Abstract
BACKGROUND:
Phaxan™ (PHAX, Chemic Labs, Canton, MA) is an aqueous solution of 10 mg/mL alphaxalone and 13% 7-sulfobutylether β-cyclodextrin (betadex). In preclinical studies, PHAX is a fast onset–offset IV anesthetic like propofol, but causes less cardiovascular depression. This first-in-man study was designed to find the anesthetic dose of PHAX and to compare it with an equivalent dose of propofol for safety, efficacy, and quality of recovery from anesthesia and sedation.
METHODS:
The study adhered to compliance with Good Clinical Practices regulations (clinical trials registry number, ACTRN12611000343909). This randomized, double-blind study compared PHAX and propofol using a Bayesian algorithm to determine dose equivalence for effects on the bispectral index (BIS). Male volunteers, ASA physical status I, gave written informed consent (n = 12 per group; PHAX or propofol). Parameters assessed for 80 minutes after drug injection (single bolus dose) were pain on injection, involuntary movement, BIS, blood pressure, need for airway support, and, as measures of recovery from sedation, the Richmond Agitation and Sedation Scale and the Digit Symbol Substitution Test. Arterial blood was withdrawn for biochemistry, hematology, and complement levels.
RESULTS:
No subject complained of pain on injection with PHAX, whereas 8 of the 12 subjects given propofol did. Nine PHAX and 8 propofol subjects reached BIS values of ≤50: median (interquartile range [IQR]) mg/kg dose = 0.5 (0.5–0.6) for PHAX and 2.9 (2.4–3.0) for propofol. The lowest median BIS reached was 27 to 28 for both agents with no significant differences between them for timing of onset and recovery of BIS. The concomitant median changes in systolic and diastolic blood pressures were −11% vs −19% for systolic and −25% vs −37% for diastolic in PHAX- and propofol-treated subjects, respectively. Nine of the 12 propofol-treated subjects and none of 12 PHAX-treated subjects required airway support. For subjects reaching an equivalent BIS of ≤50: a Richmond Agitation and Sedation Scale score of 0 was reached at a median of 5 (IQR, 5–10) and 15 (IQR, 10–20) minutes after PHAX and propofol, respectively; BIS returned to 90 at a mean of 21 (SD, 10.1) and 21 (SD, 9.2) minutes after PHAX and propofol, respectively; and Digit Symbol Substitution Test scores returned to predrug injection values at median of 50 (IQR, 35–72.5) and 42.5 (IQR, 35–76.3) minutes after PHAX and propofol, respectively. There was no increase in C3 and C4 complement fractions after either drug.
CONCLUSIONS:
PHAX causes fast-onset, short-duration anesthesia with fast cognitive recovery similar to propofol, but with less cardiovascular depression, or airway obstruction and no pain on injection.
Published ahead of print July 29, 2015
Alphaxalone is an analog of progesterone and its metabolite 3α-hydroxy-5α-pregnan-20-one (allopregnanolone). Allopregnanolone is a neurosteroid that has sedating, anesthetic, anticonvulsant, and neuroprotective properties.1–4 Alphaxalone also has these attributes5–9 and the same receptor-binding properties and actions at GABAA receptors.10,11 However, it is devoid of progestational, estrogenic, mineralocorticoid or thymolytic activity.12
Althesin® (Glaxo Laboratories Ltd., Greenford, Middlesex, UK) was a mixture of alphaxalone with a small amount of a related compound, alphadolone dispersed in water with the aid of Cremophor EL® (CAS registry 61791-12-6). It was used in clinical anesthetic practice for induction and maintenance of anesthesia from 1972 to 1984 in many countries. The anesthetic properties of this preparation were ascribed to the alphaxalone content. The properties of Althesin®, also known as alfadione, were reviewed by Gyermek and Soyka13 in Anesthesiology in 1975, and later in an editorial in the British Journal of Anaesthesia in 1980 by Prys-Roberts and Sear.14 The common view was that Althesin anesthesia was characterized by rapid onset and offset of action, no irritating effects on blood vessels, and minor cardiovascular and respiratory depression with a wide safety margin. Althesin was withdrawn from the market in 1984 because of hypersensitivity reactions caused by the Cremophor EL excipient. There were 3 main types of reactions:
Histaminoid: Peripheral vasodilatation, skin flushes, edema, and wheals
Bronchospasm: Usually accompanied by vasodilatation or hypotension
Cardiovascular collapse not usually accompanied by other features of histaminoid reactions.
The hypersensitivity reactions were investigated extensively. Activation of complement by the classic and indirect pathways was found to be common in cases of hypersensitivity reactions to Althesin.15 Gyermek and Soyka13 had suggested before this that alphaxalone should be reformulated in a water base to avoid the Cremophor EL excipient and the hypersensitivity reactions it caused.
Dissolution of neuroactive steroid anesthetics in an alternative vehicle suitable for human use has proved to be difficult. Lipid emulsions were tried, but failed to produce an agent with suitable onset and offset characteristics.16,17 When Althesin was withdrawn, 2,6, di-isopropyl phenol (also formulated previously in Cremophor EL) was reformulated successfully using lipid emulsion to produce propofol (Diprivan®, AstraZeneca, New South Wales, Australia). Propofol lipid emulsion is the current standard against which all new anesthetic and sedative drugs must be measured. The reformulated propofol has fulfilled the need for a fast-onset, short-action drug, but with the disadvantage of greater cardiovascular and respiratory depression compared with Althesin.18 Further, the lipid in that formulation has caused new safety issues with infections, contamination, and lipid toxicity.19–22 An alternative excipient for lipid-soluble IV anesthetics is clearly warranted.
7-Sulfobutylether β-cyclodextrin (SBECD) is an excipient with a low toxicity and hypersensitivity profile, which has been used to dissolve hydrophobic drugs in water for IV injections suitable for human use.23–27 It has not been previously investigated as an excipient for alphaxalone. Such a preparation, alphaxalone in aqueous solution with SBECD, Phaxan™ (PHAX), has been made and tested in preclinical studies.28 These revealed that PHAX is a fast-onset, short-duration IV anesthetic with times to induce anesthesia and recover that are equal with propofol and Althesin. Furthermore, those studies revealed that PHAX has a higher safety profile (higher therapeutic index) than propofol or Althesin and, in particular, PHAX was shown to cause less cardiovascular depression than propofol.28
The phase 1c human study reported here set out to compare the anesthetic properties of PHAX with the standard lipid preparation of propofol in common clinical use. The following objectives were addressed in this double-blind, dose-finding comparison of PHAX with propofol:
What doses of PHAX and propofol cause the same depth of anesthesia (a BIS value <50)?
When those equivalent doses of PHAX and propofol are administered, is there a difference between the 2 drug treatments for:
Time to induce hypnosis and recover,
Cardiovascular effects,
Respiratory depression and airway obstruction,
Pain on injection,
Involuntary movements or,
Speed and quality of recovery?
Apart from routine biochemical screening, subjects were also screened for complement activation because the previous alphaxalone preparation (Althesin) caused hypersensitivity reactions.15
METHODS
This research was approved by Monash Health Hospital Research Ethics Committee, Approval Number 10327A, and it was also entered into the Australian and New Zealand clinical trials registry on April 1, 2011 (ACTRN12611000343909; A/Prof C Goodchild, principal investigator). This study was performed in compliance with Good Clinical Practices, including the archiving of essential documents.
