Abstract
Rationale
The ventral tegmental area (VTA) mediates the local stimulating effects of ethanol (EtOH) in a region-dependent manner, with EtOH administration in the posterior but not anterior VTA stimulating the mesolimbic system. The serotonin-3 (5-HT3) receptor has been involved in the effects of EtOH on the mesolimbic system.
Objectives
The current study tested the hypothesis that EtOH would stimulate mesopallidal and mesocortical dopamine neurons in the posterior but not anterior VTA and that the stimulating effects of EtOH in the VTA would involve activation of local 5-HT3 receptors.
Methods
Wistar female rats were surgically implanted with two cannulae, one in one subregion of the VTA for microinjection and the other in the ventral pallidum (VP) or medial prefrontal cortex (mPFC) for microdialysis. Artificial CSF or EtOH (200 mg%; 44 mM) was microinjected in the anterior or posterior VTA, and extracellular dopamine was measured in the VP or mPFC with microdialysis-HPLC.
Results
EtOH injections in the posterior, but not anterior VTA, significantly increased extracellular dopamine levels in the VP and mPFC. Co-injections of the 5-HT3 receptor antagonist ICS-205,930 with EtOH in the posterior VTA significantly reduced the effects of EtOH on extracellular dopamine levels in the VP and mPFC.
Conclusions
The results indicate that posterior VTA dopamine neurons projecting to the VP and mPFC are stimulated by local administration of EtOH and that the local stimulating effects of EtOH are mediated, at least in part, by 5-HT3 receptors.
Keywords: dopamine; ethanol; 5-HT3 receptor; ICS-205,930; medial prefrontal cortex; microdialysis; ventral pallidum; ventral tegmental area
Introduction
Dopamine (DA) neurons in the ventral tegmental area (VTA) and their projections to the forebrain cortico-limbic regions have been implicated in mediating the effects of drugs of abuse, including ethanol (EtOH) (Koob and Volkow 2010; McBride et al. 1999). Systemic administration of EtOH activates the mesolimbic DA system and increases extracellular DA levels in the nucleus accumbens (NAc) (Imperato and Di Chiara 1986; Weiss et al. 1993). This effect of EtOH is thought to be largely due to EtOH actions in the VTA. This notion is supported by several lines of studies. Electrophysiological studies showed that both systemic and local administration of EtOH stimulated VTA DA neurons (Brodie et al. 1990, 1999; Foddai et al. 2004; Gessa et al. 1985). In addition, intra-VTA microinjections of EtOH increased DA release in the NAc (Ding et al. 2009b). Furthermore, the stimulating effects of EtOH were demonstrated only in the posterior VTA (pVTA), but not the anterior VTA (aVTA), suggesting a regional difference in the action of EtOH (Ding et al. 2009b). This finding is consistent with behavioral studies that EtOH was reinforcing in the pVTA, but not aVTA (Rodd-Henricks et al. 2000; Rodd et al. 2004). Overall, the current evidence suggests that EtOH can stimulate pVTA DA neurons projecting to the NAc.
VTA DA neurons also project to the ventral pallidum (VP) and medial prefrontal cortex (mPFC) (Oades and Halliday 1987). These two regions, along with the VTA and NAc, form a complex circuit involved in mediating the rewarding and reinforcing effects of EtOH (Koob and Volkow 2010). However, the stimulating effects of EtOH on the mesopallidal and mesocortical systems have not been fully explored. Previous studies showed that systemic administration of EtOH increased extracellular DA levels in the VP (Melendez et al. 2003, 2004). No study thus far has examined whether this effect is due to the activation of mesopallidal DA neurons in the VTA, and/or is due to EtOH effects on DA terminals in the VP. On the other hand, the effects of systemic EtOH on the mesocortical DA system are not so evident. Several studies indicated that intraperitoneal (i.p.) injection of EtOH did not alter extracellular DA levels in the mPFC (Engleman et al. 2006; Hegarty and Vogel 1993). However, a number of studies have linked the mPFC DA transmission in mediating the effects of ethanol. For example, pharmacological manipulations of DA receptor function in the mPFC or 6-hydroxydopamine lesion of the mPFC altered voluntary ethanol consumption (Nielsen et al. 1999) or operant responding for EtOH in rats (Hodge et al. 1996; Samson and Chappell 2003). In addition, the alcohol-preferring P rat has lower basal extracellular DA concentrations in the mPFC compared to the Wistar rat, suggesting lower DA transmission in the mPFC may be associated with alcohol preference and drinking (Engleman et al. 2006). Given these results, further studies are warranted to explore the neurochemical effects of EtOH on the mesocortical DA system.
