Abstract
Purpose of review
The purpose of this study is to provide an update on the role HDL apolipoprotein A-I plays in reducing the risk of cardiovascular disease (CVD) and how it relates to reverse cholesterol transport (RCT).
Recent findings
Despite numerous studies showing that plasma HDL cholesterol concentrations are correlated with a reduced risk of CVD, pharmacologic elevation of HDL has not shown any beneficial effects to date. In contrast, studies correlating the measure of an individual's plasma cholesterol efflux capacity show greater promise as a tool for assessing CVD risk. Although ATP-binding cassette transporter 1-mediated lipidation of apoA-I is considered the principal source of plasma HDL, it represents only one side of the RCT pathway. Equally important is the second half of the RCT pathway in which the liver scavenger receptor class B1 selectively removes HDL cholesteryl esters for excretion. The combined action of the two enzyme systems is reflected in the overall steady-state concentration of plasma HDL cholesterol. For example, reduced ATP-binding cassette transporter 1-mediated production of nascent HDL lowers plasma HDL concentration, just as an increase in cholesteryl ester uptake by scavenger receptor class B1 reduces HDL levels. Thus, the complexity of intravascular HDL metabolism suggests that steady-state plasma HDL concentrations do not provide adequate information regarding an individual's HDL quality or function. Herein, we describe a new player, procollagen C-endopeptidase enhancer 2, which shows atheroprotective function and influences both sides of RCT by enhancing production and catabolism of HDL cholesteryl esters.
Summary
The discovery of a new molecule, procollagen C-endopeptidase enhancer 2, implicated in the regulation of HDL cholesteryl ester concentrations suggests that the extracellular matrix and the proteins that regulate its function represent a new and as yet unexplored realm of HDL cholesterol metabolism.
Keywords: cholesteryl esters, extracellular matrix, HDL, procollagen C-endopeptidase enhancer 2, scavenger receptor class B1
INTRODUCTION
For over 40 years, it has been assumed that higher plasma levels of HDL cholesterol are associated with a reduced risk for cardiovascular disease (CVD) [1]. This belief is based on HDL's repeatedly documented role in reverse cholesterol transport (RCT), although detailed mechanisms explaining how HDL protects against cholesterol accumulation in the artery wall remain controversial. Compounding this, recent attempts to pharmacologically increase HDL levels have not led to increased benefit [2], suggesting that HDL concentration does not accurately reflect HDL function. Leading the focus on HDL function, two studies show that the efficiency of cholesterol efflux to LDL-depleted plasma [3] correlates with reduced risk for CVD in humans [4,5▪▪]. In these studies, cholesterol efflux was mostly mediated by the ATP-binding cassette transporter A1 (ABCA1) and correlated with the concentration of lipid-poor preβ HDL particles [3]. How this rare cholesterol-depleted HDL particle is continuously generated from mature HDL at the artery wall is not entirely understood [6,7,8▪▪,9,10], leaving this and other important questions unanswered.
Box 1.
no caption available
BODY
HDL concentration, reverse cholesterol transport and atherosclerosis
Several thorough reviews of cholesterol efflux, RCT and atherosclerosis have been published over the last several years [11–13]. In this review, we will emphasize recent studies into the role of the newly discovered player, procollagen C-endopeptidase enhancer 2 (PCPE2), which resides in the extracellular matrix (ECM) and plays a role in both RCT and atherosclerosis. Given that steady-state plasma HDL concentrations do not provide sufficient information on either cholesterol efflux capacity or overall HDL function, it follows that other factors that impart atheroprotective function should be examined whether they reside on the HDL particle or enable optimal processing of HDL's cholesterol cargo.
Accessory proteins, cholesterol efflux and nascent HDL production
Several reports suggest that accessory proteins located on the plasma membrane assist in ABCA1-mediated assembly of nascent HDL (nHDL) [14–19], where nHDL represents the product(s) of lipid-poor apoA-I's interaction with ABCA1 [20]. Many years ago, it was discovered that newly synthesized apoA-I contained a hexapeptide pro-segment as well as a signal peptide (prepeptide). The presegment was removed at the time of synthesis, but the pro-segment was removed during secretion [21–23]. Over 9 years since the pro-apoA-I sequence was described, many questions about this process have been answered [24–29]. It is now known that the removal of the pro-peptide stimulates the rate of apoA-I intracellular translocation and that bone morphogenetic protein 1 (BMP1) cleaves the apoA-I pro-peptide [17,24] at the surface of the cell affecting the rate of lipidation in vitro, and thus the production rate of nHDL.
