfq009. [DOI] [PubMed] [Google Scholar]
- 21.Farney AC, Rogers J, Orlando G, al-Geizawi S, Buckley M, Farooq U, et al. Evolving experience using kidneys from deceased donors with terminal acute kidney injury. J Am Coll Surg. 2013;216(4):645–655. doi: 10.1016/j.jamcollsurg.2012.12.020. discussion 55-6. [DOI] [PubMed] [Google Scholar]
- 22.Jung CW, Park KT, Kim SY, Kim SJ, Kim MG, Jo SK, et al. Clinical outcomes in kidney transplantation patients from deceased donors with acute kidney injury. Transplant Proc. 2013;45(8):2941–2945. doi: 10.1016/j.transproceed.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Anil Kumar MS, Khan SM, Jaglan S, Heifets M, Moritz MJ, Saeed MI, et al. Successful transplantation of kidneys from deceased donors with acute renal failure: Three-year results. Transplantation. 2006;82(12):1640–1645. doi: 10.1097/01.tp.0000250908.62948.8f. [DOI] [PubMed] [Google Scholar]
- 24.Lee MH, Jeong EG, Chang JY, Kim Y, Kim JI, Moon IS, et al. Clinical outcome of kidney transplantation from deceased donors with acute kidney injury by Acute Kidney Injury Network criteria. Journal of critical care. 2014;29(3):432–437. doi: 10.1016/j.jcrc.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigo E, Minambres E, Pinera C, Llorca J, Fernandez-Fresnedo G, Vallejo A, et al. Using RIFLE criteria to evaluate acute kidney injury in brain-deceased kidney donors. Nephrol Dial Transplant. 2010;25(5):1531–1537. doi: 10.1093/ndt/gfp622. [DOI] [PubMed] [Google Scholar]
- 26.Yuan XP, Han M, Wang XP, Zhou J, Jiao XY, Wang CX, et al. Kidney transplantation from cardiac death donors with terminal acute renal failure. Transplant Proc. 2014;46(4):1057–1060. doi: 10.1016/j.transproceed.2013.11.055. [DOI] [PubMed] [Google Scholar]
- 27.Kasiske BL, Gaston RS, Gourishankar S, Halloran PF, Matas AJ, Jeffery J, et al. Long-term deterioration of kidney allograft function. Am J Transplant. 2005;5(6):1405–1414. doi: 10.1111/j.1600-6143.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 28.Pascual J, Marcen R, Zamora J, Fernandez AM, Burgos FJ, Villafruela JJ, et al. Very early serum creatinine as a surrogate marker for graft survival beyond 10 years. J Nephrol. 2009;22(1):90–98. [PubMed] [Google Scholar]
- 29.Cooper JT, Chin LT, Krieger NR, Fernandez LA, Foley DP, Becker YT, et al. Donation after cardiac death: the university of wisconsin experience with renal transplantation. Am J Transplant. 2004;4(9):1490–1494. doi: 10.1111/j.1600-6143.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 30.Singh RP, Farney AC, Rogers J, Zuckerman J, Reeves-Daniel A, Hartmann E, et al. Kidney transplantation from donation after cardiac death donors: lack of impact of delayed graft function on post-transplant outcomes. Clin Transplant. 2011;25(2):255–264. doi: 10.1111/j.1399-0012.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- 31.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR. Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol. 2012;7(8):1224–1233. doi: 10.2215/CJN.00310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall IE, Bhangoo RS, Reese PP, Doshi MD, Weng FL, Hong K, et al. Glutathione S-transferase iso-enzymes in perfusate from pumped kidneys are associated with delayed graft function. Am J Transplant. 2014;14(4):886–896. doi: 10.1111/ajt.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollmen ME, Kyllonen LE, Inkinen KA, Lalla ML, Merenmies J, Salmela KT. Deceased donor neutrophil gelatinase-associated lipocalin and delayed graft function after kidney transplantation: a prospective study. Crit Care. 2011;15(3):R121. doi: 10.1186/cc10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field M, Dronavalli V, Mistry P, Drayson M, Ready A, Cobbold M, et al. Urinary biomarkers of acute kidney injury in deceased organ donors--kidney injury molecule-1 as an adjunct to predicting outcome. Clin Transplant. 2014;28(7):808–815. doi: 10.1111/ctr.12383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.