Abstract
Hypothesis. The growth hormone (GH) excess displayed in acromegaly induces insulin resistance up to diabetes mellitus (DM). The somatostatin analogues (as octreotide LAR) are useful in controlling the GH levels but disturbances of glucose metabolism might be seen.
Objective. This study evaluates the acromegalic glycemic profile under octreotide.
Methods & Results. Out of the total number of patients (N=34) diagnosed with active acromegaly, only some were followed (N=25; male/ female ratio: 6/ 19; mean age: 51.8 years) by testing GH, IGF1 (Insulin Growth Factor 1), basal glucose and oral glucose tolerance test (OCGTT) at baseline, 6 and 12 months under Octreotide (first 6 months with 20 mg/ 28 days + 6 months with 30 mg/ 28 days). Pre-treatment values were 17.6% of patients had DM, 14.7% - impaired glucose tolerance, 26.5% - impaired fasting glucose, and 41.2% - normal assays. From the statistical point of view, the DM patients were significantly older and had higher GH levels than the acromegalic without glycaemia disturbances. They did not achieve significant changes in basal blood glucose and glycated hemoglobin after 6 months, neither after 12 months.
After 6 months, there were no significant changes in basal glycaemia in patients with normal baseline glycaemia but 2-hours OGTT glucose values were significantly lower than initially (82.35 mg/ dl vs. 93 mg/ dl, p=0.005) consistent with reduced levels of GH and IGF1. After 12 months, both basal and 2-hours glucose levels in OGTT were similar to baseline despite the significant lower GH (3.3 vs. 6.61 ng/ mL, p=0.003) and IGF1 (332 vs. 713 ng/ mL, p=0.001).
Conclusions. Octreotide therapy induces an improvement in glycemic profile in patients with active acromegaly without diabetes mellitus consistent with decreased levels of GH and IGF1. In patients with diabetes, partial control of glucose metabolism is due to inadequate suppression of GH and IGF1 after one year of treatment.
Keywords: acromegaly, octreotide, glycaemia, diabetes mellitus
Introduction & Aim
Acromegaly is a classical endocrine disorder due to growth hormone excess seen after the growth cartilages are closed [1-3]. It is twice more frequent in women according to some studies and its prevalence is 5-7 cases in 100,000 people [1-4]. The condition associates metabolic disturbances in glucose and lipids metabolism increasing the cardiovascular risk [1]. In case of normal subjects, the growth hormone (GH) causes an increase of gluconeogenesis and lipolysis with consecutive high levels of glucose and free fatty acids, which further stimulates the insulin secretion despite augmented glycaemia [2]. Opposite to GH actions, the Insulin Growth Factor 1 (IGF1) increases insulin sensibility especially at muscle levels [2]. In acromegaly, the glucose tolerance is frequently altered; diabetes mellitus and impaired glucose tolerance are registered in 40-50% of the patients [5-7]. Persistent exposure to GH excess induces insulin resistance with secondary anomalies of hepatic and peripheral insulin activity [8]. The therapy in acromegaly is focused on lower GH levels and thus the insulin resistance and the associated cardio-metabolic risk [9,10]. After the first line option, which is pituitary surgery, the medical approach controls the GH and IGF1 levels in 60% of the cases [9,10]. The general data related to the precise effect on glucose metabolism of somatostatin analogues are still a matter of debate since in lower GH, we might expect a glycaemia improvement but by mimicking the physiological somatostatin, the pancreatic insulin is inhibited [11]. The study is aimed at observing the effect of the medical therapy in acromegalic glucose metabolism.
