Abstract
Environmental and lifestyle factors are considered significant components of the increasing breast cancer risk in the last 50 years. Specifically, exposure to environmental endocrine disrupting compounds is correlated with cancer susceptibility in a variety of tissues. In both human and rodent models, the exposure to ubiquitous environmental estrogens during early life has been shown to disrupt normal mammary development and cause permanent adverse effects. Recent studies indicate that environmental estrogens not only have the ability to disrupt estrogen receptor (ER) signaling, but can also reprogram the epigenome by altering DNA and histone methylation through rapid, nongenomic ER actions. We have observed xenoestrogen-mediated activation of several nongenomic signaling pathways and have identified a target for epigenetic reprogramming in MCF-7 breast cancer cells. These observations, in addition to data from the literature, support the hypothesis that activation of rapid signaling by environmental estrogens can lead to epigenetic reprogramming and contribute to the progression of breast cancer.
Keywords: Breast cancer, epigenetics, environmental estrogens, histone modifiers
1. Introduction
1.1 Early life exposure to environmental estrogens and breast cancer risk
Breast cancer risk is now understood to have both genetic and environment/lifestyle components [1, 2]. Inherited defects in tumor suppressor genes are known risk factors for cancer, and genome-wide association studies have identified more than 20 common genetic susceptibility variants of genes conferring increased breast cancer risk [3]. Less than a quarter of all breast cancers, however, can be attributed to these known susceptibility variants [4]. It has been hypothesized that the increase in the incidence of breast cancer during the last 50 years may be due to environmental exposures, most notably to hormonally active chemicals often categorized as endocrine disrupting compounds (EDCs) [5]. In fact, such compounds have been identified as priority chemicals, among others, for future breast cancer research [6].
It is well known that the exposure of tissues or organs to an adverse environment or stimulus during critical developmental periods can increase the susceptibility to many diseases in adulthood such as cardiovascular disease, obesity, and cancer [7, 8]. According to the developmental origins of health and disease (DOHaD) hypothesis, these adverse exposures reprogram normal physiologic responses, leading to lifelong changes in disease susceptibility [9, 10]. Gestation/neonatal life is a critical period of mammary gland development likely to be altered by EDC exposure [11] and evidence has emerged that early life EDC exposure disrupts normal mammary gland development, and modulates susceptibility to breast cancer [reviewed in [12]]. For example, prenatal exposure to the xenoestrogen bisphenol A (BPA) results in increased mammary tumor multiplicity, decreased tumor latency, and a shifted window of susceptibility to 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary tumorigenesis in rats [13, 14]. Although a variety of compounds have been linked to breast cancer development/progression[6], here we focus on environmental estrogens, EDCs (such as BPA) that have estrogenic properties, where there is a known correlation between exposure and breast cancer risk [15, 16].
2. Epigenetic modifications in breast development and disease
Exposure to environmental EDCs is now thought to disrupt the epigenome in the breast [reviewed in [17]] and understanding how “epigenetic” dysregulation by EDCs may contribute to breast tumorigenesis is crucial for gaining insights into the etiology of this disease, and will be important for addressing breast cancer prevention.
The term “epigenetics” refers to “heritable changes in gene expression that are not caused by alteration in the DNA sequence of the genome”. Epigenetic alterations can occur via changes in DNA methylation, histone modifications and non-coding RNAs. Specifically, histone modification involves an elegant network of epigenetic modifiers that include enzymes that add specific histone marks (“writers”), proteins that can recognize the modifications (“readers”) and enzymes that can remove the modifications (“erasers”). These modifiers dynamically and tightly regulate chromatin conformation and gene transcription, are regulated by internal and external environmental signals and can have a significant impact on normal mammary gland development and perhaps even mammary tumorigenesis. Although few studies have examined the epigenetic effects of xenoestrogens in breast cancer, there is evidence that BPA treatment of human mammary progenitor and epithelial cells in vitro promotes hypermethylation of putative tumor suppressor genes [18, 19] and alters microRNA expression[20]. Phytoestrogens have been reported to modify the methylation of breast cancer-related genes [21, 22]. Furthermore, in utero exposure to BPA or diethylstilbestrol (DES) increases expression of enhancer of zeste homolog 2 (EZH2), the histone methyltransferase for the histone 3 (H3) lysine 27 (K27) trimethyl (me3) mark (H3K27me3) in mammary tissues [23]. Interestingly, over-expression of EZH2 has been reported in breast cancer and is a marker of aggressive breast cancer [24]. The latter study offers a tantalizing clue that histone modifier proteins are indeed altered by EDC exposure.