The enzyme that cleaves apoA-I's propeptide, BMP1, is an astacin metalloprotease with diverse substrates including ECM proteins and antagonists of the transforming growth factorβ (TGFβ) superfamily [30,31]. It is best known for cleaving C-terminal propeptides from procollagen precursors. This cleavage is essential for self-assembly of mature collagen monomers into fibrils within the ECM [32,33]. Also involved in this process is the enhancer protein, procollagen proteinase enhancer protein 1 or PCPE1 (PCOLCE gene). This protein stimulates the procollagen C-proteinase activity of BMP1 [34]. PCPE2 (PCOLCE2 gene) is related to PCPE1 sharing 43% amino acid identity with similar domain structure, but having markedly different glycosylation than PCPE1, and assisting BMP1 in modifying collagen [35,36]. However, the tissue distribution of the PCPEs is somewhat different with PCPE2 more highly expressed in heart, aorta and adipose, while PCPE1 shows a wider expression pattern. Both are glycoproteins having two Complement C1r/C1s, Uegf, Bmp1 (CUB) domains (Complement C1r/C1s, Uegf, Bmp1) separated by a short linker region, with each domain containing a β-sandwich fold that mediates a variety of protein–protein interactions [37–41]. The CUB domains have a homologous Ca2+-binding site that mediates ionic interactions between protein partners [38], similar to that described for the LDL receptor family [42,43]. PCPE2 also has a netrin-like (NTR) domain [32,33,44,45] that binds cell surface heparan sulphate proteoglycans (HSPGs) anchoring it to the ECM. Once believed to inhibit BMP1, the NTR region is now known to stimulate enhancer activity in the presence of HSPG [33]. From these studies, it appears that PCPE2 binds to HSPG in the ECM and then through one or both of its CUB domains coordinates the enzymatic activity of BMP1 whether it be that of procollagen or the six amino acids from proApoA-I. In addition to these functions, several recent reports link PCPE2 as a contributor to disorders characterized by fibrosis of the pancreas [46], as well as to TGF-β1 stimulation of human amniotic fluid derived mesenchymal cells [47], arachidonic acid abundance in red blood cells [48▪] and collagen accumulation associated with chronic pressure overload in mouse heart [49].
Procollagen C-endopeptidase protein 2, nascent HDL formation and cholesterol efflux
To explore the relationship between PCPE2 and nHDL formation, Zhu et al. [50] investigated the binding interactions between PCPE2, BMP1 and proapoA-I using surface plasmon resonance (SPR) and selective immunoprecipitation. Their studies showed that PCPE2 forms a high-affinity complex with apoA-I and BMP1 and more importantly that PCPE2 serves as a cofactor reducing the K d. This study provided strong biophysical support for the notion that PCPE2 plays a role in propeptide cleavage. In addition, clinical studies show significant correlations between PCPE2 polymorphisms and HDL [51], suggesting that PCPE2 may have other significant physiologic consequences from its interactions with apoA-I.
PCPE2 was originally a candidate gene for glaucoma [52], but now best known for its relationship with HDL concentrations in three independent population cohorts [50,51,53]. Associations in these studies were modest, but led investigators to carry out studies in PCPE2-/- mice [54], which revealed elevated concentrations of enlarged HDL particles. In addition, Francone et al. [53] showed that apo B depleted plasma from PCPE2-/- mice was defective in its ability to mediate in-vitro cholesterol efflux via ABCA1 and when combined with their enlarged HDL led to the hypothesis that HDL particles were dysfunctional. These seminal studies provided the first critical proof-of-concept data showing that PCPE2 plays a pivotal role in HDL metabolism. More recent support for the role of PCPE2 in HDL metabolism comes from genome-wide association studies showing that PCPE2 is a significant modulator of hepatic apoA-I secretion [55].