Material & Method
This is an observational study in subjects admitted in a single tertiary endocrinology centre. The inclusion criteria were confirmation of acromegaly; therapy with somatostatin analogues: octreotide LAR in the first 6 months with 20 milligrams (mg) at every 28 days and then another 6 months with a single dose of 30 mg at every 28 days. The exclusion criteria were medical therapy of less than 1 year. The assays were blood GH (RadioImmunoAssay or RIA method), IGF1 (RIA method, age matched values, levels under 300 mg/ dL have been considered as normal), fasting glycaemia, and 75 grams (g) oral glucose tolerance test (OGTT) with both glucose and GH assays. All the measurements were performed at baseline (pre-treatment levels), and during follow-up after 6, respective 12 months of octreotide therapy. OGTT was not performed if the patient previously had diabetes mellitus, instead glycated haemoglobin (A1c) was measured and also 24-hours GH profile (a blood assay at every 4 hours). All the blood tests were performed with a day prior to the next octreotide injection. The American Diabetes Association (ADA) 2014 criteria were used to define diabetes mellitus (DM) as A1c ≥ 6.5% or fasting plasma glucose (FPG) ≥ 126 mg/ dL or 2-hour PG (plasma glucose) ≥ 200mg/ dL or a random PG ≥ 200 mg/ dL [12]. The categories between normal glucose profile and DM are pre- diabetes meaning impaired fasting glucose (IFG) with a FPG of 100 to 125 mg/ dL or impaired glucose tolerance (IGT) with a 2-h PG in 75-g OGTT of 140 to 199 mg/ dL [12]. Statistical analysis (Excel, SPSS) used student t-test with statistical significance at p<0.01.
Results
Baseline analysis (pre-treatment)
Out of 34 patients (male/ female ratio of 7/ 27) with active acromegaly, 25 were followed for 1 year (male/ female ratio of 6/ 19, mean age at baseline of 51.8 years. Applying ADA criteria 44% had normal glucose parameters; 28% had IFG, 16% had IGT and 12% DM (Table 1).
Table 1.
The glucose metabolism profile at baseline (N=25) in acromegalic patients (pre-treatment values)
| Acromegaly | Normal Glucose Profile | IGT+IFG | DM |
|---|---|---|---|
| N=25 | N=11 (44%) | N=4+7 (16%+28%) | N=3 (12%) |
| women N=19 | N=9 (36%) | N=3+5 (12%+20%) | N=2 (8%) |
| men N=6 | N=2 (8%) | N=1+2 (4%+8%) | N=1 (4%) |
| IGT = Impaired Glucose Tolerance, IFG = Impaired Fasting Glucose, DM = Diabetes Mellitus |
The mean age at diagnosis in patients with DM was 56 years versus 49 years in subjects with normal glycaemia (p<0.005). 3 groups were formed based on age at diagnosis: 30-50 years, 51-60 years, 61-70 years (most of subjects were in the first group) (Table 2).
Table 2.
The glucose metabolism profile at baseline (N=25) in acromegalic patients (pre-treatment values)
| Age groups (years) | Number of patients N=25 | Normal glucose profile N=11 | IGT+IFG N=4+7 | DM N=3 |
|---|---|---|---|---|
| 30-50 | 14 | 8 (57%) | 6 (42.8%) | 1 (7%) |
| 51-60 | 4 | 2(50%) | 3 (75%) | 0 |
| 61-70 | 7 | 1 (14%) | 2 (28.6%) | 2(28%) |
| IGT = Impaired Glucose Tolerance, IFG = Impaired Fasting Glucose, DM = Diabetes Mellitus |
A higher frequency of pre- diabetes was seen in 51-60 years group and DM was twice more frequent as normal glucose profile in the group with oldest subjects (Table 2).
Follow-up analysis (at 6 and 12 months)
After 6 months of therapy, the subjects with normal glucose profile at diagnosis did not significantly change the fasting glycaemia (89.71 mg/ dL vs. 93 mg/ dL, p=0.65) but the 2-h PG (OGTT) was statistically significantly lower than baseline (82.35 mg/ dL vs. 93 mg/ dL, p=0.005) according to the lowering of the GH and IGF1 levels, but only one subject had a complete control of acromegaly (by normal IGF1 and a GH level less than 1 ng/ mL in OGTT) (Fig. 1).
Fig. 1.
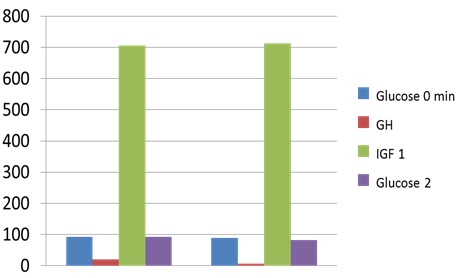
Fasting Glucose (Glucose 0 min; mg/ dL) and 2-hour Plasma Glucose (Glucose 2; mg/ dL) in 75 g OGTT; GH (ng/ mL) and IGF1 (ng/ mL) levels after 6 months of octreotide LAR (a dose of 20 mg at every 28 days) in patients with active acromegaly and normal glucose metabolism at baseline (pre-treatment)
The patients with DM had no statistically significant changes in A1c although GH considerably improved from a 24-h mean value of 33.12 ng/ mL to 6.92 ng/ mL (p<0.005) (Fig. 2).