In the present study, effects of EtOH on extracellular DA levels in the mPFC and VP were examined with local application of EtOH in the VTA. Given the stimulating effects of EtOH on mesolimbic DA neurons, we hypothesize that EtOH would activate VTA DA neurons projecting to the VP and mPFC and the effects would be evident in the pVTA but not aVTA.
The reinforcing and stimulating effects of EtOH appear to involve the 5-HT3 receptor. Systemic administration of 5-HT3 receptor antagonists suppressed voluntary EtOH consumption in rats under 24-hr free-choice conditions (Knapp and Pohorecky 1992; McKinzie et al. 1998). Co-infusion of 5-HT3 receptor antagonists with EtOH attenuated EtOH self-infusions in the pVTA (Rodd-Henricks et al. 2003). Furthermore, both systemic and local application of the 5-HT3 receptor antagonist ICS-205,930 attenuated the systemic EtOH-induced increase of extracellular DA levels within the mesolimbic system (Campbell et al. 1996; Campbell and McBride 1995; Wozniak et al. 1990). However, no study has examined the involvement of 5-HT3 receptors in the local stimulating effects of EtOH on DA neurons in the VTA. Therefore, we hypothesize that the local stimulating effects of EtOH on DA neurons in the pVTA would involve the activation of 5-HT3 receptors.
Methods and Materials
Subjects
Adult female Wistar rats (250 to 320 g, Harlan, Indianapolis IN, USA) were housed in a temperature- and humidity-controlled room maintained on a reversed 12-hr light-dark cycle (light off at 9:00 am). Rats were housed in pairs upon arrival and individually after the surgery. Food and water were freely available except in the testing chambers. Female rats were used because they maintain their head size better than male rats for more accurate stereotaxic placements (Ding et al. 2009b; Ikemoto et al. 1997a; Rodd-Henricks et al. 2000). Although the estrous cycle was not monitored, counterbalanced experiments were conducted on different days so that any effect of a given phase of the estrous cycle was distributed across experiments. Protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and were in accordance with principles in the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Chemical agents
KCl, CaCl2, MgSO4 and NaC2H3O2 were purchased from Fisher Scientific (Fair Lawn, NJ, USA). NaCl, Na2HPO4·7H2O, MgCl2, d-glucose, sodium octyl-sulfate (SOS), ethylenediaminetetraacetic acid (EDTA), acetonitrile, ascorbate and the 5-HT3 receptor antagonist tropisetron monohydrochloride (ICS-205,930) were purchased from Sigma (St. Louis, MO, USA). KH2PO4 and NaHCO3 were purchased from Acros Organics (NJ, USA). EtOH (190 proof) was obtained from McCormick Distilling, Weston, MO.
Surgery and probe insertion procedures
Guide cannulae were implanted in the ipsilateral side of the brain as previously described (Ding et al. 2009b). The target regions were: aVTA (AP − 4.8 mm, ML + 2.1 mm, DV − 9.0 mm); pVTA (AP − 5.6 mm, ML + 2.1 mm, DV − 9.0 mm); VP (AP + 0.1 mm, ML + 2.3 mm, DV − 9.0 mm); and mPFC (AP + 3.0 mm, ML +0.7 mm, DV − 5.0 mm) (Paxinos and Watson 1998). Cannulae were inserted into the VTA with a 10° angle to vertical and into the VP or mPFC with no angle. Probes (Inner Diameter: 200 μm, Molecular Weight Cut Off: 13,000, Spectrum Laboratories, Inc, Rancho Dominguez, CA) were inserted into the VP (1.5-mm active membrane) or mPFC (2.0-mm active membrane) as described previously (Ding et al. 2009b).
Microinjection procedure
EtOH and ICS-205, 930 were dissolved in artificial cerebrospinal fluid (aCSF) to the desired concentrations prior to use. Microinjections were conducted with an electrolytic micro-infusion transducer (EMIT) system, following the procedure described previously (Ding et al. 2009b). Briefly, the EMIT system was calibrated to inject a 100-nl solution in 5 seconds; a 15-sec timeout period followed each 5-sec injection. The injection-timeout cycle was repeated thirty times over a 10-min period. After the injection, the injector remained in place for one minute before being removed.