Data Storage and Verification
Data were collected on paper clinical record forms at the bedside during the trial. The original clinical record forms remained at all times with the principal investigator at Monash Health. The data were extracted from shadow records and entered into a 21cfr part 11 compliant database (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11), and data analysis was performed after data lock. All experiments were also recorded on high-definition video by an independent contractor who time and date stamped all video records by live satellite data feed. The video record was used to verify the accuracy of data entered by the clinical investigators onto the paper clinical record forms, which were the primary source documents in compliance with Good Clinical Practices regulations.
Subjects
Only males were recruited for this preliminary study to avoid data variability introduced by gender-related physiological factors, such as generally lower body weight and organ size, higher percentage of body fat, and lower glomerular filtration rate in women compared with men. Twenty-four male volunteers were recruited by Internet advertising. After screening for satisfaction of inclusion and exclusion criteria, they gave informed written consent to take part in the study. They were randomly assigned to either propofol or PHAX treatment (n = 12 each).
Inclusion and Exclusion Criteria
Men aged 18 to 40 years of only ASA physical status I were included
Body mass index (BMI) 18 to 25
Subjects had nothing to eat or drink after midnight on the day before the study
Subjects had no history of asthma, allergy, or substance abuse
Subjects were not taking regular medication and consumed no alcohol for 24 hours before the study
Subjects with a medical history of muscular dystrophy were excluded
Drug Preparations
The propofol used in these studies was Diprivan (10 mg/mL propofol in 10% soya bean oil emulsion). The alphaxalone was prepared to Current Good Manufacturing Practices standard by Chemic Labs (Canton, MA) supplied by Davos Pharma (Upper Saddle River, NJ) who also sourced and supplied Current Good Manufacturing Practices SBECD. These were combined at a compounding pharmacy (Slade Pharmacy, Richmond, Victoria, Australia). The method used was first dissolution of the SBECD in the final volume of water by stirring and then adding the alphaxalone while continuing to stir. No heating or ultrasonication was necessary to dissolve the alphaxalone in this solution. Sterilization was by filtration using a 0.2-μm Millipore® filter (Sterlitech Corporation, Kent, WA). The resulting PHAX (50-mg vials of alphaxalone 10 mg/mL in aqueous solution with 13% SBECD) and propofol were stored in the clinical trials store in the pharmacy department at Monash Health and dispensed on the day of the trial.
General Conduct of the Study
On arrival in hospital, the height and weight of the subjects were measured to calculate body mass index. The subject performed Digit Symbol Substitution Test (DSST; see in Digit Symbol Substitution Test section below) before being taken to the operating theatre.
Two anesthetists were allocated to the study: the “caring anesthetist” remained on the dominant side of the subject to provide general supportive care and monitoring plus record keeping and the “drug-administering” anesthetist on the nondominant side of the subject. The role of the drug-administering anesthetist was to prepare and administer anesthetic drugs according to a computerized randomization schedule. On arrival, an Allen test was performed in the nondominant hand to check for adequacy of arterial blood supply to the hand from the ulnar artery. An IV cannula (22g) was inserted into a vein on the back of the nondominant hand, and a 20g cannula was inserted into the radial artery of the same hand. The following physiological monitoring was initiated:
Blood pressure;
Pulse oximetry (Drager Primus, Draeger Medical Australia Pty Ltd, Notting Hill VIC Australia; Nellcor probe, Covidien, Dublin, Ireland; and OxySure algorithm, Oxy-Sure Company Ltd., Orofino, ID), electrocardiography, and BIS (BIS Vista 1850151; Aspect Medical Systems, Inc., Norwood, MA) monitor;
Video recording using 2 cameras, one filming the subject and the caring anesthetist plus the data displayed on the monitoring equipment and the other filming the actions of the drug-administering anesthetist.
A cloth barrier was erected with the subject and caring anesthetist on one side and the drug-administering anesthetist on the other side. Thus the nondominant arm with the arterial cannula and IV cannula could not be observed by the subject and caring anesthetist. This barrier blocked from sight of the subject and caring anesthetist what was happening with respect to drug administration. Further, the drug-administering anesthetist could not see the drug effects on monitored parameters.
Dose Titration and Equivalence: Dose and Randomization Procedures
The drug and dose to be administered were determined by the drug-administering anesthetist from a computerized randomization schedule (randomization of 2 treatments into 4 blocks of 6 subjects from Randomization.com). This allocated the subject randomly to receive propofol or PHAX. The computer further calculated the dose to be administered from the subject’s body weight and the planned dose per kilogram according to the Bayesian design, which is shown in Figure 1. The dose of anesthetic drug to be given to a subject was determined by whether the previous subject given that drug was anesthetized (BIS < 50). This design set out to define equivalent doses of each anesthetic that caused anesthesia (BIS < 50) in 100% of subjects. Dose–response relationships for the anesthetic effects of both drugs have been published.29,30 Althesin was used by Clarke et al.29 in their study on alphaxalone. Preclinical studies28 have revealed that PHAX is a fast-onset, short-duration IV anesthetic with doses and times to induce anesthesia and recover that are equal with Althesin. Thus, it was thought appropriate to use data from the study by Clarke et al.29 to estimate the starting dose of PHAX. Therefore, the doses judged to be most likely to be safe and to cause close to 100% subjects being anesthetized (BIS < 50) were taken from those published data. Thus, the initial doses were set at propofol 3 mg/kg and alphaxalone 0.55 mg/kg. The dose decrement and increment for the Bayesian calculations were set at 10% and 20%, respectively (see Statistics). The identity of the drug and dose given were recorded in the clinical record form and kept secret from the caring anesthetist, subject, and postanesthesia care unit (PACU) nurse until the end of the trial and data lock.
Figure 1.
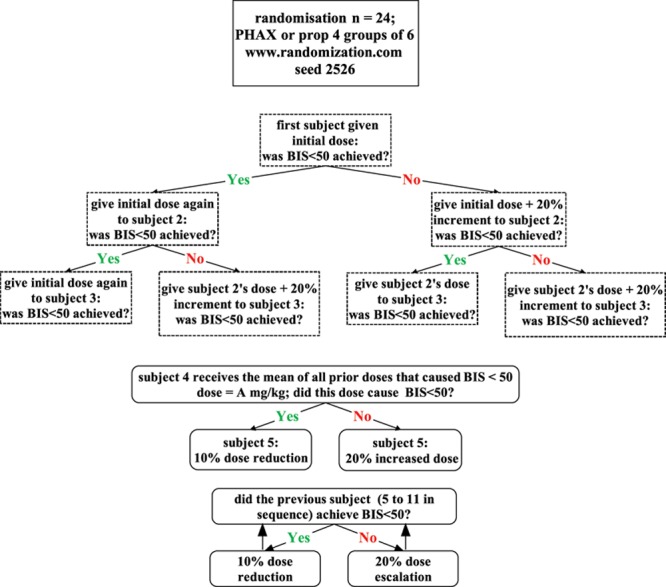
Dose finding design with titration of dose to a common effect (BIS < 50). BIS = bispectral index; PHAX = Phaxan™.