Fig. 2.
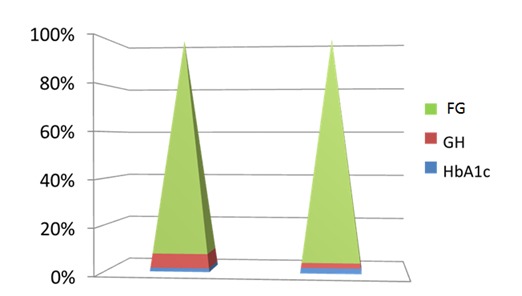
Fasting Glucose (FG, in mg/ dL); GH (Growth Hormone) levels (ng/ mL) and HbA1c (glycated haemoglobin, in %) after 6 months of octreotide LAR (a dose of 20 mg at every 28 days) in patients with active acromegaly and diabetes mellitus at baseline
After 12 months of therapy, the patients with normal glycaemia pre-treatment profile had no statistically significant changes in FG and 2-h BG (in OGTT) despite the relevant lowering of GH: 3,3 ng/ mL vs. 6,16 ng/ mL, (p=0.003) and IGF1: 332 ng/ mL vs. 713 ng/ mL (p=0.001) (Fig. 3a,b).
Fig. 3a.
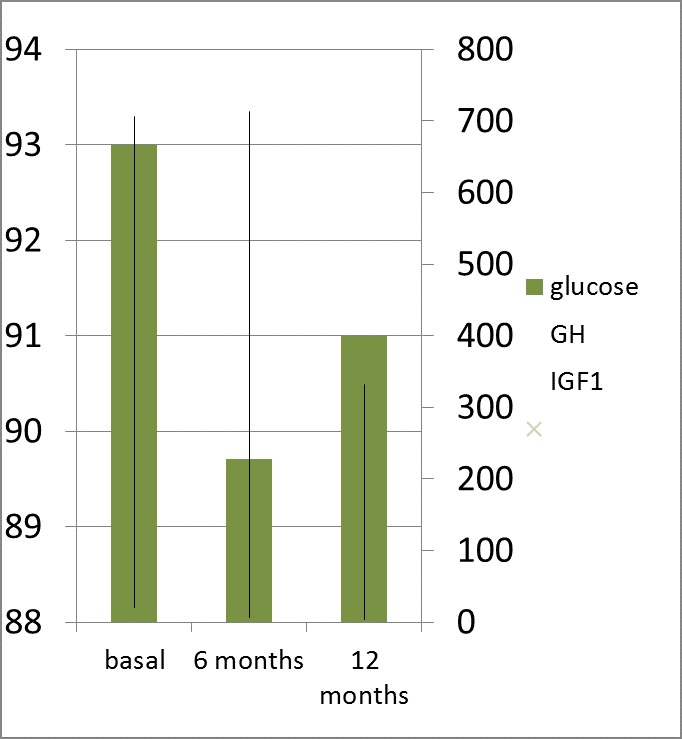
Fasting Glucose (Glucose; mg/ dL); GH (ng/ mL) and IGF1 (ng/ mL) levels after 12 months of octreotide LAR (a dose of 20 mg at every 28 days for 6 months followed by 6 months of 30 mg at every 28 days) in patients with active acromegaly and normal glucose metabolism at baseline (pre-treatment)
Fig. 3b.
