Abstract
Purpose of review
Microvesicles, in general, and exosomes together with their delivered content in particular, are now being widely recognized as key players in atherosclerosis. We have previously reviewed the role of microvesicles in atherosclerosis pathogenesis, diagnosis and therapy. Here, we focus on the roles of exosomes and discuss their emergent role in mediating activation and response to inflammation, vessel infiltration and induction of coagulation. We will finally give an outlook to discuss novel detection techniques and systems biology based data analyses to investigate exosome-mediated cell-to-cell communication.
Recent findings
Recent research points to a role of exosomes in delivering apoptotic and inflammatory content between blood cells and vascular cells, with a potential contribution of exosomes secreted by adipose tissue. An atheroprotective role of exosomes in response to coagulation that may contrast with the procoagulatory role of platelet-derived larger microvesicles is envisaged. New detection and separation methods and systems biology techniques are emerging.
Conclusion
We project that the development of novel detection, separation and analysis mechanism and systems-based analysis methods will further unravel the paracrine and endocrine ‘communication protocol’ between cellular players in atherosclerosis, mediating inflammation, oxidative stress and apoptosis.
Keywords: atherosclerosis, exosomes, blood mononuclear cells, endothelial cells, systems biology
INTRODUCTION
Atherosclerosis is a chronic condition whereby vessel walls thicken through infiltration of inflammatory cells, fat deposits and cell proliferation. Unstable atherosclerosis, resulting from apoptosis and necrosis, can lead to thrombus formation, causing myocardial infarction and stroke [1]. Several genetic, behavioural, metabolic, environmental and nutritional factors have been linked to its pathogenesis, including familial history, obesity, diabetes mellitus, tobacco smoking, dyslipidemia and ageing. Atherosclerosis is commonly associated with intracellular stress conditions such as systemic inflammation [2], hyperactivation of inflammatory response through release of inflammatory cytokines and deregulated levels of M1/M2 macrophages [3], and oxidative stress [4]. However, to fully appreciate mechanisms contributing to this viscous circle of molecular maladaptation between these conditions [5], it is necessary to understand how the different cellular players communicate between each other during pathogenesis by other ways than by secreting cytokines. Hence, we focus on the role of exosomes that are known to contain proteins and genetic material.
Box 1.
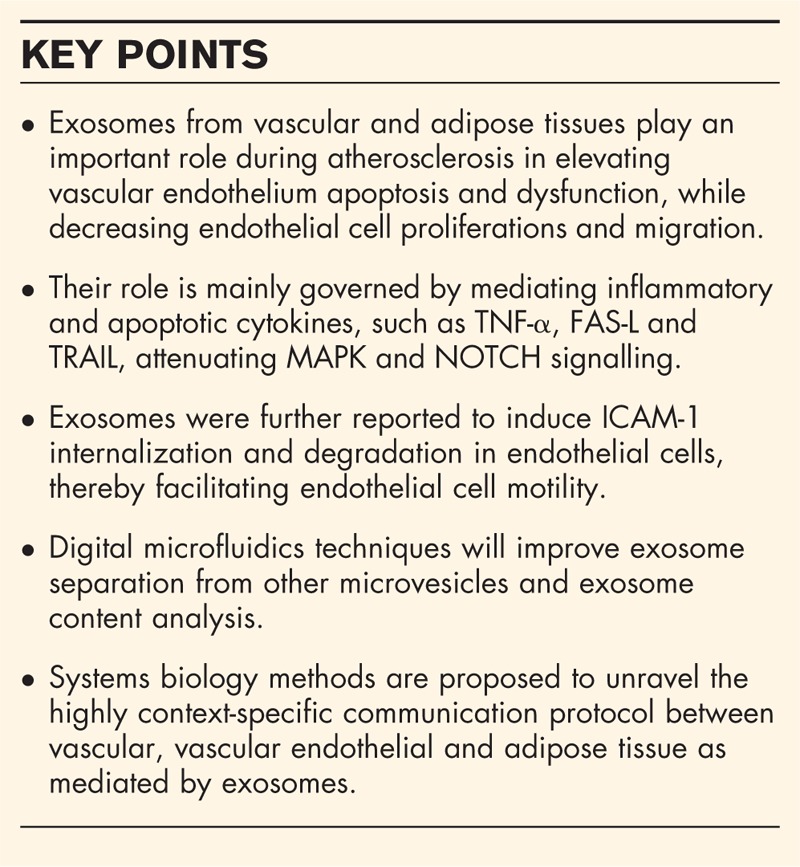
no caption available
EXOSOMES AND THEIR ROLE IN HORIZONTAL GENE TRANSFER
Exosomes are bi-lipid membranous vesicles containing protein, lipid and nucleic acid contents that are excreted from cells. They are distinguished from other cell-secreted particles, collectively called microvesicles, such as shedding microvesicles (SMVs) and apoptotic bodies by their smaller size [approximately 40–100 nm of exosomes compared with 100 nm–1 μm for SMV and 1–4 μm for apoptotic bodies] and by their intracellular origin. Several cells of the vascular system can secrete exosomes such as red blood cells, platelets, endothelial cells, monocytes, lymphocytes, dendritic cells, mast cells and cells from the tumour vasculature. Exosomes are found in blood plasma, breast milk, cerebrospinal fluid, saliva and urine, and are extracted using ultracentrifugation [6,7]. They are characterized and identified by integrins and tetraspanins CD63, CD89, CD81, CD9 and CD82, by maturation-related proteins flotillin and annexin, and the heat shock proteins hsp70 and hsp90. Although originally considered as cellular debris, it now becomes clear that exosomes are not a result of casual sampling; instead, they contain selective loads via dedicated packing mechanisms [8▪,9▪], deliver these loads to targeted cells and contain unique trafficking probabilities. All these mechanisms were found to be dependent on the individual cell, their cellular state and different under physiological and pathological and stress conditions [10].