3. Rapid, nongenomic signaling through the estrogen receptor
Despite the aforementioned findings, few studies have investigated the mechanisms by which environmental estrogens disturb the epigenome, specifically histone modification, and drive breast carcinogenesis. There are a number of EDCs (or their metabolites) that can bind and activate estrogen receptors (ERs), including the pharmaceutical DES, organochlorine pesticides such as methoxychlor, industrial chemicals such as BPA, and phytoestrogens such as genistein [reviewed in [25]]. Activation of ER elicits biological responses in target tissues through both genomic (i.e. binding to DNA) and nongenomic, or more precisely, pregenomic (i.e. rapid signaling not requiring DNA binding) mechanisms. Genomic ER action involves the binding of ligand to the receptor, which induces a conformational change that promotes dissociation from chaperone protein complexes, and binding of receptors to hormone response elements on DNA. This binding is followed by dynamic and sequential recruitment of coregulator proteins to regulate gene transcription [reviewed in [26]].
Nongenomic ER action, usually extranuclear and membrane localized, involves rapid activation of kinases and resulting downstream signaling pathways that mediate biological responses independent of ER nuclear localization [reviewed in [26]]. Studies have shown that ER-mediated nongenomic activation of phosphoinositide 3-kinase/Akt (PI3K/Akt), c-Src/mitogen-activated protein kinase (Src/MAPK), and MAPK occurs in endothelial, breast and pancreatic β cells, respectively [reviewed in [27]]. These effects are initiated at the membrane, presumably through the classical ER [either ERα or ERβ; reviewed in [27]], which can localize to the membrane due to a conserved palmitoylation domain [28]. Recently, Pedram et al. generated a knock-in mouse that expresses a mutant ER incapable of being palmitoylated (hence, does not localize to the membrane) and demonstrated that membrane ER signaling plays crucial roles in hormone-mediated gene transcription, as well as reproductive tract and breast development [29]. Specifically, the homozygous, but not heterozygous, knock-in mice exhibited diminished ductal side branching and formation of blunted duct termini [29], highlighting the importance of nongenomic signaling in mammary gland development (and possibly disease). It is important to note that although nongenomic signaling does not directly involve ER binding to target genes, altered gene transcription may still occur as the final downstream impact of this activation.
Importantly, EDCs that are not categorized as environmental estrogens may disrupt ER signaling indirectly through the activation of the aryl hydrocarbon receptor (AhR) or through modification of estrogen metabolism [reviewed in [25]]. Studies to date have focused on the transcriptional effect of AhR activation and subsequent disruption of ER activity in response to EDCs [reviewed in [25]], but AhR signaling can also occur through nongenomic pathways [30–35] highlighting the need to examine activation of nongenomic signaling pathways through receptors other than ER.
4. Activation of nongenomic signaling and epigenetic reprogramming by environmental estrogens in breast cancer cells
4.1 Epigenetic modifier proteins as targets of nongenomic signaling
Studies have shown that activating nongenomic signaling pathways can regulate the function of epigenetic programmers in a variety of model systems [reviewed in [36] and Treviño LS et al. Progress in Biophysics & Molecular Biology in press]. For example, activation of protein kinase A (PKA) signaling results in phosphorylation and activation of the JmjC demethylase PHD finger protein 2 (PHF2), leading to association of PHF2 with AT-rich interactive domain 5B (ARID5B) [37]. This association results in the demethylation of methylated ARID5B and targeting of the PHF2-ARID5B complex to promoters of target genes where it removes the histone H3 (H3) lysine 9 dimethyl (me2) mark (H3K9me2) [37]. Evidence suggests a possible tumor suppressor role for PHF2 in breast cancer due to the fact that PHF2 alterations (deletion/methylation) were shown to be associated with poor patient survival [38].