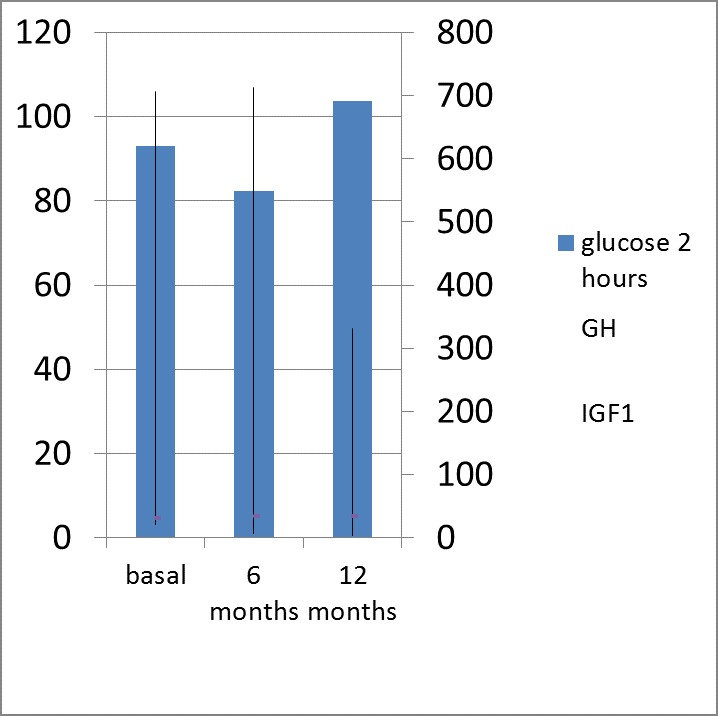
2-hour Plasma Glucose (Glucose 2 hours; mg/ dL) in 75 g OGTT; GH (ng/ mL) and IGF1 (ng/ mL) levels after 12 months of octreotide LAR (a dose of 20 mg at every 28 days for 6 months followed by 6 months of 30 mg at every 28 days) in patients with active acromegaly and normal glucose metabolism at baseline (pre-treatment)
Acromegalic patients with pre-treatment glucose metabolism considered as IGT or IFG had a statistically significantly reduction of FG after 6, respective 12 months according to GH and IGF1 lowering (Fig. 4).
Fig. 4.
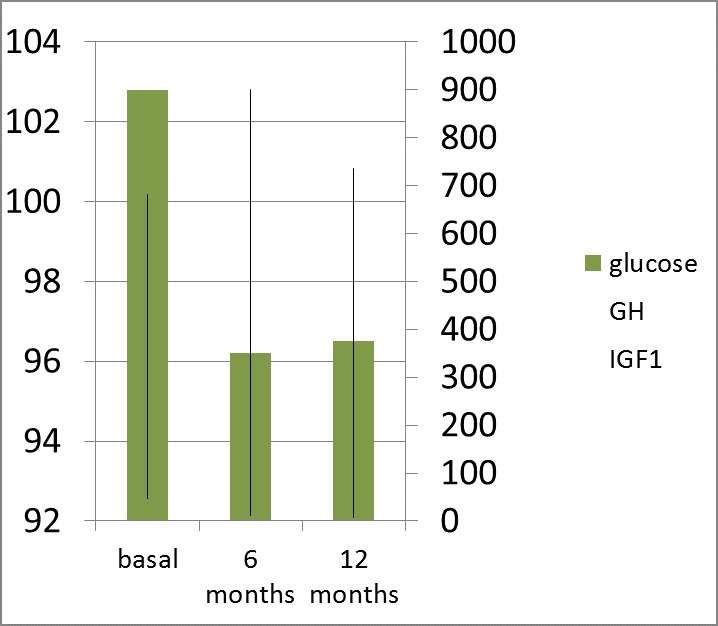
Fasting Glucose (Glucose; mg/ dL); GH (ng/ mL) and IGF1 (ng/ mL) levels after 12 months of octreotide LAR (a dose of 20 mg at every 28 days for 6 months followed by 6 months of 30 mg at every 28 days) in patients with active acromegaly and pre-diabetes (IGF+IGT) at baseline (pre-treatment)
The 2-h BG in OGTT after an initially (within first 6 months) decreasing later (during the next 6 months) increased back to the pre-treatment levels so no significant differences were seen after 1 year (p<0.07) (Fig. 5).
Fig. 5.
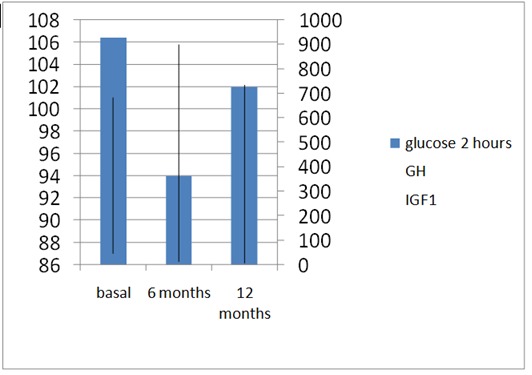
2-hour Plasma Glucose (Glucose 2 hours; mg/ dL) in 75 g OGTT; GH (ng/ mL) and IGF1 (ng/ mL) levels after 12 months of octreotide LAR (a dose of 20 mg at every 28 days for 6 months followed by 6 months of 30 mg at every 28 days) in patients with active acromegaly and pre-diabetes (IGF+IGT) at baseline (pre-treatment) (after 1 year: p<0.07)
After 12 months both basal and 2-hours OGTT glucose levels were similar to baseline despite significant lower levels of GH (3.3 vs. 6.61 ng/ mL, p=0.003) and IGF1 (332 vs. 713 ng/ mL, p=0.001).