Procollagen C-endopeptidase protein 2, atherosclerosis and HDL cholesteryl ester catabolism
Intrigued by the paradoxical observations of Francone et al. [53], Pollard et al. [56] investigated whether the higher concentration of enlarged HDL associated with the loss of PCPE2 was atherogenic or atheroprotective. To do this, they crossed PCPE2-/- with LDLr-/- mice to obtain LDLr-/- PCPE2-/- mice. After feeding LDLr-/-, PCPE2-/- and LDLr-/- mice a Western diet for 12 weeks, the aortic root was examined for neutral lipid and immune cell content. Interestingly, LDLr-/- PCPE2-/- mice had a greater extent of aortic root neutral lipid and CD68+ accumulation than did LDLr-/- mice. Furthermore, these mice showed a similar extent of neutral lipid staining and immune cell accumulation as LDLr-/- ApoA-I-/- mice that possess no HDL apoA-I. Taken together, this suggested that in the absence of PCPE2, the increased levels of enlarged HDL were completely dysfunctional, showing for the first time that PCPE2, an extracellular matrix associated protein, confers atheroprotective function to HDL particles in vivo.
To explore the basis for the increased concentrations of enlarged HDL, catabolic studies were conducted using HDL isolated from both diet-fed LDLr-/- PCPE2-/- and LDLr-/- mice. The plasma decay of 125-I apoA-I labelled HDL was monitored as a function of time and found to be delayed in LDLr-/- PCPE2-/- compared with LDLr-/- mice, regardless of the source of HDL particles. These studies suggested that PCPE2 was acting at the level of the tissues/cells and not by virtue of the HDL particle or its cargo. To examine the effect of reduced HDL catabolism on RCT, [3H]-cholesterol loaded J774 cells were intraperitoneal injection injected into mice and their appearance in plasma and faeces monitored. Mice lacking PCPE2-/- showed significant attenuation of RCT [56], suggesting that liver scavenger receptor class B1 (SR-B1) may be impaired.
In mice, increased HDL size and concentration is a hallmark of SR-B1 deficiency [57,58]. Hepatic-specific SR-B1 deficient mice show greater aortic lesion formation and reduced macrophage to liver RCT [59], despite higher HDL concentrations. As reported in genetically modified mice, humans with genetic variants in SR-B1 show a reduced capacity to efflux cholesteryl ester, which leads to greater HDL plasma concentrations and a greater risk for CVD [60,61▪▪,62▪▪,63]. The PCPE2-deficient mice showed a macrophage to faecal RCT rate significantly lower than control LDLr-/- mice and similar to that reported for SR-BI knockout mice. Interestingly, this lower rate occurred despite a two-fold higher level of SR-BI protein in the livers of LDLr-/-, PCPE2-/- mice [56], prompting a closer look at how PCPE2 might influence SR-BI function.
To imagine how PCPE2 might influence SR-BI function, an understanding of SR-B1 structure, is necessary. Details of SR-B1's conformation have been inferred by analogy to the structure of LIMP-2 [64], although a much needed NMR structure nears completion [65▪]. What is known, SR-B1's N- and C-terminal domains span the plasma membrane [66], while the heavily N-glycosylated central region loops out into the extracellular matrix wherein it interacts with HDL particles. The exact mechanism explaining how and where SR-BI selectively removes HDL cholesteryl ester and in the process releasing lipid-poor apoA-I remains to be fully explained [67,68▪]. However, mounting evidence suggests that SR-B1 achieves selective cholesteryl ester uptake following oligomerization [69,70], while the cytoplasmic C-terminal region of SR-B1 bound to Na(+)/H(+) exchange regulatory cofactor NHE-RF3 protein encoded by the PDZK1 gene, a four PDZ domain containing adaptor protein, confers localization [71].
To understand how PCPE2, an enhancer protein found in the ECM, might influence SR-B1 function, Pollard et al. [56] overexpressed PCPE2 in Chinese hamster ovary cells and measured a two-fold increase in the uptake of HDL 3H-cholesteryl oleyl ether. In these studies, SR-B1 protein levels were unchanged but a shift to a higher fluorescence intensity was noted, suggesting that the presence of PCPE2 on the cell surface may induce conformational shift(s) in the extracellular loop region of SR-B1 corresponding to amino acids 38–440 either through direct protein–protein interaction or by enhancing SR-B1 oligomerization on the membrane surface.