Few studies have investigated if and how environmental estrogens activate these pathways to drive epigenetic reprogramming in breast cancer. We have previously shown that treatment of MCF7 cells with DES results in a global decrease in the repressive histone mark H3K27me3 [39]. This decrease is likely through rapid, DES-mediated activation of the PI3K/Akt pathway, which results in a site-specific phosphorylation on serine 21 and subsequent inactivation of the H3K27me3 methyltransferase EZH2 [39]. Consistent with loss of this repressive epigenetic mark, hormone-responsive gene expression is increased [39].
To date, only EZH2 has been identified as target of nongenomic signaling in breast cancer cells and it is clear that further study is needed to identify additional targets. Prediction algorithms can aid in this process. For example, the histone H3 lysine 9 (H3K9) demethylase lysine (K)-specific demethylase 4B (KDM4B/JMJD2B) has been shown to promote breast cancer proliferation and tumorigenesis by facilitating transcription of ER-responsive genes [40, 41], but no studies have examined its regulation by site-specific phosphorylation in breast cancer thus far. In fact, in silico analysis of the histone H3 lysine 9 (H3K9) demethylase lysine (K)-specific demethylase 4B (KDM4B/JMJD2B) reveals that it may be a putative substrate for Akt [42].
Preliminary data from our lab suggests that two additional signaling pathways, PKA and p44/p42 mitogen-activated protein kinase (MAPK), might be activated by DES in MCF-7 cells as well (data not shown). Further experiments are necessary to determine whether these pathways are indeed activated by ER-mediated nongenomic signaling and to identify epigenetic targets of this signaling. Verification that DES can activate multiple cell signaling pathways would expand the opportunity for nongenomic signaling to engage epigenetic “readers, writers and erasers” in addition to EZH2, and modify their activity.
4.2 Recommendations for future in vitro experiments
The aforementioned in vitro studies suggest that xenoestrogens, such as DES, may activate nongenomic signaling pathways to disrupt the epigenome in breast cancer cells, however, more studies are required to determine the effect of this disruption on breast tumorigenesis. Breast cancer is a complex and heterogeneous disease with a variety of subtypes, each exhibiting different prognosis and treatment response. Since MCF-7 cells represent just one of those subtypes (luminal A)[43], studies in multiple breast cancer cell lines are warranted. These additional studies are especially important in light of the fact that breast cancer cell lines exhibit diversity in estrogen receptor expression [43], which will impact the ability of xenoestrogens to activate nongenomic signaling pathways in a particular cell line.
Thus far, the discussion has focused on activation of nongenomic signaling pathways by the pharmaceutical DES, a model test compound for ER signaling. However, present-day exposure to DES is limited and investigation of other EDCs is needed. DES has been shown to be associated with increased breast cancer risk in rodents and humans, but this association is still a matter of debate [16, 44–47]. Despite this debate, concerns have been raised that other endogenous and exogenous estrogens and estrogen mimics could contribute to breast cancer etiology and/or progression.