Microdialysis procedure
Microdialysis was conducted following procedures described previously (Ding et al. 2009b). Briefly, microdialysis started 16–18 hr after the probe insertion. Rats were placed into microdialysis chambers and connected to a Harvard pump. Microdialysis started with a 90-min washout period with the perfusion of aCSF through probes at a rate of 1.0 μl/min. After the washout period, four to five baseline samples were collected, which was followed by microinjections of either vehicle or the drug for 10 min in the sub-region of the VTA. Five samples were collected thereafter. Samples were collected at 10-min intervals.
Sample analysis
DA was analyzed with a reversed-phase high performance liquid chromatography coupled with electrochemical detection, as described previously (Thielen et al. 2004). Briefly, 5-ul samples were delivered onto an analytical column (BDS Hypersil C18, 3 μm, 100 mm × 1 mm, Thermo) with a mobile phase containing 77.0 mM NaC2H3O2, 0.5 mM EDTA, 3.4 mM SOS, 9.9 mM NaCl, and 6.0% acetonitrile, pH 4.0. DA was detected with a glassy-carbon electrode and an amperometric detector with the oxidation potential set at + 450 mV and sensitivity set at 1 nA/V. The signal then was analyzed with a ChromPerfect data station. The detection limit for DA was approximately 100 pmol/l with a signal-to-noise ratio of 3:1.
The effects of EtOH in different sub-regions of the VTA on extracellular DA levels in the VP
Rats were randomly assigned to the following groups (n = 5–8/group). Two groups received either aCSF or EtOH (200 mg%; 44 mM) in the aVTA; and the other two groups received either aCSF or 200 mg% EtOH in the pVTA. Extracellular DA was sampled in the VP. For female Wistar rats, the 200 mg% EtOH (44 mM) appeared to be an optimal concentration to be self-infused into the p-VTA (Rodd-Henricks et al. 2000) and to increase DA release in the NAc after being injected into the pVTA (Ding et al. 2009b).
The second experiment examined the involvement of the 5-HT3 receptor in the effects of EtOH in the pVTA where local administration of EtOH increased extracellular DA levels in the VP. Two groups of rats received co-infusion of the 5-HT3 receptor antagonist ICS-205,930 (100 or 200 μM, n = 5–8 per dose) and 200 mg% EtOH in the pVTA during the microdialysis. ICS-205,930 (100 μM) has been shown to attenuate self-infusion of EtOH into the pVTA (Rodd-Henricks et al. 2003). A third group received microinjections of the antagonist alone (200 μM, n = 5) in the pVTA. To reduce the number of rats, groups receiving either EtOH (200 mg%) or aCSF alone in the pVTA were not repeated in this experiment because the first experiment outlined above already contained these groups. However, results from the first experiment were adopted for statistical analysis in this experiment.
The effects of EtOH in different sub-regions of the VTA on extracellular DA levels in the mPFC
Rats were randomly assigned to the following groups (n = 5–8/group). Two groups received microinjections of either aCSF or 200 mg% EtOH in the aVTA; and the other two groups received microinjections of either aCSF or 200 mg% EtOH in the pVTA. Extracellular DA levels in the mPFC were measured.
In the pVTA, where local administration of EtOH increased extracellular DA levels in the mPFC, the effects of co-infusion of the 5-HT3 receptor antagonist ICS-205,930 with 200 mg% EtOH were examined, following the same procedure described above in experiment# 2 in the study performed above.
Histology
At the end of experiments, rats were euthanized and bromophenol blue (1%) was delivered into the target regions. Brains were removed and sections (40 μm) were prepared and stained, as described previously (Ding et al. 2009b). The placement of injection sites and probes were determined in accordance with the rat brain atlas of Paxinos & Watson (1998).
Statistical analysis
DA levels were expressed as percent of baseline averaged from the last three baseline samples prior to the microinjection. Analyses of variance (ANOVA) with repeated measures were conducted to analyze the time-course data. Area under the curve (AUC) coupled with one-way ANOVA were used to analyze data from the ICS-205,930 co-infusion experiments. The AUC values were derived from time-course data of percentage DA levels. The calculation included all time points and used 100% as baseline. Significant difference was set at p < 0.05. Post-hoc tukey’s b tests were performed following significant major effects.