Discussions
The glucose metabolism profile seems to be more related to the patients’ age than to the GH and IGF control [9]. In this study, DM had a greater incidence in subjects older than 61 years corresponding to similar values of GH and IGF1 from different age groups. Pre-treatment values of GH and IGF1 were not different if the patients had DM, IFG, IGT or normal glucose profile at baseline. While literature data pointed a good outcome of glucose metabolism after pituitary surgery, the somatostatin analogues had a variant effect. Initially, the insulin suppression caused glucose increasing despite GH suppression and later higher or lower glycaemia levels could be seen, regardless [13,14]. In acromegalic with DM, the fasting glucose and glycated haemoglobin A1c did not differ after 6 months of octreotide although the mean GH values were half as initial. IGF1 did not differ between either baseline or follow-up. Studies showed a previous hyperinsulinism mechanism in diabetic subjects with acromegaly characterised by normal fasting glucose with rapid and persistent insulin response in OGTT, which became normal again later than seen in normal conditions or IGT/ IFG [15-17]. This stage is reversible if adequate acromegaly therapy is introduced but later it will progress to a maximal beta pancreatic cells fasting response without further insulin enhance which is considered irreversible despite treatment [18-20]. This previous high insulin status would be able to explain the partial glucose answer to octreotide therapy in diabetic subjects although somatostatin analogues increase the insulin sensitivity by blocking the hepatic production of glucose [18-20]. These results are similar to those in literature according to which the glucose profile is mainly dependent on the baseline glycaemia status and the GH changes under octreotide. Moreover, we did not find higher glucose levels under therapy by beta cells inhibition caused by somatostatin analogue as some studies previously reported [21,22]. As limits of our study, we should mention the fact that we did not measure the insulin resistance indexes.
Conclusions
The therapy with octreotide in subjects with acromegaly improves the glucose profile if normal glycaemia is found at baseline according to GH and IGF1 lowering and, if the patients already have diabetes mellitus, only partial glucose control is found, as GH and IGF1 are inadequately suppressed.
Conflict of interest
None.
Acknowledgement
We thank the entire medical team involved in this study.
References
- 1.LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nature Clinical Practice. Endocrinology & Metabolism. 2007;3:302–310. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- 2.Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocrine Reviews. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shlomo A, Melmed S. Acromegaly. Endocrinology and Metabolism Clinics of North America. 2008;37:101–122. doi: 10.1016/j.ecl.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoskuldsdottir GT, Fjalldal SB, Sigurjonsdottir HA. The incidence and prevalence of acromegaly, a nationwide study from 1955 through 2013. Pituitary. 2015 Apr 17; doi: 10.1007/s11102-015-0655-4. [DOI] [PubMed] [Google Scholar]
- 5.Sonksen PH, Greenwood FC, Ellis JP, Lowy C, Rutherford A, Nabarro JD. Changes of carbohydrate tolerance in acromegaly with progress of the disease and in response to treatment. Journal of Clinical Endocrinology and Metabolism. 1967;27:1418–1430. doi: 10.1210/jcem-27-10-1418. [DOI] [PubMed] [Google Scholar]
- 6.Rose DR, Clemmons DR. Growth hormone receptor antagonist improves insulin resistance in acromegaly. Growth Hormone & IGF Research. 2002;12:418–424. doi: 10.1016/s1096-6374(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 7.Dal J, List EO, Jørgensen JO, Berryman DE. Glucose and Fat Metabolism in Acromegaly: From Mice Models to Patient Care. Neuroendocrinology. 2015 Apr 29; doi: 10.1159/000430819. [DOI] [PubMed] [Google Scholar]