Exosomes have been considered as means of horizontal, paracrine gene transfer, delivering microRNA and mRNA between cells of different origin. Transfer processes have been associated to induce gene silencing via modulation of RISC-complex related proteins and microRNA delivery [11], and activate inflammatory response within the target cells [12]. However, the amount as to how much content is delivered remains controversial. On the one hand, it has been reported that the majority of microRNAs that is detectable in serum and saliva is concentrated in exosomes [13] and that small nucleic acid content is enriched [14]. On the other hand, however, a previous study using state-of-the art sequencing and digital microfluidics techniques reported that only far less than one nucleotide copy of a specific microRNA is present per exosome [15▪], suggesting that exosome-mediated gene transfer is dependent on a high amount of exosome trafficking. To this end, databases are emerging that aim to catalogue exosome proteins, RNA and lipids, specific to secreting cell types, organisms and pathophysiological state [16,17]. Apart from their role in paracrine signalling, exosomes also play an endocrine role in communication between adipocytes via vascular tissues, wherein they may act as a carrier of atherogenic content [12,18]. As such, plasma exosomes were found to be modulators of the adiponectin pathway via upregulation of miR-326 and downregulation of let-7a and let-7f in type 2 diabetic patients [19▪].
ROLE OF EXOSOMES IN VESSEL WALL INFILTRATION AND ENDOTHELIAL CELL MIGRATION
The role of microvesicles derived from monocytes and their cellular descendants in intercellular communication during inflammation and immune response is well established [6,20,21] and their activation may be in part induced by exosomes from neighbouring cells in a Toll-like receptor-dependent manner [22]. Monocytes are the major progenitor cells of macrophages and dendritic cells and their activation is required for proper immune response, while pathological deregulation leads to inflammation and oxidative stress [5,23,24].
Macrophages and macrophage-derived microvesicles are overrepresented in the vicinity of the arterial endothelium. Monocytes may secrete exosomes to directly induce intima infiltration. As such, Aharon et al. [25] found monocyte-derived exosomes to induce apoptotic effects in endothelial cells and to promote coagulatory pathways by tissue factor release. Leading to further disarrangement of the endothelium, monocyte-derived exosomes can promote endothelial cell migration by miR-150 delivery [26], although exosomes from macrophages have also been reported by Lee et al. [27▪] to suppress integrin trafficking mediated endothelial cell migration. Their study demonstrates that exosomes released from human macrophages negatively regulate endothelial cell migration through control of integrin trafficking.
Specifically, macrophage-derived exosomes promote internalization of integrin β1 in primary human umbilical vein endothelial cells (HUVEC) and stimulate trafficking of internalized integrin β1 to lysosomal compartments, resulting in its proteolytic degradation. Exosome-mediated integrin degradation was blocked by bafilomycin A, a lysosomal degradation inhibitor. Apart from mediating integrin surface expression, macrophage-derived exosomes were also shown to effectively suppress collagen-induced activation of the mitogen-activated protein kinase/extracellular signal regulated kinase signalling pathway and HUVEC migration, which are both dependent on integrin β1. These observations provide new insight into the functional significance of exosomes in the regulation of integrin trafficking.
Several studies raise the possibility that exosome-mediated coregulation and common inflammatory activation of macrophages and lymphocytes may allow their wall infiltration. Macrophages are, together with lymphocytes, the first cells that enter the injury site and synergize with them in infiltration of the vessel intima [1]. Macrophage-derived larger microvesicles may promote leukocyte rolling and adherence via elevation of intracellular adhesion molecule (ICAM-1) and NF-κB activation [28]. Direct exosome-mediated communication between lymphocytes and macrophages was also observed [29]. In this study, authors found that exosomes derived from the supernatant of activated, and potentially atherogenic, CD4+ T lymphocytes enhanced cholesterol load accumulation in cultured monocytes in a phosphatidylserine receptor dependent manner. Exosomes may also have a coregulatory role of both cells, as they were shown to be able to transfer exogenous siRNA (against mitogen-activated protein kinase-1, which was introduced into exosomes in their setting) to both monocytes and lymphocytes [30] (Fig. 1).