Sequence of Events
Having prepared the drug and dose to be administered, the drug-administering anesthetist struck a bell at the start of the IV injection of anesthetic, PHAX or propofol. This was administered through the 22g cannula in the back of the nondominant hand at a constant rate over a period of 15 seconds. On hearing the bell, the caring anesthetist said to the subject: “We are going to give the drug now. Tell us if you have any discomfort.” The measurements described below were made with no verbal or other communication between the drug-administering anesthetist and the caring anesthetist. Thirty minutes after drug injection, the subject was moved to the PACU, where a qualified recovery nurse continued to monitor vital signs. When the subject was fully recovered, he was returned to the ward and allowed to eat, drink, and move freely around the ward. No alcoholic beverages were allowed. The subject was interviewed and discharged from hospital the following morning and interviewed further by telephone 1 week later.
Measurements
The arterial pressure, systolic and diastolic blood pressures, heart rate, oxygen saturation by pulse oximetry, and BIS values were recorded in the clinical record form every 30 seconds for 5 minutes and every minute for a further 5 minutes and then every 5 minutes thereafter until the subject was discharged to PACU.
Time to Induce Hypnosis and Recover
The BIS values recorded in the clinical record forms for the subjects who received the near equivalent doses of PHAX (n = 9) and propofol (n = 8) were combined according to drug treatment and plotted against time to compare the drugs for time of onset of hypnosis, the depth of BIS depression, and the recovery of BIS values toward the normal awake value of 100. The precise time from the start of the drug injection to when the BIS reached 50 was measured for each of these subjects from the digital video records. These were combined to calculate means for time of onset of hypnosis. The lowest BIS value for each subject and time to recover BIS > 90 were taken from the clinical record form and expressed as means (SD) for each drug treatment to compare PHAX and propofol for depth of hypnosis and time to recover.
Cardiovascular Effects
The values for systolic and diastolic blood pressures and heart rate written in the clinical record forms for the subjects who received the near equivalent doses of PHAX (n = 9) and propofol (n = 8) were combined according to drug treatment and plotted against time to compare the drugs for magnitude of depth and duration of cardiovascular depression.
Respiratory Depression and Airway Obstruction
The caring anesthetist who was unaware of which drug or dose had been given to the subject made continuous clinical assessments of the presence of respiratory effort and upper airway patency when the subject was lying supine. Apnea was scored as present if no breathing efforts were clinically discernible continuously for 30 seconds. Upper airway obstruction was scored for a subject if there was upper airway obstruction for more than 30 seconds requiring airway support in the form of jaw lift. The number of subjects with apnea and airway obstruction was entered into a contingency table for comparison of treatment effect.
Pain on Injection
The drugs were administered through a 22g IV cannula in the dorsum of the nondominant hand in all cases. The injection was given in 15 seconds in all subjects in the absence of any concurrent IV fluid. This injection site was obscured from sight by the subject and caring anesthetist who asked the subject a standardized question inviting a pain report for discomfort in the hand as the drug was injected. The subject was also asked about pain on injection in a structured interview after 24 hours and 7 days. A report by the subject of pain on injection was scored as “yes” or “no.” The incidences of pain on injection were calculated and entered into a contingency table.
Involuntary Movements
The caring anesthetist who was unaware of which drug or dose had been given to the subject made a note in the clinical record form if any involuntary or nonpurposeful movements occurred at any time after drug administration. These were scored as present or absent, and the numbers in each treatment group were entered into a contingency table.
Speed and Quality of Recovery
The speed and quality of recovery were assessed with 2 instruments: The Richmond Agitation and Sedation Scale (RASS)31 and the DSST.32,33
Richmond Agitation and Sedation Scale This assessment was completed 6 times; every 5 minutes starting 5 minutes after drug injection by the caring anesthetist who remained unaware of the drug and dose given to the subject. This is a 10-point numerical rating scale:
+ 4 = Subject is combative, violent, danger to staff
+ 3 = Subject pulls or removes tube(s) or catheters; aggressive
+ 2 = Subject makes frequent nonpurposeful movements
+ 1 = Subject is anxious, apprehensive, but not aggressive
0 = Subject is alert and calm
−1 = Subject awakens to voice (eye opening/contact) >10 seconds
−2 = Subject is lightly sedated, briefly awakening to voice (eye opening/contact) <10 seconds
−3 = Subject is moderately sedated, with some movement or eye opening. No eye contact
−4 = Subject is deeply sedated, with no response to voice, but movement or eye opening to physical stimulation
−5 = Subject is unrousable, no response to voice or physical stimulation
Digit Symbol Substitution Test
A DSST32,33 was completed 4 times at 15-minute intervals before anesthetic drug injection and again 4 more times at 15-minute intervals in the PACU from 35 to 80 minutes after anesthetic drug injection. The 4 tests before PHAX and propofol injection were administered by the caring anesthetist and the 4 tests in PACU were administered by the PACU nurse.
The DSST is a paper-based test. At the top of the page are printed 9 digits, 1 to 9 each paired with a different symbol (e.g., 1/-,2/┴ ... 7/Λ,8/X,9/=). The rest of the page is covered with a list of digits in a random order (e.g., [7][3][4]....) with a blank space under each digit. The subject is asked to write down the corresponding symbol in each blank space, as fast as possible in 90 seconds. The number of correct symbols within the allowed time is measured. The pairing of digit and symbol was kept constant during the study, but the order in which the digits were presented in the list to be completed with symbols was different from one testing time to the next. However, the same list was used for all subjects at a particular test time. The test was repeated on 4 occasions separated by 15 minutes, because it is well known that repeat testing causes improved scores because of learning.34 This produced a “best” prestudy score on the fourth test. The aim of this design was to compare the postsedation scores in each subject with his last best predrug score to assess the time taken to recover from subtle sedating effects of PHAX and propofol.
The times after anesthetic injection for the subjects who received the near equivalent doses of PHAX (n = 9) and propofol (n = 8) to achieve DSST scores in the PACU equal to or greater than the last predrug score were combined to produce medians (IQR) for comparison of the PHAX and propofol in achieving early recovery.
Biochemistry and Hematology
Venous blood was taken for the measurement of total blood count, liver function test, and renal function test before drug administration and once on the morning of discharge. Venous blood was also taken for the measurement of complement fractions (C3 and C4) at 1 and 10 minutes after anesthetic drug injection. Arterial blood was withdrawn for blood gas measurements at 2 and 5 minutes after drug administration.
Statistics
Because this was a phase 1c study with the primary outcome of dose equivalency, a statistical analysis was performed to determine the probability of success using the Bayesian design with the parameters of dose decrement at 10% and increment at 20%. One hundred experiments were performed in silico using published dose response curves29,30 to calculate the probability of anesthesia being produced by the doses of propofol and alphaxalone generated by the Bayesian algorithm. This showed that the dose titration method, with the starting doses set at 3 mg/kg propofol and 0.55 mg/kg PHAX, plus the increment of 10% and decrement of 20%, gave a more than 95% chance of achieving 6 results from 12 experiments that were within 20% of a mean dose, while at the same time allowing for titration below the lowest effective dose.