- 8.Urbani C, Sardella C, Calevro A, Rossi G, Scattina I, Lombardi M, Lupi I, Manetti L, Martino E, Bogazzi F. Effects of medical therapies for acromegaly on glucose metabolism. Eur J. Endocrinol. 2013;169(1):99–108. doi: 10.1530/EJE-13-0032. [DOI] [PubMed] [Google Scholar]
- 9.Colao A, Auriemma R, Savastano S, Galdiero M, Grasso L, Lombardi G, Pivonello R. Glucose Tolerance and Somatostatin Analog Treatment in Acromegaly: A 12-Month Study. J Clin Endocrinol Metab. 2009 Aug;94(8):2907–2914. doi: 10.1210/jc.2008-2627. [DOI] [PubMed] [Google Scholar]
- 10.Katznelson L, Laws ER Jr., Melmed S, Molitch ME, Murad MH, Utz A, Wass JA. Endocrine Society. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014 Nov;99(11):3933–3951. doi: 10.1210/jc.2014-2700. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulou O, Bex M, Kamenicky P, Mvoula AB, Chanson P, Maiter D. Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: a study in 148 patients. Pituitary. 2014 Feb;17(1):81–89. doi: 10.1007/s11102-013-0471-7. doi: 10.1007/s11102-013-0471-7. [DOI] [PubMed] [Google Scholar]
- 12.Standards of Medical Care in Diabetes-2014. American Diabetes Association. Diabetes Care. 2014;37(S1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 13.Mazziotti G, Floriani I, Bonadonna S, Torri V, Chanson P, Giustina A. Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. Journal of Clinical Endocrinology and Metabolism. 2009;94:1500–1508. doi: 10.1210/jc.2008-2332. [DOI] [PubMed] [Google Scholar]
- 14.Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis and management. Endocr Rev. 2004;25:102–152. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 15.Koop BL, Harris AG, Ezzat S. Effect of octreotide on glucose tolerance in acromegaly. European Journal of Endocrinology. 1994;130:581–586. doi: 10.1530/eje.0.1300581. [DOI] [PubMed] [Google Scholar]
- 16.Pereira AM, Biermasz NR, Roelfsema F, Romijn JA. Pharmacologic therapies for acromegaly. A review of their effects on glucose metabolism and insulin resistance. Treat Endocrinol. 2004;4:43–53. doi: 10.2165/00024677-200504010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Parkinson C, Drake WM, Roberts ME, Meeran K, Besser GM, Trainer PJ. A comparison of the effects of pegvisomant and octreotide on glucose, insulin, gastrin, cholecystokinin, and pancreatic polypeptide responses to oral glucose and a standard mixed meal. Journal of Clinical Endocrinology and Metabolism. 2002;87:1797–1804. doi: 10.1210/jcem.87.4.8432. [DOI] [PubMed] [Google Scholar]
- 18.Baldelli R, Battista C, Leonetti F, Ghiggi MR, Ribaudo MC, Paoloni A, D’Amico E, Ferretti E, Baratta R, Liuzzi A, et al. Glucose homeostasis in acromegaly: effects of long-acting somatostatin analogues treatment. Clinical Endocrinology. 2003;59:492–499. doi: 10.1046/j.1365-2265.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- 19.Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D. Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2005;90:4465–4473. doi: 10.1210/jc.2005-0260. [DOI] [PubMed] [Google Scholar]
- 20.Melmed S, Casanueva FF, Cavagnini F, Chanson P, Frohman L, Grossman A, Ho K, Kleinberg D, Lamberts S, Laws E, Lombardi G, Vance ML, Werder K, Wass J, Giustina A. For the Acromegaly Treatment Consensus Workshop Participants Guidelines for acromegaly management. J Clin Endocrinol Metab. 2002;87:4054–4058. doi: 10.1210/jc.2002-011841. [DOI] [PubMed] [Google Scholar]
- 21.Kasayama S, Otsuki M, Takagi M, Saito H, Sumitani S, Kouhara H, Koga M, Saitoh Y, Ohnishi T, Arita Impaired beta-cell function in the presence of reduced insulin sensitivity determines glucose tolerance status in acromegalic patients. Clin Endocrinol. 2000;52:549–555. doi: 10.1046/j.1365-2265.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 22.Lamberts SW, Uitterlinden P, Verschoor L, van Dongen KJ, del Pozo E. Long-term treatment of acromegaly with the somatostatin analogue SMS 201– 995. N Engl J Med. 1985;313:1576–15807. doi: 10.1056/NEJM198512193132504. [DOI] [PubMed] [Google Scholar]