FIGURE 1.
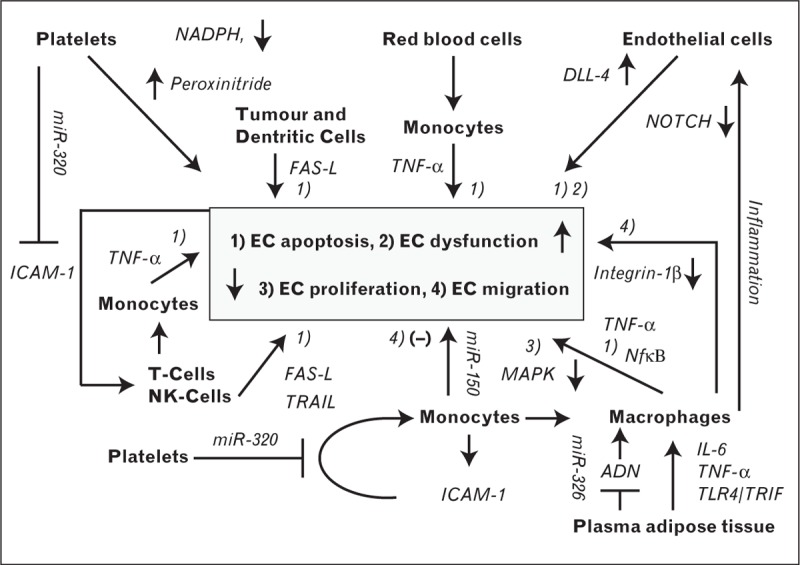
Role of exosomes in affecting endothelial cell function. Platelet-derived exosomes reduce ICAM-1 expression, reducing monocyte adhesion, while platelet microvesicles under hyperglycaemic conditions induce EC monocyte adhesion via decreased NADPH oxidase activity and increased peroxynitrite. Several blood and vascular cells release exosomes that contain cytokines and apoptosis ligands, promoting inflammation and EC apoptosis. Dendritic cells (DC), T-lymphocytes, NK cells, and some tumour cells, directly release apoptotic ligand FAS-L via exosomes, the first two cell types also in combination with TRAIL. Exosomes from T-cells can also induce FAS-L and TNF-α release from monocytes. Monocytes may also release TNF-α when exposed to exosomes from red blood cells. In turn, endothelial cell apoptosis and dysfunction may exacerbate inflammatory conditions, leading to further T-cell activation or sensitization. Monocyte-derived exosomes can promote endothelial cell migration by miR-150 delivery, while they, however, can also attenuate it via reduced integrin 1β action and collagen expression, suggesting a tightly regulated process. Similarly, exosomes from monocyte-derive macrophages can reduce proliferation by negatively impacting MAPK-signalling. Exosomes from adipose tissue were shown to activate macrophages at the vessel wall, secreting IL-6 and TNF-α in a TLR4/TRIF-dependent way, while plasma-derived exosomes attenuated adiponectin signalling. Endothelial cells secrete exosomes containing the NOTCH ligand DLL-4 that negatively regulates NOTCH signalling and this NOTCH-suppression may be exacerbated by inflammation.
EXOSOMES AS A CARRIER AND AN INDUCER OF APOPTOTIC SIGNALS
Exosomes from different vascular cells may induce endothelial cell apoptosis and thereby contribute to vascular dysfunction. As such, platelet-derived exosomes may elevate endothelial cell apoptosis by deregulation of redox metabolism via elevated NADPH activity [31] or by peroxynitrite generation [32]. By similar means, exosomes from red blood cells enhanced tumour necrosis factor-alpha (TNFα) production in monocytes and augmented mitogen-induced CD4+ and CD8+ T-cell proliferation [in an antigen-presenting cell (APC)-dependent manner] [33▪]. Several lines of evidence exist that elevated exosome-mediated apoptosis occur under inflammatory conditions. As such, exosomes derived from activated T-cells were shown to increase levels of the apoptosis-inducing ligand FAS-L [34]. Likewise, elevated TNF-α levels were found in supernatant of human monocytes exposed to exosomes from the conditioning medium of activated CD4+ lymphocytes [29]. Degranulation of lysosome-derived, multivesicular bodies (as a means of exosome release) in activated T-lymphocytes was observed to increase FAS cell surface death receptor-ligand (FAS-L) and TRAIL (TNF-α related apoptosis ligand) expression in the vicinity of their cell membrane [35], although elevation of FAS-L was observed in T-lymphocytes and natural killer (NK) cells [36]. Likewise, exosomes from dendritic cells express on their surface apoptosis inducing ligands that may further contribute to elevated apoptosis in the endothelium [37]. Apart from the apoptotic role of vascular cell derived exosomes as a response to inflammation, other cells may excrete exosomes that induce an inflammation-mediated apoptotic response. As an example, elevated exosome-mediated FAS-L release (negatively regulated by diaglycerol kinase alpha) has been observed in tumour cells and attributed as a stress-response to immune attack [38]. Finally, exosome-like vesicles released from adipose tissue were shown to activate macrophages at the vessel wall that can secrete IL-6 and TNF-α in a TLR4/TRIF-dependent way, mediating insulin resistance [39] (Fig. 1).