Model of PCPE2 influence on SR-BI function and physiological implications
SR-BI belongs to the scavenger receptor protein family of which there are at least eight classes [72]. Using accumulated information, it is believed that class B receptors form multimolecular complexes or signalosomes in which they mediate chaperone functions and ligand internalization, despite the absence of discernable signalling motifs. Several different approaches have shown that SR-BI employs its PDZ-interaction domain and the C-terminal transmembrane domain for HDL-initiated signalling [73], which involves cholesterol sensing. Thus, the physiological implications of SR-BI mediated HDL signalling have been implicated in a variety of systems related to the maintenance of haematopoietic stem cell [74▪] and lymphocyte cholesterol homeostasis [75], which could play an important role in the development of atherosclerosis. To better understand how an extracellular matrix localized collagen processing enhancer protein impacts SR-BI function, a hypothetical model shown in Fig. 1 is described. PCPE2 binds to HSPGs, which are found in the extracellular matrix. One or both CUB domains found in PCPE2 bind to HDL apoA-I and assist in localizing or altering particle stability assisting in the movement of HDL cholesteryl ester to SR-BI for selective uptake. Lipid-poor apoA-I is then released by PCPE2 and it is either remodelled [76] or can bind ABCA1 and initiate the formation of nHDL particles.
FIGURE 1.
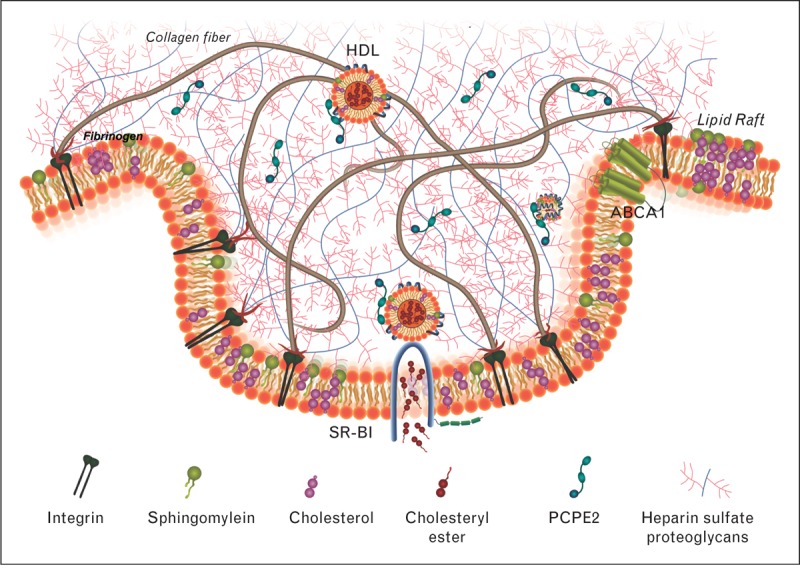
The role of procollagen C-endopeptidase protein 2 (PCPE2) in reverse cholesterol transport. PCPE2 binds to the extracellular matrix through heparin sulphate proteoglycans. One hypothesis of how PCPE2 stimulates scavenger receptor class B1 (SR-BI) cholesteryl ester uptake suggests that PCPE2 binds to plasma HDL thereby inducing conformational changes to the lipoprotein particle promoting the removal of HDL cholesteryl ester.
CONCLUSION
The identification of a new player in the RCT pathway underscores the lack of understanding of HDL cholesteryl ester homeostasis in atherosclerosis. Because RCT and HDL play a pivotal role in cholesterol homeostasis and modulating atherosclerosis, increased attention to mechanistic details will yield new information for predicting risk and eventually show how to pharmacologically control the development of CVD.
Acknowledgements
Instrumentation used for these studies were procured through Grants from the North Carolina Biotechnology Center 2007-IDG-1021 and NIH Shared Instrumentation Grants 1S10RR027940 and 1S10RR17846.
Financial support and sponsorship
This work was supported by Grants from the National Institutes of Health NHLBI HL127649, HL112270 and HL-112276 (M.S.T.), and by a NIH Diversity Supplement HL112270A1-S1 (R.D.P.) and by American Heart Association Grants 09GRNT2280053 and 14GRNT20500029 (M.J.T.).