One such exogenous estrogen is the ubiquitous industrial chemical bisphenol A (BPA). CDC studies have shown that virtually all Americans have detectable levels of BPA in their urine [48] and unconjugated BPA is detectable in human cord blood[49]. A limited number of studies have examined the effects of BPA in mammary gland development and tumorigenesis. Mice exposed to BPA in utero exhibit altered fetal mammary gland morphogenesis, an increase in ductal extension and a delay in lumen formation at embryonic day 18 [50]. In utero exposure to BPA also has the ability to cause adverse effects in adult life. Mammary tissue of exposed mice is hypersensitive to hormones, with an increase in the number of terminal end buds (TEB), as well as increased TEB area and density [51–53]. In CD-1 female mice, intraductal hyperplasias (pre-cancerous lesions that give rise to mammary adenocarcinomas) were observed exclusively in BPA-exposed animals [54]. As mentioned previously, early-life BPA exposure alters susceptibility to DMBA-induced carcinogenesis in rats [13, 14]. These experimental findings point to a possible association between BPA exposure and breast tumorigenesis, however, the mechanisms underlying this association have not been elucidated. It is possible that, similar to DES, BPA (and other xenoestrogens) can also activate nongenomic signaling pathways leading to post-translational modifications of epigenetic modifiers and subsequent reprogramming in breast cancer cells.
5. Concluding remarks
In vitro studies in breast cancer cells serve as a foundation for the hypothesis that xenoestrogens activate nongenomic signaling pathways, leading to post- translational modification of histone modifiers and subsequent epigenetic reprogramming in the developing mammary gland to increase susceptibility to breast cancer (see Figure 1 for model). Estrogen exposure has been shown to activate PI3K/Akt signaling, resulting in phosphorylation and inactivation of EZH2, as well as decreased H3K27me3 and increased target gene expression in mouse mammary epithelial cells [29]. Interestingly, this activation is not seen in mammary epithelial cells from knock-in mice that express a mutant ER incapable of being palmitoylated (hence, does not localize to the membrane) [29]. These data highlight a role for membrane ER signaling in normal mammary gland development and possibly breast tumorigenesis since the effect PI3K/Akt activation on epigenetic reprogramming is conserved between normal breast and breast cancer cells. Future in vivo studies utilizing models that exhibit susceptibility to mammary tumors are needed.
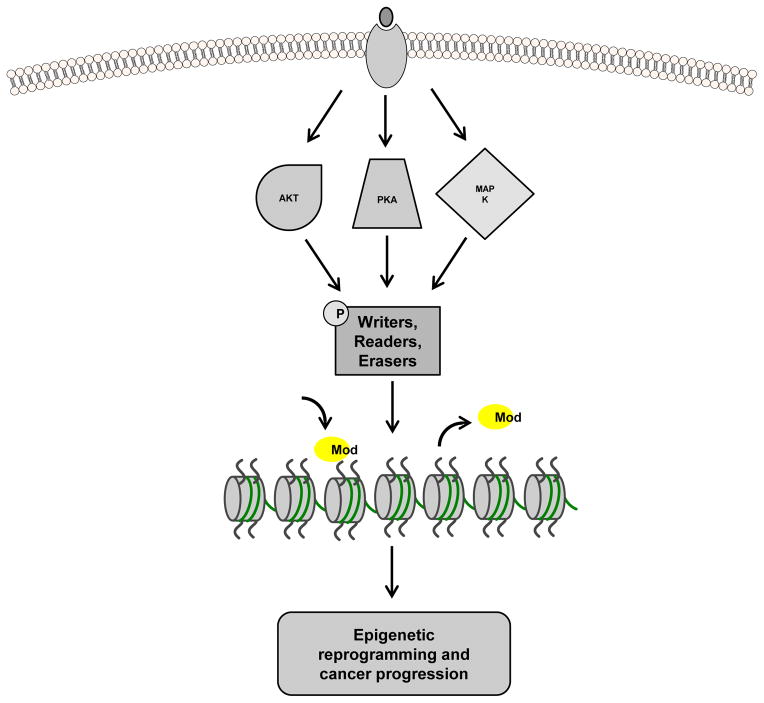
Figure 1.
Model of epigenetic reprogramming by xenoestrogens and breast cancer risk. Xenoestrogens activate cell-signaling pathways, including the PI3K/Akt, PKA, and MAPK pathways (among others), leading to phosphorylation of “writer”, “reader”, and “eraser” epigenetic modifier proteins. These phosphorylation events can serve to activate or inactivate the modifier proteins resulting in alteration (either addition or loss) of specific histone modifications (Mod) and epigenetic reprogramming of target genes (i.e. oncogenes and tumor suppressors) that could ultimately contribute to breast tumorigenesis.