ROLE OF EXOSOMES IN MEDIATING COAGULATORY PATHWAYS
Platelet-derived microvesicles are responsible for about 70% of the microparticle content in blood serum and affect atherogenesis by activating ECs [40], initiating DC maturation [41] and inducing vascular smooth muscle cell (VSMC) proliferation [42]. Their most prominent role is, however, activation of other platelets and promoting their adherence to the vessel wall, providing a positive feedback that leads to thrombus formation [43]. Heijnen et al. [44] were the first to distinguish platelet-derived exosomes from their larger microvesicles relatives. The studies of Gidlof et al. [45] and Srikanthan et al. [46▪] suggest that unlike platelet-derived shedding microvesicles, platelet-derived exosomes act ratheras anticoagulants or in response to coagulation. Srikanthan et al. [46▪] provided preclinical evidence that exosomes derived from platelet-rich plasma (and after removing nucleated cells by size exclusion filtering) reduced lipid loading and decreased platelet coagulation. This was mediated by decreased CD36 expression, a major mediator of the procoagulative and foam cell-inducing role of oxidized LDL in platelets [46▪]. Using RNA sequencing, Gidlof et al. [45] found that platelet-derived exosomal miR-320 reduced endothelial ICAM-1 expression, thereby facilitating endothelial cell motility, reducing inflammation and thrombus formation, potentially as a means of protection against further injury. Exosome dependency was validated by the lysosomal inhibitor brefeldin A, which ablated microvesicles expression. They further demonstrate that miR-22, miR-185, miR-320b and miR-4235p were elevated in the supernatant of platelets upon induction of aggregation. Likewise, mast cell exosomes were found to be thrombolytic by stimulating endothelial cells to induce plasminogen activator type I, not only contributing to plaque reduction through fibrinolysis but also to their instability [47].
ROLES OF ENDOTHELIAL CELLS AND ENDOTHELIAL-DERIVED EXOSOMES AND MICROVESICLES
Endothelial-derived exosomes and other microvesicles present only a sparse fraction of the circulating microvesicles. Yet, they have been ascribed a multifaceted role including atherosclerotic effects such as increasing arterial stiffness, inflammation and thrombosis, or atheroprotective roles such as promoting angiogenesis, endothelial cell differentiation and cell survival [48]. Endothelial-derived microvesicles may be activated under hyperglycemic conditions to induce monocyte-adhesion in other endothelial cells via increased NADPH oxidase activity [49]. They may further act as diagnostic markers in atherosclerosis and endothelial cell functions [50,51]. As such, the amount of endothelium-derived microparticles, considered as markers of endothelial cell (dys-)function, was positively correlated with incidence of atherosclerosis in hypercholesterolemia [52] and with incidence of chronic kidney disease in children who also show increased arterial stiffness and atherosclerosis [53].
Endothelial-derived exosomes may contain RNA and proteins that are reflective of the undergoing specific stress conditions [12] and have further implicated in vascular development through attenuation of NOTCH signalling [54]. Specifically, authors found that endothelial and tumour cells express the NOTCH ligand Delta-like 4 (Dll4), which negatively regulates NOTCH. It thereby changes endothelial cells to a tip-cell phenotype, supporting vessel branching. Likewise, as NOTCH signalling in endothelial cells is necessary for protection of EC exposed to stress to prevent endothelial senescence and inflammation, exosomes containing DLL-4 and inflammation-induced NOTCH depression may contribute to EC dysfunction and apoptosis [55,56].
FURTHER ROLE EXOSOMES UNDER ATHEROGENIC STRESS CONDITIONS
Exosomes and other microparticles have been shown to play an atheroprotective or atherogenic role in several conditions accompanying atherosclerosis [57]. Exosomes from mature dendritic cells may contribute to immune system hyperactivation during atherosclerosis, as they transfer the ability to induce naive T-cell priming in an ICAM-1 dependent way to B lymphocytes [58], potentially reducing their reactiveness to ox-LDL. Maturation of plasmacytoid dendritic cells can, in turn, occur by endothelial microvesicles, leading to T-cell stimulation and the secretion of inflammatory cytokines [59]. Furthermore, an exosome-mediated link between systemic calcium deregulation, smooth muscle cells calcification and increased vessel stiffness has also been made by analysing data in chronic kidney disease patients [60]. In contrast, exosome-mediated communication between endothelial and smooth muscle cells may be atheroprotective under endothelial shear stress conditions due to turbulent blood flow near bends and bifurcations by containing expression of the miR-143/145 cluster and dependent on the expression of the shear stress responsive Krüppel like factor 2 (KLF2) [61].