The doses for each drug that caused the BIS to decrease to ≤50 were combined and expressed as medians and IQR. Subjects who received doses of drug within the IQR of the respective median for each drug were used to compare the effects and side effects of PHAX and propofol.
All statistical analyses were performed using GraphPad Prism version 6.05 for Windows (GraphPad Software, Inc., La Jolla, CA). Unpaired t-test, Wilcoxon matched pairs test, and Mann-Whitney U test, as appropriate, were used to compare the treatments for effects on BIS and cardiovascular parameters. Systolic and diastolic blood pressure measurements were standardized by expressing all values as a percentage of the starting value in that subject, before any statistical analysis. This was done to negate the effects of interindividual variation in starting values.
The incidences of side effects (respiratory obstruction, injection pain, etc.) were entered into a contingency table for all 12 subjects in each treatment group, the treatment effects being compared using Fisher exact test. Each P value was calculated using a corresponding exact method, accurate to greater than 2 significant figures.
RESULTS
PHAX-treated subjects (n = 12) were of mean age 25 (range, 18–29) years and average BMI of 22.1 (range, 18–24.9). Propofol subjects (n = 12) were of mean age 26 (range, 19–33) years and average BMI of 22.3 (range, 18.9–25). The 2 groups did not differ significantly with respect to age (P = 0.38) or BMI (P = 0.80) when analyzed by unpaired t-test.
Dose Titration and Equivalence
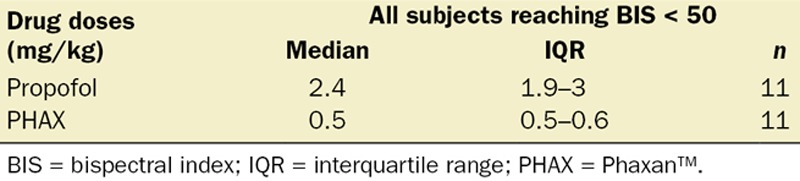
Figure 2 presents the doses of PHAX and propofol administered to each subject in the study. The starting doses caused hypnosis to BIS < 50 in all subjects with both drugs. Progressive decrements in dose eventually led to BIS < 50 not being reached (subject 15 PHAX; subject 21 propofol). Thus, 11 subjects in each group were sedated to a BIS < 50. The median and IQR of these doses are presented in Table 1. Nine doses of the PHAX and 8 doses of the propofol were within the IQR of the median. Data from these subjects, PHAX (median, 0.5 [IQR, 0.5–0.6] mg/kg; n = 9) and propofol (median, 2.9 [IQR, 2.4–3] mg/kg; n = 8) were considered near equipotent and used to make subsequent comparisons between groups.
Figure 2.
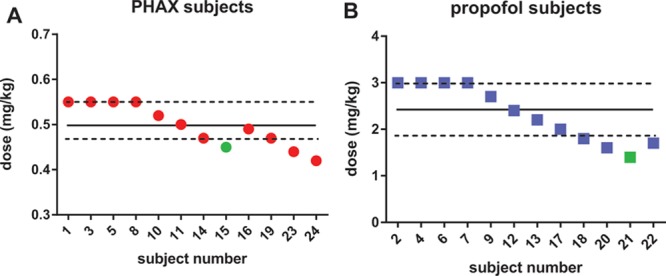
Dose titration to achieve a BIS score below 50 for PHAX (A, red dots, n = 12) and propofol (B, blue squares, n = 12). Symbols in green represent doses that did not cause the BIS to decrease below 50. The solid and dotted lines represent the median and 25th and 75th interquartiles, respectively. BIS = bispectral index; PHAX = Phaxan™.
Table 1.
Dose Titration to Achieve a Bispectral Index <50
Time to Induce Hypnosis and Recover: BIS Measurements
Figure 3 shows the BIS values measured in those subjects (n = 9 PHAX; n = 8 propofol). The solid lines are the mean values plotted against time after the IV bolus injection of anesthetic. The dashed lines are 95% confidence intervals, and the points are the individual values. The lowest BIS values attained were, median (IQR), 28 (21–31; n = 8) and 27 (24–33; n =9) for propofol and PHAX, respectively (P = 0.74; Mann-Whitney U test). The times taken by subjects to reach a BIS < 50, time being measured precisely in seconds from the end of drug injection using the digital video records, were, mean (SD), 34 (13; n = 8) and 34 (12; n = 9) seconds for propofol and PHAX, respectively (P = 1.0; unpaired t-test). The times taken by subjects to recover to a BIS > 90 were, mean (SD), 21 (9.2; n = 8) and 21 (10.1; n = 9) minutes after drug injection for propofol and PHAX, respectively (P = 1.0; unpaired t-test].
Figure 3.

BIS scores over time for subjects treated with PHAX (A, red, n = 9) and propofol (B, blue, n = 8). The solid and dotted lines represent the median and 25th and 75th interquartiles, respectively. BIS = bispectral index; PHAX = Phaxan™.
Cardiovascular Effects
Figure 4 shows the cardiovascular effects of IV injection of 0.5 mg/kg PHAX (median dose; n = 9) in red and 2.9 mg/kg propofol (median dose; n = 8) in blue. The solid lines are medians, and the broken lines IQR. The black points show individual responses. Systolic and diastolic blood pressures have been standardized as a percentage of the preanesthetic starting value for that parameter in that individual. Heart rates are shown as raw values. Comparison of these graphs reveals that propofol caused greater decreases in systolic and diastolic blood pressures than PHAX. This difference occurred even though the same depth and duration of cortical depression was present in both treatment groups evidenced by the same BIS values shown in Figure 3. The maximum decreases in systolic blood pressure, median (IQR), were 12 (11–22) mm Hg for PHAX and 25 (17–28) mm Hg for propofol and diastolic blood pressure were 14 (9–16) mm Hg for PHAX and 26 (22–30) mm Hg for propofol. Further, these differences in pressure occurred when the heart rate increases after administration of anesthetic injection, median (IQR) were: 21 (16–24) beats/minute for PHAX and 15 (11–25) beats/minute for propofol (P = 0.49; Mann-Whitney U test).
Figure 4.
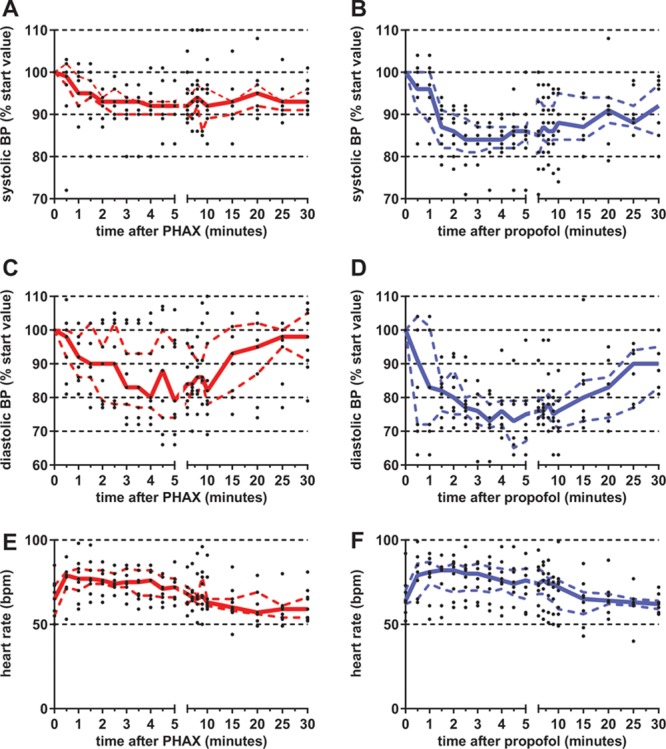
Systolic and diastolic BPs and heart rate after similar hypnotic doses of PHAX (median dose = 0.5 mg/kg; n = 9) and propofol (median dose = 2.9 mg/kg; n = 8). Data are presented as percentages of starting, presedation measurements. Individual measurements are presented as black dots. The solid and dashed lines represent the median and 25th and 75th interquartiles, respectively (red for PHAX and blue for propofol). BP = blood pressure; PHAX = Phaxan™.