Conflicts of interest
M.S.T. has received honoraria from Eli Lilly & Co. and Merck & Co. M.J.T. and R.D.P. have no conflicts to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1. Castelli WP, Doyle JT, Gordon T, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977; 55:767–772. [DOI] [PubMed] [Google Scholar]
- 2. Sorci-Thomas MG, Thomas MJ. Why targeting HDL should work as a therapeutic tool, but has not. J Cardiovasc Pharmacol 2013; 62:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, et al. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 2010; 30:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011; 364:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪▪. Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014; 371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]; HDL cholesterol efflux capacity was inversely associated with cardiovascular events in about 3000 adults who participated in the Dallas Heart Study.
- 6. Mulya A, Lee JY, Gebre AK, et al. Minimal lipidation of prebeta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol 2007; 27:1828–1836. [DOI] [PubMed] [Google Scholar]
- 7. Mulya A, Lee JY, Gebre AK, et al. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J Lipid Res 2008; 49:2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪▪. Du X, Kim MJ, Hou L, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res 2015; 116:1133–1142. [DOI] [PubMed] [Google Scholar]; This is a comprehensive study that examines the role of HDL size and cholesterol efflux and finds that smaller HDLs are more efficient mediators of efflux. This study compliments the work of Mulya et al. [6,7], Favari et al. [9] and Asztalos et al. [10].
- 9. Favari E, Lee M, Calabresi L, et al. Depletion of prebeta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J Biol Chem 2004; 279:9930–9936. [DOI] [PubMed] [Google Scholar]
- 10. Asztalos BF, de la Llera-Moya M, Dallal GE, et al. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res 2005; 46:2246–2253. [DOI] [PubMed] [Google Scholar]
- 11. Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem 2014; 289:24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuteja S, Rader DJ. High-density lipoproteins in the prevention of cardiovascular disease: changing the paradigm. Clin Pharmacol Ther 2014; 96:48–56. [DOI] [PubMed] [Google Scholar]
- 13. Rosenson RS, Brewer HB, Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Getz GS, Reardon CA. Cubbing in proapolipoprotein maturation. J Lipid Res 2011; 52:1861–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fielding CJ, Fielding PE. Caveolae and intracellular trafficking of cholesterol. Adv Drug Deliv Rev 2001; 49:251–264. [DOI] [PubMed] [Google Scholar]
- 16. Denis M, Landry YD, Zha X. ATP-binding cassette A1-mediated lipidation of apolipoprotein A-I occurs at the plasma membrane and not in the endocytic compartments. J Biol Chem 2008; 283:16178–16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chau P, Fielding PE, Fielding CJ. Bone morphogenetic protein-1 (BMP-1) cleaves human proapolipoprotein A1 and regulates its activation for lipid binding. Biochemistry 2007; 46:8445–8450. [DOI] [PubMed] [Google Scholar]
- 18. Chau P, Nakamura Y, Fielding CJ, Fielding PE. Mechanism of prebeta-HDL formation and activation. Biochemistry 2006; 45:3981–3987. [DOI] [PubMed] [Google Scholar]
- 19. Sviridov D. Maturation of apolipoprotein A-I: unrecognized health benefit or a forgotten rudiment? J Lipid Res 2009; 50:1257–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sorci-Thomas MG, Owen JS, Fulp B, et al. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three ApoA-I monomers. J Lipid Res 2012; 53:1890–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gordon JI, Smith DP, Andy R, et al. The primary translation product of rat intestinal apolipoprotein A-I mRNA is an unusual preproprotein. J Biol Chem 1982; 257:971–978. [PubMed] [Google Scholar]
- 22. Brewer HB, Jr, Fairwell T, Kay L, et al. Human plasma proapoA-I: isolation and amino-terminal sequence. Biochem Biophys Res Commun 1983; 113:626–632. [DOI] [PubMed] [Google Scholar]
- 23. Zannis VI, Karathanasis SK, Keutmann HT, et al. Intracellular and extracellular processing of human apolipoprotein A-I: secreted apolipoprotein A-I isoprotein 2 is a propeptide. Proc Natl Acad Sci U S A 1983; 80:2574–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLeod RS, Robbins C, Burns A, et al. Deletion of the propeptide of apolipoprotein A-I impairs exit of nascent apolipoprotein A-I from the endoplasmic reticulum. Biochem J 1994; 302:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sviridov D, Fidge N. Efflux of intracellular versus plasma membrane cholesterol in HepG2 cells: different availability and regulation by apolipoprotein A-I. J Lipid Res 1995; 36:1887–1896. [PubMed] [Google Scholar]