OUTLOOK: NOVEL DETECTION TECHNIQUES
Currently, only a few studies focus specifically on exosomes. We therefore expect that improvement and standardization of separation procedures and co-culturing protocols will further increase inter-study comparability and exosome role clarification. Exosome-specific labels or, likewise, of their nucleic acid content that may trace them after in-vivo re-injection may further shed light into their pathophysiological role during atherogenesis [62▪]. Novel detection methods going beyond ultracentrifugation or size exclusion techniques will increase sensitivity of exosome and microparticle detection and speed up analyses cycles [62▪]. These techniques will include microfluidics devices for on-chip isolation, quantification and characterization of circulating microparticles [63]. They will further include droplet-based segmented-flow microfluidics for extraction of nucleic acids [64], microchip-based RNA extraction, droplet digital and analogue RT-PCR for mRNA and miR amplification [65], and plasmonics for detecting their biomarker-relevant content [66].
OUTLOOK: SYSTEMS BIOLOGY BASED DATA ANALYSIS
As intracellular communication is complex and highly dependent on cellular context, systems biology techniques will help to generate context-specific hypotheses of the role of specific microvesicles dependent on state of the disease, the expression status of the cell, the secretion frequency and trafficking properties of microparticles, and the type and amount of content delivered. Promising methods therefore are cue-signal-response studies using partial least square regression (PLSR) methods which is a statistical procedure combining multivariate regression with parameter reduction. PLSR allows us to study how signals (exosome content) translate cues from the secreting cell (its gene/protein expression state) to elicit a specific response in the recipient cells. To this end, Gray et al. [67▪] demonstrated that depending on the preconditioning of cardiac progenitor cells (hypoxia vs. normoxia at different time points), different clusters of miRs were packed into exosomes derived from these cells. They then identified that these specific clusters gave rise to differential physiological responses in recipient endothelial cells such as assessing their capability for tube formation, thereby suggesting a way how changes of miR composition could be used to direct outcome in target cells.
We propose here to use this method to study how hypoxic and reperfusion-induced atherogenic stress in monocytes would translate into specific endothelial cell responses mediated by specific exosome loading (Fig. 2a). As such, we propose to determine how a physiological stress condition 1 consisting mainly of a mixture of acute and chronic hypoxia induced macrophages to release an exosome content E1 (e.g. containing a certain combination of miR#3 and miR#4), which translates to a specific response in endothelial cells such as reactive oxygen species (ROS) production, while a stress condition 2 that is mainly determined by inflammation and hyperlipidemia would lead to an exosome content E2 and induces an endothelial phenotype consisting of deregulated ICAM-1 expression and EC migration.
FIGURE 2.
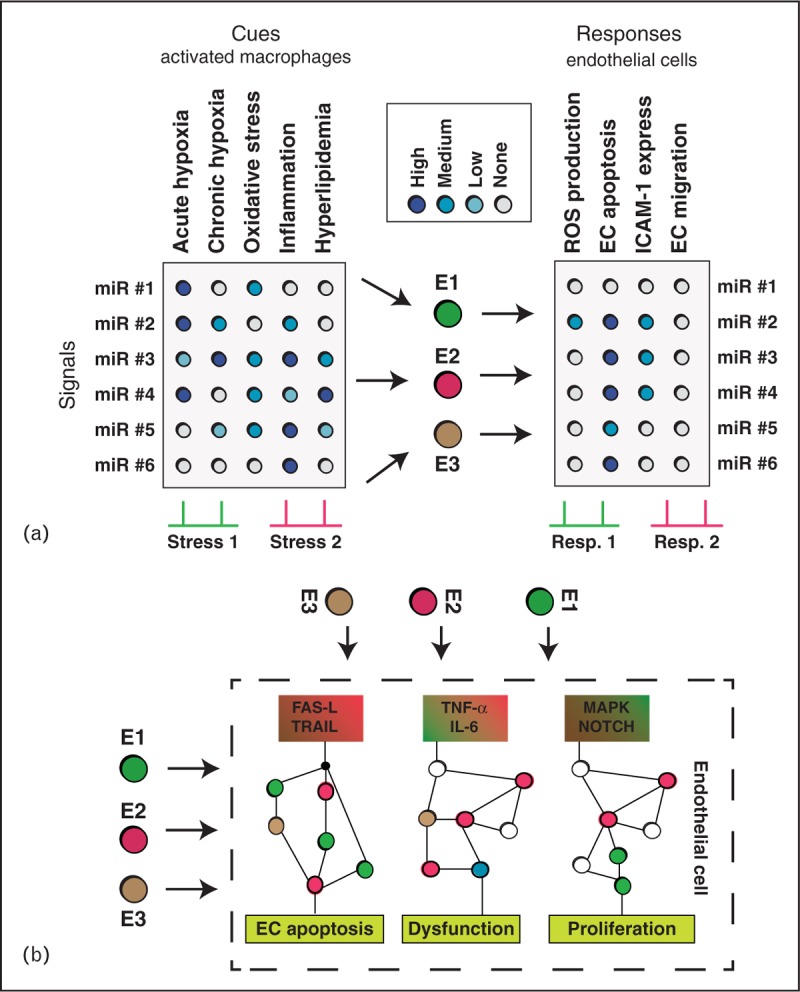
Systems biology approaches to study exosome-mediated signalling during atherosclerotic conditions. (a) Proposed cue-signal-response analysis to investigate how different stress conditions in macrophages translate into changes in endothelial cell phenotype using partial least square regression (PLSR) similar to Gray et al. [67▪]. The method seeks for canonical types of exosome loadings (E1, E2, E3; each containing a certain combination of miRs) that are characteristic for in-vivo conditions wherein different combinations of stressors simultaneously apply. Thereby the method aims to separate effective exosome mRNA from bystander content. (b) Once most important exosome content and most affected pathways in endothelial cells have been identified from (a), computational signal transduction analyses may help to assess the effect of exosome content on a detailed level. Content released from exosomes may trigger specific pathways relevant to EC function (apoptosis, dysfunction, proliferation), while further exosome content may also exacerbate or attenuate the signalling response. Computational models can assess the net effect of exosome content on signalling by including quantitative expression levels from pathway proteins and exosome content.