When these values are expressed as percentage changes from the preanesthetic values, the median (IQR) for maximum depression of systolic blood pressure was 11% (9–17) for PHAX and 19% (15–23) for propofol (P = 0.043; Mann-Whitney U test). The median (IQR) for maximum depression of diastolic blood pressure was 25% (12–26) for PHAX and 37% (31–39) for propofol (P = 0.0012; Mann-Whitney U test). These differences between PHAX and propofol on blood pressure occurred in the absence of any significant changes in the percentage of increase of heart rate (P = 0.49).
The cardiovascular data were analyzed further by measurement of the area under the curve (AUC) of the plot of time after anesthetic injection versus heart rate, and systolic and diastolic blood pressures were expressed as a percentage of starting (preanesthetic level) in Figure 4. Only the first 10 minutes of the anesthetic were included in this comparison, i.e., the time when the BIS value was <75 as shown in Figure 3. Thus, each subject produced 1 number for AUC for each parameter (n = 9 for PHAX-treated subjects and n = 8 for propofol-treated subjects). These values were combined for each parameter and drug treatment, expressed as median (IQR). Comparison of the AUC values with Mann-Whitney U tests revealed that propofol treatment caused greater depression of systolic (P = 0.046) and diastolic (P = 0.021) blood pressures but the changes in heart rate were the same for both treatments (P = 0.89).
Respiratory Depression and Airway Obstruction
The incidences of side effects noted in all 24 subjects are shown in Table 2. No apneas occurred that required ventilatory support, but 9 of the 12 propofol-treated subjects did require airway support. Comparison of treatments statistically using Fisher exact test confirmed that propofol caused more upper airway obstruction than PHAX (P = 0.0028; Table 2).
Table 2.
Side-Effect Profile for Propofol and PHAX

Pain on Injection
There was no pain on injection reported during PHAX administration or reported by the PHAX-treated subjects in the postoperative interviews. Pain on injection was reported by 8 of the 12 subjects who received propofol (P = 0.0013, Fisher exact test; Table 2).
Involuntary Movements
Involuntary movements occurred only in propofol-treated subjects (Table 2). Further, emergence delirium (defined as a RASS score >0) occurred only in propofol-treated subjects. Subject 9 scored +3 on the RASS scale and subjects 13 and 14 scored +1 5 minutes after propofol injection. The course of hypnosis induction and recovery from sedation was smoother with PHAX than propofol.
Other Side Effects
No nausea or vomiting was noted by research staff or reported in the follow-up questionnaires at 24 hours and 1 week after study (Table 2). No hallucinations were reported in either group.
Speed and Quality of Recovery Richmond Agitation and Sedation Scale
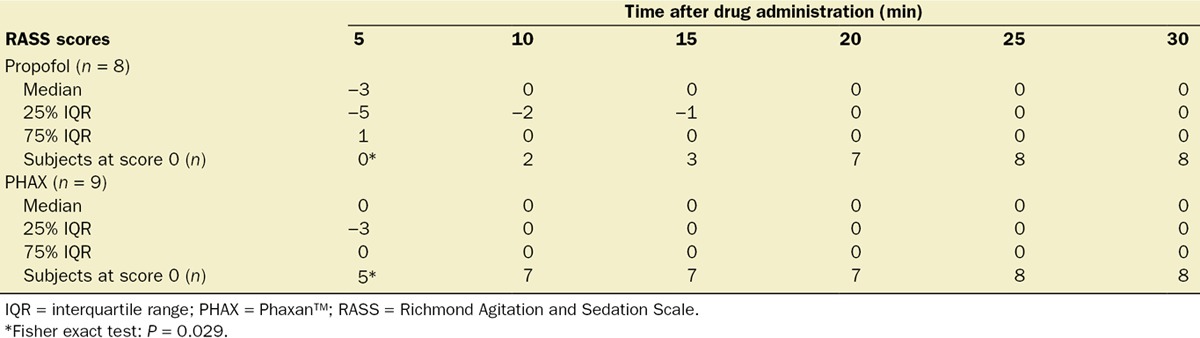
The RASS scores that were measured at 5-minute intervals from 5 to 30 minutes after IV anesthetic injection are shown in Table 3. The scores are shown as medians (IQR) and also as the number of subjects scoring 0 (meaning awake and calm) in this test at each time point. There were no significant differences between treatments in the RASS scores except at 5 minutes postanesthetic when a greater proportion of PHAX-treated subjects were awake and calm (P = 0.029; Fisher exact test).
Table 3.
Richmond Agitation and Sedation Scale Scores After IV Injection of PHAX and Propofol
Digit Symbol Substitution Test
Results of the DSST are presented in Figure 5. After sedation, the time that the DSST score was equal to or greater than the last DSST score before sedation was recorded for each subject. There were no significant differences between PHAX- and propofol-treated subjects in the time taken after drug injection for DSST scores to return to presedation levels (P = 0.88; Mann-Whitney U test).
Figure 5.
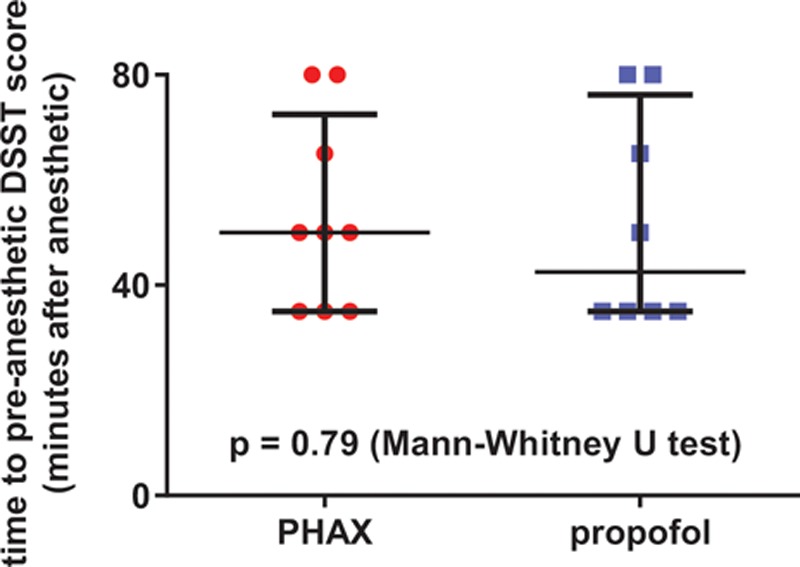
Recovery times for Digit Symbol Substitution Test scores to return to preanesthetic levels (PHAX, red circles; median dose = 0.5 mg/kg, n = 9; propofol, blue squares, median dose = 2.9 mg/kg, n = 8). Horizontal bars represent medians and interquartiles. PHAX = Phaxan™.