There is evidence, however, that epigenetic reprogramming induced by environmental estrogens can influence tumor development in other tissues. For example, neonatal exposure to BPA sensitized the rat prostate gland to adult induced hormonal carcinogenesis and significantly increased incidence of prostatic intraepithelial neoplasia (PIN), a precursor to prostate cancer [55]. Furthermore, differentially methylated candidate genes were identified, a subset of which are members of signal transduction pathways, including MAPK/ERK, phosphokinase C and cAMP pathways [55]. In a separate study, Prins et al. reported that BPA stimulates human prostate stem cell renewal and activates PI3K/Akt and ERK in these cells [56]. In addition, similar to the study in rats, BPA enhances estrogen-driven carcinogenesis of human prostate epithelial xenografts [56].
Our group has shown that the dietary estrogen genistein activates PI3K/Akt in the uterus, increases phosphorylation of EZH2, and decreases H3K27me3 levels, resulting in hypersensitivity of target genes to estrogen [57, 58]. Additionally, neonatal exposure to genistein significantly increases uterine tumor incidence and multiplicity in rats [58]. Interestingly, BPA exposure was unable to induce nongenomic signaling in the uterus, and in contrast to DES, did not increase uterine tumorigenesis, indicating nongenomic signaling and engagement of epigenetic programmers exhibits both xenoestrogen- and tissue-specific effects [58].
Taken together, the in vitro studies and in vivo studies in the prostate and the uterus support the hypothesis that environmental estrogen exposure, in particular during early life, may indeed alter susceptibility to tumor formation in the adult through epigenetic programming of target tissues. Since the ability of EDCs to activate nongenomic signaling exhibits tissue specificity, it is not sufficient to merely extrapolate effects in other tissues to the mammary gland. As such, it is now important to move this research into the mammary gland to determine whether environmental estrogens activate nongenomic signaling to induce epigenetic reprogramming is an underlying mechanism by which these exposures increase breast cancer risk.
Supplementary Material
Highlights.
Xenoestrogens modulate histone methyl mark expression in breast cancer cells
Xenoestrogens activate multiple cell signaling pathways in breast cancer cells
Histone “writers”, “readers” and “erasers” are targets of these signaling pathways
Epigenetic reprogramming by xenoestrogens may play a role in breast carcinogenesis
Acknowledgments
C.L.W. is supported by grants from the Cancer Prevention Research Institute of Texas (CPRIT; RP120855), and the National Institutes of Health (RC2 ES018789-02 and R01 ES008263-14).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmidt CW. IOM issues report on breast cancer and the environment. Environmental health perspectives. 2012;120:a60–1. doi: 10.1289/ehp.120-a60a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IBCERCC. Breast Cancer and the Environment: Prioritizing Prevention. National Institute of Environmental Health Sciences; 2013. [Google Scholar]
- 3.Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annual review of genomics and human genetics. 2008;9:321–45. doi: 10.1146/annurev.genom.9.081307.164339. [DOI] [PubMed] [Google Scholar]
- 4.Thompson D, Easton D. The genetic epidemiology of breast cancer genes. Journal of mammary gland biology and neoplasia. 2004;9:221–36. doi: 10.1023/B:JOMG.0000048770.90334.3b. [DOI] [PubMed] [Google Scholar]
- 5.Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environmental health perspectives. 1993;101:372–7. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudel RA, Ackerman JM, Attfield KR, Brody JG. New exposure biomarkers as tools for breast cancer epidemiology, biomonitoring, and prevention: a systematic approach based on animal evidence. Environmental health perspectives. 2014;122:881–95. doi: 10.1289/ehp.1307455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public health. 2012;126:185–9. doi: 10.1016/j.puhe.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature reviews Genetics. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker DJ. The origins of the developmental origins theory. Journal of internal medicine. 2007;261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 10.Walker CL, Ho SM. Developmental reprogramming of cancer susceptibility. Nature reviews Cancer. 