In contrast to such large-scale systems biology approaches, targeted system models that focus on specific pathways may help to study how specific exosome content may change signal transduction processes in the target cells. Such analysis would allow further segregation of exosome bystander content from such relevant to intercellular signalling. An example therefore would be to study how exosome-delivered interleukin (IL)-6, TNF-α or FAS-L from monocytes/macrophages would affect pathways of endothelial cell apoptosis, dysfunction and proliferation (Fig. 2b). How system models can help to improve clinical diagnostics and treatment in the field of cancer therapy and apoptosis was recently reviewed by us [68▪]. We thereby collected studies from translational systems biology, whereby theoretical predictions helped to design molecular interventions to re-establish impaired cancer cell apoptosis and personalized dosages of chemotherapy based on molecular profiling of apoptosis proteins. It is therefore expected that similar personalized medicine approaches may assess grade of inflammation and susceptibility to coagulation from quantifying exosome content, or allowing the design of optimal exosome-content for therapeutically attenuating these processes.
CONCLUSION
Exosome-mediated intercellular signalling during atherosclerosis is a multifacet set of processes whose context-dependent roles are slowly being deciphered. Still, exosome isolation from serum and from their sister microparticles is tedious, and only a few studies are specifically focussed to study exosomes and their role in preclinical and clinical research. Nevertheless, the recent progress in the field of exosome separation and detection, the application of systems biology based data analysis to unravel the ‘communication protocol’ between the involved cells and tissues, and the potential exploitation of exosome delivery for therapeutic purposes make exosome research an attractive field for complex, systemic diseases such as atherosclerosis.
Acknowledgements
None.
Financial support and sponsorship
This work was funded by the Bijzonder Onderzoeksfonds of the KU Leuven (PF/10/014; Centre of Excellence), by the Interdisciplinair Ontwikkelingsfonds - Kennisplatform (Centre of Excellence KP/12/009) and by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (G0846.11, and Vascular Biology Network).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1. Ross R. Cell biology of atherosclerosis. Annu Rev Physiol 1995; 57:791–804. [DOI] [PubMed] [Google Scholar]
- 2. Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2003; 52:1799–1805. [DOI] [PubMed] [Google Scholar]
- 3. Hirata Y, et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol 2011; 58:248–255. [DOI] [PubMed] [Google Scholar]
- 4. Geeraert B, Crombe F, Hulsmans M, et al. Stevioside inhibits atherosclerosis by improving insulin signaling and antioxidant defense in obese insulin-resistant mice. Int J Obes (Lond) 2010; 34:569–577. [DOI] [PubMed] [Google Scholar]
- 5. Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med 2010; 14:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews. Immunology 2009; 9:581–593. [DOI] [PubMed] [Google Scholar]
- 7. van der Pol E, Boing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological reviews 2012; 64:676–705. [DOI] [PubMed] [Google Scholar]
- 8▪. Squadrito ML, et al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep 2014; 8:1432–1446. [DOI] [PubMed] [Google Scholar]; A gene expression study using qPCR arrays demonstrating that exosomes from bone marrow derived macrophages induced silencing in endothelial cells by transferring miRs. The amount of silencing miR-content was found to be partly controlled by the amount of transcripts available in the targeted cell. Relation between detected transcript repression and MiR content was corroborated by a computational analysis of miR-target interactions on a genome-wide level.
- 9▪. Villarroya-Beltri C, et al. Sorting it out: regulation of exosome loading. Semin Cancer Biol 2014; 28:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review describing ESCRT-dependent and independent mechanisms of protein, lipid and RNA cargo loading into multivesicular bodies; a description of Rab-protein dependent molecular mechanisms of movement of multivesicular bodies to the plasma membrane and exosome release; a detailing of exosome uptake via different adhesion molecules, integrins and lecting receptors or via exosome fusion to the membrane of vascular cells; and a portrayal of the effect of stress on exosome composition.