Biochemistry and Hematology
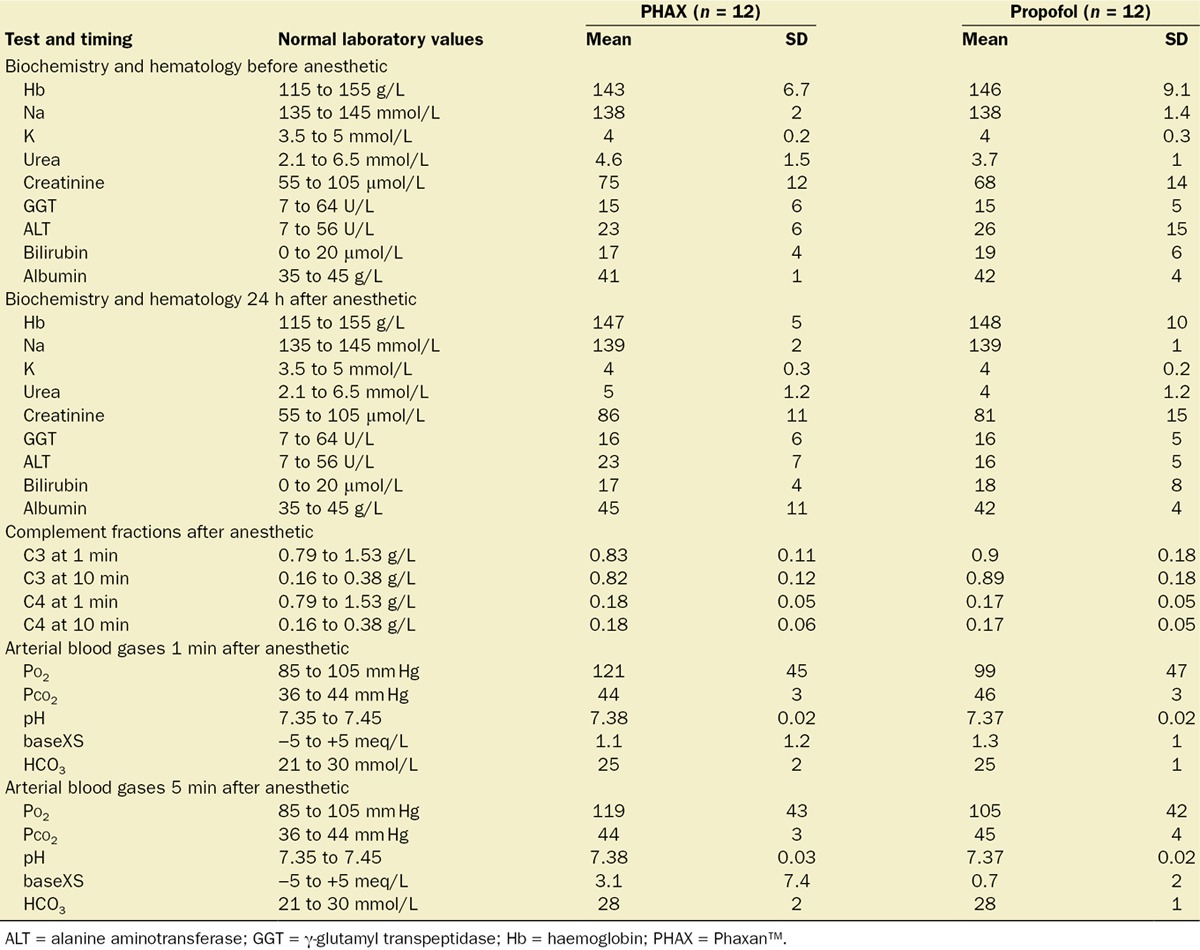
No biochemical or hematologic abnormalities were found, and no differences were noted between treatment groups. Plasma complement fraction levels (C3 and C4) measured at 1 and 10 minutes after bolus administration of each drug were normal for both PHAX- and propofol-treated subjects. The results of the laboratory analysis of the venous and arterial blood samples for all subjects are given in Table 4.
Table 4.
Biochemistry and Hematology Results
DISCUSSION
The aim of this phase 1c clinical trial was to compare the time course of effects and side effect profiles from equivalent doses of PHAX and propofol in healthy male subjects. In this preliminary report, our most important findings were that 0.5 mg/kg alphaxalone as PHAX produced sedation at an equivalent speed of onset and offset with respect to BIS depression with 2.9 mg/kg propofol. The major differences between alphaxalone and propofol were the side-effect profiles at equipotent doses. Specifically, we found the following results:
No pain on injection with PHAX compared with pain on injection with propofol in 8 of 12 subjects (Table 2)
No difference in involuntary movements during induction
No difference in time to return of responsiveness and normal cognition
Less cardiovascular depression with PHAX compared with propofol (Fig. 4)
No airway obstruction requiring jaw lift intervention with PHAX compared with airway obstruction with propofol in 9 of 12 subjects (Table 2)
Previous work exploring cardiovascular depression caused by propofol and alphaxalone has reported similar findings. For example, in one study, an induction dose of propofol (as Diprivan; 1–2 mg/kg) was compared with an induction dose alphaxalone (as Althesin; 0.5 mg/kg).18 In that study, the authors found that propofol produced more respiratory and cardiovascular depression than Althesin.18 In another study exploring the hemodynamic impact of Althesin and propofol in elderly vascular patients, the authors found that Althesin produced less hemodynamic compromise compared with equi-hypnotic doses of propofol.35,36 Although preliminary, the clinical implication of the smaller decrease in blood pressure with PHAX versus propofol suggests that PHAX may be better tolerated in patients with cardiovascular instability or advanced age.
The mechanisms behind why equipotent doses of alphaxalone and propofol that produce a similar time course in cerebral cortical depression, yet yield very different effects in blood pressure and airway patency, are not well understood. One potential explanation for the difference in airway patency is the distribution of GABAA subunits targeted by PHAX and propofol. Previous work has established that there is a relative paucity of GABAA subunits necessary for neurosteroid action in the brainstem compared with the cerebral cortex37–39 and that alphaxalone has little activity in the brainstem.40 A potential explanation for the difference in blood pressure is that propofol increases capacitance and decreases venous return by a direct action on the smooth muscle of veins41,42 and alphaxalone does not. These differences in side effects warrant further study.
The RASS and DSST analysis revealed that the rates of recovery were similar for both sedatives. Our RASS data showed that subjects sedated with alphaxalone were more awake and calm at 5 minutes after drug administration compared with propofol (Table 3), but after 5 minutes, the recovery profile was similar between groups. Similarly, our DSST analysis revealed no difference between groups in the time required for DSST scores to return to presedation levels (Fig. 5). However, our RASS analysis did detect emergence delirium (scores > 0) in 3 subjects sedated with propofol but reported no emergence delirium in subjects sedated with PHAX.