2012;12:479–86. doi: 10.1038/nrc3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 12.Macon MB, Fenton SE. Endocrine disruptors and the breast: early life effects and later life disease. Journal of mammary gland biology and neoplasia. 2013;18:43–61. doi: 10.1007/s10911-013-9275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environmental health perspectives. 2010;118:1614–9. doi: 10.1289/ehp.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamartiniere CA, Jenkins S, Betancourt AM, Wang J, Russo J. Exposure to the Endocrine Disruptor Bisphenol A Alters Susceptibility for Mammary Cancer. Hormone molecular biology and clinical investigation. 2011;5:45–52. doi: 10.1515/HMBCI.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109:2667–711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- 16.Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter W, et al. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1509–14. doi: 10.1158/1055-9965.EPI-06-0109. [DOI] [PubMed] [Google Scholar]
- 17.Knower KC, To SQ, Leung YK, Ho SM, Clyne CD. Endocrine disruption of the epigenome: a breast cancer link. Endocrine-related cancer. 2014;21:T33–55. doi: 10.1530/ERC-13-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng YI, Hsu PY, Liyanarachchi S, Liu J, Deatherage DE, Huang YW, et al. Epigenetic influences of low-dose bisphenol A in primary human breast epithelial cells. Toxicology and applied pharmacology. 2010;248:111–21. doi: 10.1016/j.taap.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin XY, Fukuda T, Yang L, Zaha H, Akanuma H, Zeng Q, et al. Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer biology & therapy. 2012;13:296–306. doi: 10.4161/cbt.18942. [DOI] [PubMed] [Google Scholar]
- 20.Tilghman SL, Bratton MR, Segar HC, Martin EC, Rhodes LV, Li M, et al. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PloS one. 2012;7:e32754. doi: 10.1371/journal.pone.0032754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King-Batoon A, Leszczynska JM, Klein CB. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environmental and molecular mutagenesis. 2008;49:36–45. doi: 10.1002/em.20363. [DOI] [PubMed] [Google Scholar]
- 22.Bosviel R, Dumollard E, Dechelotte P, Bignon YJ, Bernard-Gallon D. Can soy phytoestrogens decrease DNA methylation in BRCA1 and BRCA2 oncosuppressor genes in breast cancer? Omics: a journal of integrative biology. 2012;16:235–44. doi: 10.1089/omi.2011.0105. [DOI] [PubMed] [Google Scholar]
- 23.Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Hormones & cancer. 2010;1:146–55. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chemical research in toxicology. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trevino LS, Weigel NL. Phosphorylation: a fundamental regulator of steroid receptor action. Trends in endocrinology and metabolism: TEM. 2013;24:515–24. doi: 10.1016/j.tem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489–95. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. The Journal of biological chemistry. 2007;282:22278–88. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 29.Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor alpha is required for normal organ development and function. Developmental cell. 2014;29:482–90. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Matsumura F. Significance of the nongenomic, inflammatory pathway in mediating the toxic action of TCDD to induce rapid and long-term cellular responses in 3T3-L1 adipocytes. Biochemistry. 2008;47:13997–4008. doi: 10.1021/bi801913w. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura F. The significance of the nongenomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochemical pharmacology. 2009;77:608–26. doi: 10.1016/j.bcp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Sciullo EM, Dong B, Vogel CF, Matsumura F. Characterization of the pattern of the nongenomic signaling pathway through which TCDD-induces early inflammatory responses in U937 human macrophages. Chemosphere. 2009;74:1531–7. doi: 10.1016/j.chemosphere.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong B, Nishimura N, Vogel CF, Tohyama C, Matsumura F. TCDD-induced cyclooxygenase-2 expression is mediated by the nongenomic pathway in mouse MMDD1 macula densa cells and kidneys. Biochemical pharmacology. 2010;79:487–97. doi: 10.1016/j.bcp.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Vogel CF, Wu D, Matsumura F. Non-genomic action of TCDD to induce inflammatory responses in HepG2 human hepatoma cells and in liver of C57BL/6J mice. Biological chemistry. 2010;391:1205–19. doi: 10.1515/BC.2010.126. [DOI] [PubMed] [Google Scholar]