- 26. Sviridov D, Pyle L, Fidge N. Identification of a sequence of apolipoprotein A-I associated with the efflux of intracellular cholesterol to human serum and apolipoprotein A-I containing particles. Biochemistry 1996; 35:189–196. [DOI] [PubMed] [Google Scholar]
- 27. Sviridov D, Pyle LE, Jauhiainen M, et al. Deletion of the propeptide of apolipoprotein A-I reduces protein expression but stimulates effective conversion of prebeta-high density lipoprotein to alpha-high density lipoprotein. J Lipid Res 2000; 41:1872–1882. [PubMed] [Google Scholar]
- 28. Pyle LE, Sviridov D, Fidge NH. Characterization of the maturation of human pro-apolipoprotein A-I in an in vitro model. Biochemistry 2001; 40:3101–3108. [DOI] [PubMed] [Google Scholar]
- 29. Sviridov D, Fidge N, Beaumier-Gallon G, Fielding C. Apolipoprotein A-I stimulates the transport of intracellular cholesterol to cell-surface cholesterol-rich domains (caveolae). Biochem J 2001; 358:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asharani PV, Keupp K, Semler O, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet 2012; 90:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hartigan N, Garrigue-Antar L, Kadler KE. Bone morphogenetic protein-1 (BMP-1). Identification of the minimal domain structure for procollagen C-proteinase activity. J Biol Chem 2003; 278:18045–18049. [DOI] [PubMed] [Google Scholar]
- 32. Ricard-Blum S, Bernocco S, Font B, et al. Interaction properties of the procollagen C-proteinase enhancer protein shed light on the mechanism of stimulation of BMP-1. J Biol Chem 2002; 277:33864–33869. [DOI] [PubMed] [Google Scholar]
- 33. Bekhouche M, Kronenberg D, Vadon-Le Goff S, et al. Role of the netrin-like domain of procollagen C-proteinase enhancer-1 in the control of metalloproteinase activity. J Biol Chem 2010; 285:15950–15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldsmith EC, Bradshaw AD, Spinale FG. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol 2013; 304:C393–C402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steiglitz BM, Greenspan DS. Assignment of the mouse Pcolce2 gene, which encodes procollagen C-proteinase enhancer protein 2, to chromosome 9 and localization of PCOLCE2 to human chromosome 3q23. Cytogenet Cell Genet 2001; 95:244–245. [DOI] [PubMed] [Google Scholar]
- 36. Steiglitz BM, Keene DR, Greenspan DS. PCOLCE2 encodes a functional procollagen C-proteinase enhancer (PCPE2) that is a collagen-binding protein differing in distribution of expression and posttranslational modification from the previously described PCPE1. J Biol Chem 2002; 277:49820–49830. [DOI] [PubMed] [Google Scholar]
- 37. Kronenberg D, Vadon-Le Goff S, Bourhis JM, et al. Strong cooperativity and loose geometry between CUB domains are the basis for procollagen c-proteinase enhancer activity. J Biol Chem 2009; 284:33437–33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blanc G, Font B, Eichenberger D, et al. Insights into how CUB domains can exert specific functions while sharing a common fold: conserved and specific features of the CUB1 domain contribute to the molecular basis of procollagen C-proteinase enhancer-1 activity. J Biol Chem 2007; 282:16924–16933. [DOI] [PubMed] [Google Scholar]
- 39. Bourhis JM, Vadon-Le Goff S, Afrache H, et al. Procollagen C-proteinase enhancer grasps the stalk of the C-propeptide trimer to boost collagen precursor maturation. Proc Natl Acad Sci U S A 2013; 110:6394–6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bernocco S, Steiglitz BM, Svergun DI, et al. Low resolution structure determination shows procollagen C-proteinase enhancer to be an elongated multidomain glycoprotein. J Biol Chem 2003; 278:7199–7205. [DOI] [PubMed] [Google Scholar]
- 41. Vadon-Le Goff S, Kronenberg D, Bourhis JM, et al. Procollagen C-proteinase enhancer stimulates procollagen processing by binding to the C-propeptide region only. J Biol Chem 2011; 286:38932–38938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Daly NL, Djordjevic JT, Kroon PA, Smith R. Three-dimensional structure of the second cysteine-rich repeat from the human low-density lipoprotein receptor. Biochemistry 1995; 34:14474–14481. [DOI] [PubMed] [Google Scholar]