By comparison with a previous formulation of alphaxalone (Althesin), our study revealed 2 important points:
Limitations of the Study
There are several limitations to this study. Although our findings are promising, by design, the group sizes were small in this phase 1c trial. Additional work is required to confirm our findings regarding the side-effect profile, safety, and efficacy of alphaxalone. Another limitation was that our subject recruitment, by design only included male subjects. Only men were recruited for this preliminary study to avoid data variability introduced by gender-related physiological factors. Future studies may show a gender difference. However, it has been reported that there were no gender differences for Althesin.46 A further limitation relates to the fact that young subjects were used. If this study were repeated in older subjects, it is possible that the dose–effect relationship for both hypnosis and cardiovascular and respiratory effects would be different.
CONCLUSIONS
In this first-in-man trial, a new aqueous formulation of alphaxalone in SBECD PHAX has a similar potency to Althesin, a similar onset and offset of sedation to propofol, and a rapid recovery in cognitive function. Unlike propofol, PHAX causes no pain on injection or emergence delirium. In conclusion, PHAX may have potential to match propofol in performance as a fast-onset, short-acting IV anesthetic but with less cardiovascular depression, airway obstruction, and pain on injection.
DISCLOSURES
Name: Colin S. Goodchild, MA, MB, BChir, PhD, FRCA, FANZCA, FFPMANZCA.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: Colin S. Goodchild has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Conflicts of Interest: Colin S. Goodchild has an equity interest in Drawbridge Pharmaceuticals Pty Ltd. He is cited as inventor on PhaxanTM patents and is also a Director and owner of Drawbridge Pharmaceuticals to which the Phaxan patents have been assigned. He was not involved with subject management, i.e., he was not a party to obtaining informed consent or the conduct of the experiment in the operating theatre or postanesthesia care unit; he was not in the hospital when the experiments were being conducted.
Name: Juliet M. Serrao, MBBS, PhD, FRCA.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: Juliet M. Serrao has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Conflicts of Interest: Juliet M. Serrao has an equity interest in Drawbridge Pharmaceuticals Pty Ltd. She is cited as inventor on PhaxanTM patents and is also a Director and owner of Drawbridge Pharmaceuticals to which the Phaxan patents have been assigned. She was not involved with subject management, i.e., she was not a party to obtaining informed consent or the conduct of the experiment in the operating theatre or postanesthesia care unit; she was not in the hospital when the experiments were being conducted.
Name: John Monagle, MBBS, MSc, FANZCA, FACHSM, FIPP (WIP).
Contribution: This author helped conduct the study.
Attestation: John Monagle has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Lyndon Siu, MBBS, FANZCA.
Contribution: This author helped conduct the study.
Attestation: Lyndon Siu has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Jodie Worrell, RN.
Contribution: This author helped conduct the study.
Attestation: Jodie Worrell has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Conflicts of Interest: The author has no conflicts of interest to declare.
This manuscript was handled by: Ken B. Johnson, MD.
ACKNOWLEDGMENTS
The authors thank Professor John Sear for his valuable advice in preparation of the manuscript and being a mentor for Drs. Goodchild and Serrao; the staff at the Pharmacy Department at Monash Medical Centre and at Jessie McPherson Private hospital for accommodating the needs of compliance with Good Clinical Practices clinical trial.
Footnotes
Funding: This work was funded entirely by Drawbridge Pharmaceuticals.
Conflict of Interest: See Disclosures at the end of the article.
Reprints will not be available from the authors.
REFERENCES
- 1.Bitran D, Purdy RH, Kellogg CK. Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol Biochem Behav. 1993;45:423–8. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- 2.Korneyev A, Costa E. Allopregnanolone (THP) mediates anesthetic effects of progesterone in rat brain. Horm Behav. 1996;30:37–43. doi: 10.1006/hbeh.1996.0006. [DOI] [PubMed] [Google Scholar]
- 3.Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di Michele F, Holsboer F, Rupprecht R. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282:1213–8. [PubMed] [Google Scholar]
- 4.Moralí G, Montes P, Hernández-Morales L, Monfil T, Espinosa-García C, Cervantes M. Neuroprotective effects of progesterone and allopregnanolone on long-term cognitive outcome after global cerebral ischemia. Restor Neurol Neurosci. 2011;29:1–15. doi: 10.3233/RNN-2011-0571. [DOI] [PubMed] [Google Scholar]
- 5.Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9:241–50. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervantes M, Ruelas R, Sánchez R, Alvarez-Reséndiz G. Brain injury following cardiorespiratory arrest in cats. Effects of alphaxalone-alphadolone. Bol Estud Med Biol. 1989;37:17–27. [PubMed] [Google Scholar]
- 7.Gran L, Bergsholm P, Bleie H. Seizure duration in unilateral electroconvulsive therapy. A comparison of the anaesthetic agents etomidate and Althesin with methohexitone. Acta Psychiatr Scand. 1984;69:472–83. doi: 10.1111/j.1600-0447.1984.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 8.Hansen SL, Sperling BB, Sánchez C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:105–13. doi: 10.1016/j.pnpbp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Munari C, Casaroli D, Matteuzzi G, Pacifico L. The use of althesin in drug-resistent status epilepticus. Epilepsia. 1979;20:475–83. doi: 10.1111/j.1528-1157.1979.tb04829.x. [DOI] [PubMed] [Google Scholar]
- 10.Cottrell GA, Lambert JJ, Peters JA. Modulation of GABAA receptor activity by alphaxalone. Br J Pharmacol. 1987;90:491–500. doi: 10.1111/j.1476-5381.1987.tb11198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–92. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 12.Child KJ, Currie JP, Dis B, Dodds MG, Pearce DR, Twissell DJ. The pharmacological properties in animals of CT1341—a new steroid anaesthetic agent. Br J Anaesth. 1971;43:2–13. doi: 10.1093/bja/43.1.2-a. [DOI] [PubMed] [Google Scholar]
- 13.Gyermek L, Soyka LF. Steroid anesthetics. Anesthesiology. 1975;42:331–44. doi: 10.1097/00000542-197503000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Prys-Roberts C, Sear J. Steroid anaesthesia. Br J Anaesth. 1980;52:363–5. doi: 10.1093/bja/52.4.363. [DOI] [PubMed] [Google Scholar]