- 35.Fujisawa Y, Li W, Wu D, Wong P, Vogel C, Dong B, et al. Ligand-independent activation of the arylhydrocarbon receptor by ETK (Bmx) tyrosine kinase helps MCF10AT1 breast cancer cells to survive in an apoptosis-inducing environment. Biological chemistry. 2011;392:897–908. doi: 10.1515/BC.2011.087. [DOI] [PubMed] [Google Scholar]
- 36.Wong RL, Walker CL. Molecular pathways: environmental estrogens activate nongenomic signaling to developmentally reprogram the epigenome. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:3732–7. doi: 10.1158/1078-0432.CCR-13-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baba A, Ohtake F, Okuno Y, Yokota K, Okada M, Imai Y, et al. PKA-dependent regulation of the histone lysine demethylase complex PHF2-ARID5B. Nature cell biology. 2011;13:668–75. doi: 10.1038/ncb2228. [DOI] [PubMed] [Google Scholar]
- 38.Sinha S, Singh RK, Alam N, Roy A, Roychoudhury S, Panda CK. Alterations in candidate genes PHF2, FANCC, PTCH1 and XPA at chromosomal 9q22.3 region: pathological significance in early- and late-onset breast carcinoma. Molecular cancer. 2008;7:84. doi: 10.1186/1476-4598-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Molecular endocrinology. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawazu M, Saso K, Tong KI, McQuire T, Goto K, Son DO, et al. Histone demethylase JMJD2B functions as a co-factor of estrogen receptor in breast cancer proliferation and mammary gland development. PloS one. 2011;6:e17830. doi: 10.1371/journal.pone.0017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi L, Sun L, Li Q, Liang J, Yu W, Yi X, et al. Histone demethylase JMJD2B coordinates H3K4/H3K9 methylation and promotes hormonally responsive breast carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7541–6. doi: 10.1073/pnas.1017374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic acids research. 2003;31:3635–41. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast cancer research: BCR. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Troisi R, Hatch EE, Titus-Ernstoff L, Hyer M, Palmer JR, Robboy SJ, et al. Cancer risk in women prenatally exposed to diethylstilbestrol. International journal of cancer Journal international du cancer. 2007;121:356–60. doi: 10.1002/ijc.22631. [DOI] [PubMed] [Google Scholar]
- 45.Verloop J, van Leeuwen FE, Helmerhorst TJ, van Boven HH, Rookus MA. Cancer risk in DES daughters. Cancer causes & control: CCC. 2010;21:999–1007. doi: 10.1007/s10552-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, et al. Preventable exposures associated with human cancers. Journal of the National Cancer Institute. 2011;103:1827–39. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. The New England journal of medicine. 2011;365:1304–14. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 48.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental health perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fenichel P, Dechaux H, Harthe C, Gal J, Ferrari P, Pacini P, et al. Unconjugated bisphenol A cord blood levels in boys with descended or undescended testes. Human reproduction. 2012;27:983–90. doi: 10.1093/humrep/der451. [DOI] [PubMed] [Google Scholar]
- 50.Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–27. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biology of reproduction. 2001;65:1215–23. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 52.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–47. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environmental health perspectives. 2007;115:592–8. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Experimental cell research. 2004;298:122–32. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer research. 2006;66:5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155:805–17. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greathouse KL, Cook JD, Lin K, Davis BJ, Berry TD, Bredfeldt TG, et al. Identification of uterine leiomyoma genes developmentally reprogrammed by neonatal exposure to diethylstilbestrol. Reproductive sciences. 2008;15:765–78. doi: 10.1177/1933719108322440. [DOI] [PubMed] [Google Scholar]
- 58.Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, et al. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Molecular cancer research: MCR. 2012;10:546–57. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.