- 43. Daly NL, Scanlon MJ, Djordjevic JT, et al. Three-dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor. Proc Natl Acad Sci U S A 1995; 92:6334–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liepinsh E, Banyai L, Pintacuda G, et al. NMR structure of the netrin-like domain (NTR) of human type I procollagen C-proteinase enhancer defines structural consensus of NTR domains and assesses potential proteinase inhibitory activity and ligand binding. J Biol Chem 2003; 278:25982–25989. [DOI] [PubMed] [Google Scholar]
- 45. Banyai L, Patthy L. The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci 1999; 8:1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ulmasov B, Oshima K, Rodriguez MG, et al. Differences in the degree of cerulein-induced chronic pancreatitis in C57BL/6 mouse substrains lead to new insights in identification of potential risk factors in the development of chronic pancreatitis. Am J Pathol 2013; 183:692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hosper NA, Bank RA, van den Berg PP. Human amniotic fluid-derived mesenchymal cells from fetuses with a neural tube defect do not deposit collagen type i protein after TGF-beta1 stimulation in vitro. Stem Cells Dev 2014; 23:555–562. [DOI] [PubMed] [Google Scholar]
- 48▪. Tintle NL, Pottala JV, Lacey S, et al. A genome-wide association study of saturated, mono- and polyunsaturated red blood cell fatty acids in the Framingham Heart Offspring Study. Prostaglandins Leukot Essent Fatty Acids 2015; 94:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using RBC fatty acid data from the Framingham Offspring Study, this study analysed over 2.5 million single-nucleotide polymorphisms (SNPs) for association with 14 RBC fatty acids. One loci, PCOLCE2 (PCPE2), was significantly associated with arachidonic acid levels.
- 49. Baicu CF, Zhang Y, Van Laer AO, et al. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol 2012; 303:H234–H240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu J, Gardner J, Pullinger CR, et al. Regulation of apoAI processing by procollagen C-proteinase enhancer-2 and bone morphogenetic protein-1. J Lipid Res 2009; 50:1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hinds DA, Seymour AB, Durham LK, et al. Application of pooled genotyping to scan candidate regions for association with HDL cholesterol levels. Hum Genomics 2004; 1:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu H, Acott TS, Wirtz MK. Identification and expression of a novel type I procollagen C-proteinase enhancer protein gene from the glaucoma candidate region on 3q21-q24. Genomics 2000; 66:264–273. [DOI] [PubMed] [Google Scholar]
- 53. Francone OL, Ishida BY, de la Llera-Moya M, et al. Disruption of the murine procollagen C-proteinase enhancer 2 gene causes accumulation of pro-apoA-I and increased HDL levels. J Lipid Res 2011; 52:1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heinzel K, Bleul CC. The Foxn1-dependent transcripts PCOLCE2 and mPPP1R16B are not required for normal thymopoiesis. Eur J Immunol 2007; 37:2562–2571. [DOI] [PubMed] [Google Scholar]
- 55. Miles RR, Perry W, Haas JV, et al. Genome-wide screen for modulation of hepatic apolipoprotein A-I (ApoA-I) secretion. J Biol Chem 2013; 288:6386–6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pollard jD, Blesso CN, Zabalawi M, et al. Procollagen C-endopeptidase enhancer protein 2 (PCPE2) reduces atherosclerosis in mice by enhancing SR-BI mediated HDL-cholesteryl ester uptake. J Biol Chem 2015; 290:15496–15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rigotti A, Trigatti B, Babitt J, et al. Scavenger receptor BI - a cell surface receptor for high density lipoprotein. Curr Opin Lipidol 1997; 8:181–188. [DOI] [PubMed] [Google Scholar]
- 58. Rigotti A, Trigatti BL, Penman M, et al. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A 1997; 94:12610–12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Da Silva JR, Reilly M, et al. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest 2005; 115:2870–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. West M, Greason E, Kolmakova A, et al. Scavenger receptor class B type I protein as an independent predictor of high-density lipoprotein cholesterol levels in subjects with hyperalphalipoproteinemia. J Clin Endocrinol Metab 2009; 94:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61▪▪. Vickers KC, Rodriguez A. Human scavenger receptor class B type I variants, lipid traits, and cardiovascular disease. Circ Cardiovasc Genet 2014; 7:735–737. [DOI] [PMC free article] [PubMed] [Google Scholar]; An editorial concerning article by Niemsiri et al. [62▪▪] on human SR-BI variants and their impact on cardiovascular disease.