- 10. de Jong OG, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 2012; 1: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melo SA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014; 26:707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res 2013; doi: 10.1093/cvr/cvt1161. [DOI] [PubMed] [Google Scholar]
- 13. Gallo A, et al. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 2012; 7:e30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zomer A, et al. Exosomes: fit to deliver small RNA. Commun Integr Biol 2010; 3:44750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪. Chevillet JR, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A 2014; 111:14888–14893. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used a stoichiometric approach to quantify exosome numbers and numbers of miR transcripts. Several state-of-the-art detection mechanisms such as digital microfluidic and chip-based PCR were used and applied to exosome extraction from human serum, cord blood, medium from ovarian carcinoma cells and semen. They found that several miRs that were previously ascribed a functional and diagnostic role contained far less than one molecule per exosome on average.
- 16. Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic acids research 2012; 40:D1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 2009; 9:4997–5000. [DOI] [PubMed] [Google Scholar]
- 18. Baron M, Boulanger CM, Staels B, Tailleux A. Cell-derived microparticles in atherosclerosis: biomarkers and targets for pharmacological modulation? Journal of cellular and molecular medicine 2012; 16:1365–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪. Santovito D, et al. Plasma exosome microRNA profiling unravels a new potential modulator of adiponectin pathway in diabetes: effect of glycemic control. J Clin Endocrinol Metab 2014; 99:E1681–E1685. [DOI] [PubMed] [Google Scholar]; Circulating miRs of type 2 diabetic patients naive to treatment were studied and a significant downregulation of let-7a and let-7f was observed in diabetic patients vs. control. A correlation between upregulated miR-326 and lowered adiponectin was obtained. MiR-326 levels were unaffected upon antidiabetic treatments, while levels of let-7a and let-7f were decreased.
- 20. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007; 110:3234–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 2011; 33:419–440. [DOI] [PubMed] [Google Scholar]
- 22. Bretz NP, Ridinger J, Rupp AK, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. The Journal of biological chemistry 2013; 288:36691–36702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J 2011; 25:2515–2527. [DOI] [PubMed] [Google Scholar]
- 24. Hulsmans M, Geeraert B, De KD, et al. Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One 2012; 7:e30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost 2008; 100:878–885. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Molecular cell 2010; 39:133–144. [DOI] [PubMed] [Google Scholar]
- 27▪. Lee HD, Kim YH, Kim DS. Exosomes derived from human macrophages suppress endothelial cell migration by controlling integrin trafficking. Eur J Immunol 2014; 44:1156–1169. [DOI] [PubMed] [Google Scholar]; Using THP-1 cell-derived macrophages, the authors demonstrated that integrin-1 was shuttled to the lysosome of HUVEC cells, promoting its degradation and attenuating endothelial cell migration. This effect was ablated by the lysosomal inhibitor bafilomycin A.
- 28. Liu ML, Scalia R, Mehta JL, Williams KJ. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arteriosclerosis, thrombosis, and vascular biology 2012; 32:2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol 2007; 212:174–181. [DOI] [PubMed] [Google Scholar]
- 30. Wahlgren J, De LKT, Brisslert M, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic acids research 2012; 40:e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janiszewski M, Do Carmo AO, Pedro MA, et al. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Critical care medicine 2004; 32:818–825. [DOI] [PubMed] [Google Scholar]
- 32. Gambim MH, do Carmo Ade O, Marti L, et al. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care 2007; 11:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪. Danesh A, Inglis HC, Jackman RP, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 2014; 123:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors exposed peripheral blood mononuclear cells to microvesicles from red blood cells, releasing pro-inflammatory cytokines and augmenting mitogen-induced CD4+ and CD8+ T-cell proliferation in an antigen-presenting cell-dependent manner. Exosomes (identified as particles <200 nm) were found to be the active fraction.
- 34. Alonso R, Mazzeo C, Rodriguez MC, et al. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell death and differentiation 2011; 18:1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez-Lorenzo MJ, Anel A, Gamen S, et al. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. Journal of immunology 1999; 163:1274–1281. [PubMed] [Google Scholar]
- 36. Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med 1999; 5:90–96. [DOI] [PubMed] [Google Scholar]
- 37. Munich S, Sobo-Vujanovic A, Buchser WJ, et al. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 2012; 1:1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andreola G, Rivoltini L, Castelli C, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. The Journal of experimental medicine 2002; 195:1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009; 58:2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. The Journal of clinical investigation 1998; 102:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaneider NC, et al. CD40 ligand-dependent maturation of human monocyte-derived dendritic cells by activated platelets. Int J Immunopathol Pharmacol 2003; 16:225–231. [DOI] [PubMed] [Google Scholar]
- 42. Weber A, Koppen HO, Schror K. Platelet-derived microparticles stimulate coronary artery smooth muscle cell mitogenesis by a PDGF-independent mechanism. Thromb Res 2000; 98:461–466. [DOI] [PubMed] [Google Scholar]
- 43. Wang H, Wang ZH, Kong J, et al. Oxidized low-density lipoprotein-dependent platelet-derived microvesicles trigger procoagulant effects and amplify oxidative stress. Molecular medicine 2012; 18:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heijnen HF, Schiel AE, Fijnheer R, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94:3791–3799. [PubMed] [Google Scholar]
- 45. Gidlof O, van der Brug M, Ohman J, et al. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 2013; 121:3908–3917.S3901–3926. [DOI] [PubMed] [Google Scholar]
- 46▪. 2014; Srikanthan S, Li W, Silverstein RL, McIntyre TM. Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions, Journal of thrombosis and haemostasis : JTH. 12:1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]; This recent study suggests that unlike their larger sister micro particles, platelet-derived exosomes suppressed platelet aggregation. Platelets were manipulated ex vivo with conditioning medium of exosomes and their anticoagulatory effect was studied in thrombocytopenic recipient mice.