- 15.Radford SG, Lockyer JA, Simpson PJ. Immunological aspects of adverse reactions to althesin. Br J Anaesth. 1982;54:859–63. doi: 10.1093/bja/54.8.859. [DOI] [PubMed] [Google Scholar]
- 16.Hering WJ, Ihmsen H, Langer H, Uhrlau C, Dinkel M, Geisslinger G, Schüttler J. Pharmacokinetic-pharmacodynamic modeling of the new steroid hypnotic eltanolone in healthy volunteers. Anesthesiology. 1996;85:1290–9. doi: 10.1097/00000542-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Høgskilde S, Wagner J, Carl P, Sørensen MB. Anaesthetic properties of pregnanolone emulsion. A comparison with alphaxolone/alphadolone, propofol, thiopentone and midazolam in a rat model. Anaesthesia. 1987;42:1045–50. doi: 10.1111/j.1365-2044.1987.tb05166.x. [DOI] [PubMed] [Google Scholar]
- 18.Kay B, Stephenson DK. ICI 35868 (Diprivan): a new intravenous anaesthetic. A comparison with Althesin. Anaesthesia. 1980;35:1182–7. doi: 10.1111/j.1365-2044.1980.tb05075.x. [DOI] [PubMed] [Google Scholar]
- 19.Devaud JC, Berger MM, Pannatier A, Marques-Vidal P, Tappy L, Rodondi N, Chiolero R, Voirol P. Hypertriglyceridemia: a potential side effect of propofol sedation in critical illness. Intensive Care Med. 2012;38:1990–8. doi: 10.1007/s00134-012-2688-8. [DOI] [PubMed] [Google Scholar]
- 20.Jensen V, Rappaport BA. The reality of drug shortages—the case of the injectable agent propofol. N Engl J Med. 2010;363:806–7. doi: 10.1056/NEJMp1005849. [DOI] [PubMed] [Google Scholar]
- 21.Wachowski I, Jolly DT, Hrazdil J, Galbraith JC, Greacen M, Clanachan AS. The growth of microorganisms in propofol and mixtures of propofol and lidocaine. Anesth Analg. 1999;88:209–12. doi: 10.1097/00000539-199901000-00039. [DOI] [PubMed] [Google Scholar]
- 22.Wong JM. Propofol infusion syndrome. Am J Ther. 2010;17:487–91. doi: 10.1097/MJT.0b013e3181ed837a. [DOI] [PubMed] [Google Scholar]
- 23.Clark AM, Kriel RL, Leppik IE, Marino SE, Mishra U, Brundage RC, Cloyd JC. Intravenous topiramate: comparison of pharmacokinetics and safety with the oral formulation in healthy volunteers. Epilepsia. 2013;54:1099–105. doi: 10.1111/epi.12134. [DOI] [PubMed] [Google Scholar]
- 24.Koltun M, Morizzi J, Katneni K, Charman SA, Shackleford DM, McIntosh MP. Preclinical comparison of intravenous melphalan pharmacokinetics administered in formulations containing either (SBE)7 m-β-cyclodextrin or a co-solvent system. Biopharm Drug Dispos. 2010;31:450–4. doi: 10.1002/bdd.725. [DOI] [PubMed] [Google Scholar]
- 25.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36:30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 26.McIntosh MP, Schwarting N, Rajewski RA. In vitro and in vivo evaluation of a sulfobutyl ether beta-cyclodextrin enabled etomidate formulation. J Pharm Sci. 2004;93:2585–94. doi: 10.1002/jps.20160. [DOI] [PubMed] [Google Scholar]
- 27.Egan TD, Kern SE, Johnson KB, Pace NL. The pharmacokinetics and pharmacodynamics of propofol in a modified cyclodextrin formulation (Captisol) versus propofol in a lipid formulation (Diprivan): an electroencephalographic and hemodynamic study in a porcine model. Anesth Analg. 2003;97:72–9. doi: 10.1213/01.ane.0000066019.42467.7a. [DOI] [PubMed] [Google Scholar]
- 28.Goodchild CS, Serrao JM, Kolosov A, Boyd BJ. Alphaxalone reformulated: a water-soluble intravenous anesthetic preparation in sulfobutyl-ether-β-cyclodextrin. Anesth Analg. 2015;120:1025–31. doi: 10.1213/ANE.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 29.Clarke RS, Dundee JW, Carson IW. Some aspects of the clinical pharmacology of Althesin. Postgrad Med J. 1972;48(suppl 2):62–5. [PubMed] [Google Scholar]
- 30.Naguib M, Sari-Kouzel A, Seraj M, el-Gammal M, Gomma M. Induction dose-responses studies with propofol and thiopentone. Br J Anaesth. 1992;68:308–10. doi: 10.1093/bja/68.3.308. [DOI] [PubMed] [Google Scholar]
- 31.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 32.Patterson KW, Casey PB, Murray JP, O’Boyle CA, Cunningham AJ. Propofol sedation for outpatient upper gastrointestinal endoscopy: comparison with midazolam. Br J Anaesth. 1991;67:108–11. doi: 10.1093/bja/67.1.108. [DOI] [PubMed] [Google Scholar]
- 33.Stone BM. Pencil and paper tests—sensitivity to psychotropic drugs. Br J Clin Pharmacol. 1984;18(suppl 1):15S–20S. doi: 10.1111/j.1365-2125.1984.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zihl J, Fink T, Pargent F, Ziegler M, Bühner M. Cognitive reserve in young and old healthy subjects: differences and similarities in a testing-the-limits paradigm with DSST. PLoS One. 2014;9:e84590. doi: 10.1371/journal.pone.0084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monk CR, Coates DP, Prys-Roberts C, Turtle MJ, Spelina K. Haemodynamic effects of a prolonged infusion of propofol as a supplement to nitrous oxide anaesthesia. Studies in association with peripheral arterial surgery. Br J Anaesth. 1987;59:954–60. doi: 10.1093/bja/59.8.954. [DOI] [PubMed] [Google Scholar]
- 36.Sear JW, Prys-Roberts C. Dose-related haemodynamic effects of continuous infusions of Althesin in man. Br J Anaesth. 1979;51:867–73. doi: 10.1093/bja/51.9.867. [DOI] [PubMed] [Google Scholar]
- 37.Wegner F, Rassler C, Allgaier C, Strecker K, Wohlfarth K. Auto-modulation of neuroactive steroids on GABA A receptors: a novel pharmacological effect. Neuropharmacology. 2007;52:672–83. doi: 10.1016/j.neuropharm.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–9. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- 39.Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- 40.Thornton C, Heneghan CP, Navaratnarajah M, Jones JG. Selective effect of althesin on the auditory evoked response in man. Br J Anaesth. 1986;58:422–7. doi: 10.1093/bja/58.4.422. [DOI] [PubMed] [Google Scholar]
- 41.Bentley GN, Gent JP, Goodchild CS. Vascular effects of propofol: smooth muscle relaxation in isolated veins and arteries. J Pharm Pharmacol. 1989;41:797–8. doi: 10.1111/j.2042-7158.1989.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 42.Goodchild CS, Serrao JM. Cardiovascular effects of propofol in the anaesthetized dog. Br J Anaesth. 1989;63:87–92. doi: 10.1093/bja/63.1.87. [DOI] [PubMed] [Google Scholar]
- 43.Clarke RS, Dundee JW, Carson IW. Some aspects of the clinical pharmacology of Althesin. Postgrad Med J. 1972;48(suppl 2):62–5. [PubMed] [Google Scholar]
- 44.Carson IW, Dundee JW, Clarke RS. The speed of onset and potency of althesin. Br J Anaesth. 1975;47:512–5. doi: 10.1093/bja/47.4.512. [DOI] [PubMed] [Google Scholar]
- 45.Hannington-Kiff JG. Comparative recovery rates following induction of anaesthesia with Althesin and methohexitone in out-patients. Postgrad Med J. 1972;48(suppl 2):116–20. [PubMed] [Google Scholar]
- 46.Swerdlow M. Studies with Althesin—a new steroid anaesthetic. Postgrad Med J. 1972;48(suppl 2):105–8. [PubMed] [Google Scholar]