- 62▪▪. Niemsiri V, Wang X, Pirim D, et al. Impact of genetic variants in human scavenger receptor class B type I (SCARB1) on plasma lipid traits. Circ Cardiovasc Genet 2014; 7:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides new information regarding the genetic role of SCARB1 in determining plasma apoB levels in addition to its established role in HDL metabolism.
- 63. Vergeer M, Korporaal SJ, Franssen R, et al. Genetic variant of the scavenger receptor BI in humans. N Engl J Med 2011; 364:136–145. [DOI] [PubMed] [Google Scholar]
- 64. Neculai D, Schwake M, Ravichandran M, et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013; 504:172–176. [DOI] [PubMed] [Google Scholar]
- 65▪. Chadwick AC, Jensen DR, Peterson FC, et al. Expression, purification and reconstitution of the C-terminal transmembrane domain of scavenger receptor BI into detergent micelles for NMR analysis. Protein Expr Purif 2015; 107:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]; These studies report essential steps in determining the structure of SR-B1 by establishing conditions for expression and NMR analysis of the C-terminal domain.
- 66. Babitt J, Trigatti B, Rigotti A, et al. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem 1997; 272:13242–13249. [DOI] [PubMed] [Google Scholar]
- 67. Meyer JM, Graf GA, van der Westhuyzen DR. New developments in selective cholesteryl ester uptake. Curr Opin Lipidol 2013; 24:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68▪. Rohrl C, Eigner K, Winter K, et al. Endoplasmic reticulum stress impairs cholesterol efflux and synthesis in hepatic cells. J Lipid Res 2014; 55:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work showed that hepatic endoplasmic reticulum stress reduced nascent HDL formation by about 80% due to impaired hepatic ABCA1 function.
- 69. Sahoo D, Peng Y, Smith JR, et al. Scavenger receptor class B, type I (SR-BI) homo-dimerizes via its C-terminal region: fluorescence resonance energy transfer analysis. Biochim Biophys Acta 2007; 1771:818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gaidukov L, Nager AR, Xu S, et al. Glycine dimerization motif in the N-terminal transmembrane domain of the high density lipoprotein receptor SR-BI required for normal receptor oligomerization and lipid transport. J Biol Chem 2011; 286:18452–18464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kocher O, Yesilaltay A, Cirovic C, et al. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J Biol Chem 2003; 278:52820–52825. [DOI] [PubMed] [Google Scholar]
- 72. Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 2013; 13:621–634. [DOI] [PubMed] [Google Scholar]
- 73. Mineo C, Shaul PW. Regulation of signal transduction by HDL. J Lipid Res 2013; 54:2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪. Gao M, Zhao D, Schouteden S, et al. Regulation of high-density lipoprotein on hematopoietic stem/progenitor cells in atherosclerosis requires scavenger receptor type BI expression. Arterioscler Thromb Vasc Biol 2014; 34:1900–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates a novel role of SR-BI in the regulation of haematopoietic stem progenitor cell proliferation and differentiation.
- 75. Feng H, Guo L, Wang D, et al. Deficiency of scavenger receptor BI leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arterioscler Thromb Vasc Biol 2011; 31:2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Curtiss LK, Valenta DT, Hime NJ, Rye KA. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler Thromb Vasc Biol 2006; 26:12–19. [DOI] [PubMed] [Google Scholar]