- 47. Al-Nedawi K, Szemraj J, Cierniewski CS. Mast cell-derived exosomes activate endothelial cells to secrete plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol 2005; 25:1744–1749. [DOI] [PubMed] [Google Scholar]
- 48. Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 2011; 31:27–33. [DOI] [PubMed] [Google Scholar]
- 49. Jansen F, Yang X, Franklin BS, et al. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovascular research 2013; 98:94–106. [DOI] [PubMed] [Google Scholar]
- 50. Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes 2012; 19:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Chen LM, Liu ML. Microvesicles and diabetic complications - novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacol Sin 2014; 35:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pirro M, Schillaci G, Paltriccia R, et al. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arteriosclerosis, thrombosis, and vascular biology 2006; 26:2530–2535. [DOI] [PubMed] [Google Scholar]
- 53. Dursun I, Poyrazoglu HM, Gunduz Z, et al. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 2009; 24:2511–2518. [DOI] [PubMed] [Google Scholar]
- 54. Sheldon H, Heikamp E, Turley H, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood 2010; 116:2385–2394. [DOI] [PubMed] [Google Scholar]
- 55. Liu ZJ, Tan Y, Beecham GW, et al. Notch activation induces endothelial cell senescence and pro-inflammatory response: implication of Notch signaling in atherosclerosis. Atherosclerosis 2012; 225:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quillard T, Devalliere J, Coupel S, Charreau B. Inflammation dysregulates Notch signaling in endothelial cells: implication of Notch2 and Notch4 to endothelial dysfunction. Biochemical pharmacology 2010; 80:2032–2041. [DOI] [PubMed] [Google Scholar]
- 57. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006; 6:508–519. [DOI] [PubMed] [Google Scholar]
- 58. Segura E, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005; 106:216–223. [DOI] [PubMed] [Google Scholar]
- 59. Angelot F, Seilles E, Biichle S, et al. Endothelial cell-derived microparticles induce plasmacytoid dendritic cell maturation: potential implications in inflammatory diseases. Haematologica 2009; 94:1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kapustin AN, Chatrou ML, Drozdov I, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circulation research 2015; 116:1312–1323. [DOI] [PubMed] [Google Scholar]
- 61. Hergenreider E, Heydt S, Treguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature cell biology 2012; 14:249–256. [DOI] [PubMed] [Google Scholar]
- 62▪. Peterson MF, Otoc N, Sethi JK, et al. Integrated systems for exosome investigation. Methods 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; A methods review about the current isolation, detection, validation and visualization methods for exosomes derived from vascular tissue.
- 63. Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab on a chip 2014; 14:1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Verbruggen B, Leirs K, Puers R, Lammertyn J, Selective DNA extraction with microparticles in segmented flow. Microfluid Nanofluid 2015; 18:293–303. [Google Scholar]
- 65. Moltzahn F, Olshen AB, Baehner L, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer research 2011; 71:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Takahashi K, Yan IK, Kim C, et al. Analysis of extracellular RNA by digital PCR. Frontiers in oncology 2014; 4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪. Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circulation research 2015; 116:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]; A systems biology study investigating the effect of hypoxic and normoxic treatment at different time points on exosome release in cardiac progenitor cells and studying the effect of stress-dependent conditioning medium on recipient endothelial cells. PLSR was applied to reduce the amount of miRs that are relevant for stress-induced signalling and to regress their expression to the presence of an observed endothelial phenotype such as their capability of tube formation.
- 68▪. Huber HJ, McKiernan RG, Prehn JH. Harnessing system models of cell death signalling for cytotoxic chemotherapy: towards personalised medicine approaches? J Mol Med (Berl) 2014; 92:227–237. [DOI] [PubMed] [Google Scholar]; A recent review that investigates how recent signal transduction models of programmed cell death can translate into molecular strategies in the clinics to overcome apoptosis resistance in cancer cells, to identify therapeutic windows and to design personalized treatment regimens.