Results
Histology
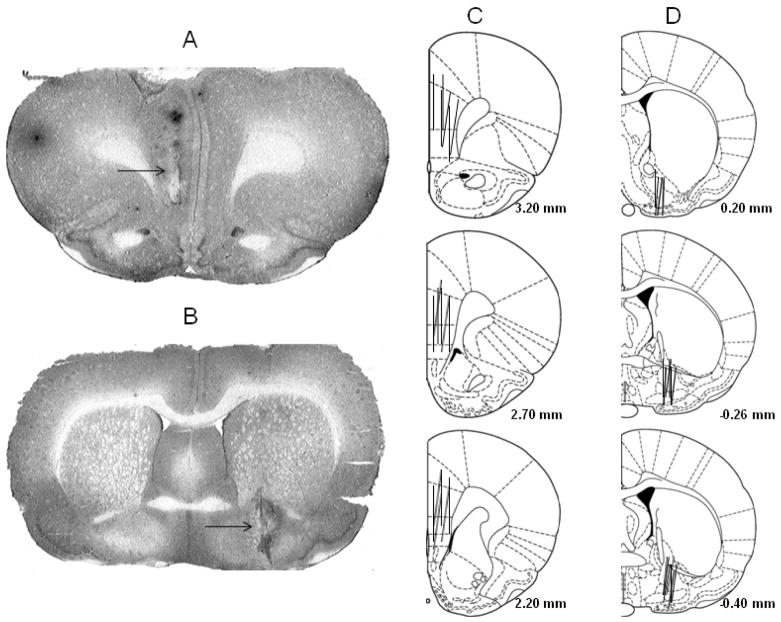
Figure 1 shows placements of probes in the mPFC and VP. In the mPFC, probes were mainly in the prelimbic and infralimbic areas of the mPFC. Some probes also covered a small portion of the anterior cingulate. In the VP, probes were mainly in the VP area underneath the anterior commissure. Some probes also covered a small portion of the lateral globus pallidus to the dorsal side and a small portion of the nucleus of horizontal limb of the diagonal band (HDB) or the magnocellular preoptic nucleus (MCPO) to the ventral side. To be included in the analysis, rats had to have at least 75% of the probe in the VP or mPFC.
Figure 1.
Photomicrographs (A & B) and schematic representation (C & D) of brain slices with microdialysis probes in the medial prefrontal cortex (mPFC, A & C) and ventral pallidum (VP, B & D). The arrow indicates the position of the probe. The lines represent the 2.0-mm length of microdialysis probes in the mPFC (C) and the 1.5-mm length of microdialysis probes in the VP (D).
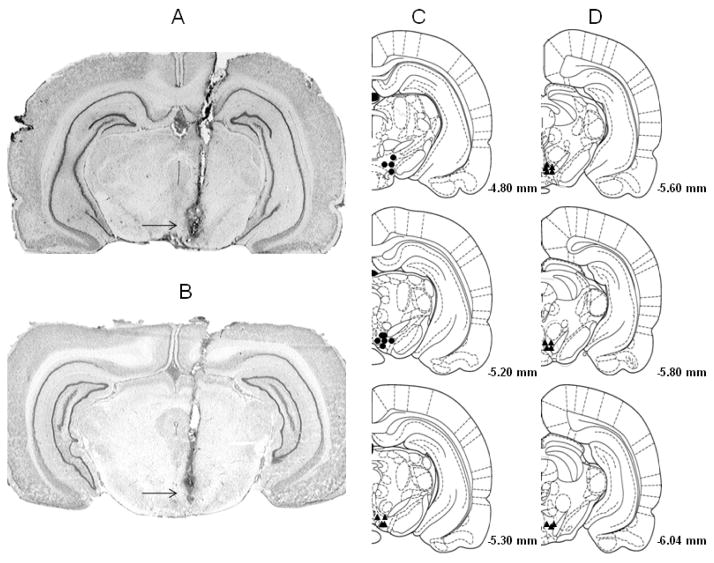
Figure 2 shows the placement of micro-injection sites within the aVTA and pVTA. Previous studies indicated that the aVTA ranges from coronal section 4.8 to 5.2 mm posterior to bregma, and the pVTA ranges from coronal section 5.3 to 6.3 mm posterior to bregma (Fig. 2) (Ding et al. 2009b; Rodd-Henricks et al. 2000). Approximately 80% of the rats fulfilled these criteria. Rats with injection sites outside of the VTA were not included in analysis.
Figure 2.
Photomicrographs (A & B) and schematic representation (C & D) of brain slices with injection sites within the anterior (A) and posterior (B) ventral tegmental area (VTA). The arrow indicates the tip of the micro-injector. The filled circles represent microinjection sites within the anterior VTA and the filled triangles represent microinjection sites within the posterior VTA, as defined by Rodd-Henricks et al (2000).
Effects on extracellular DA levels in the VP
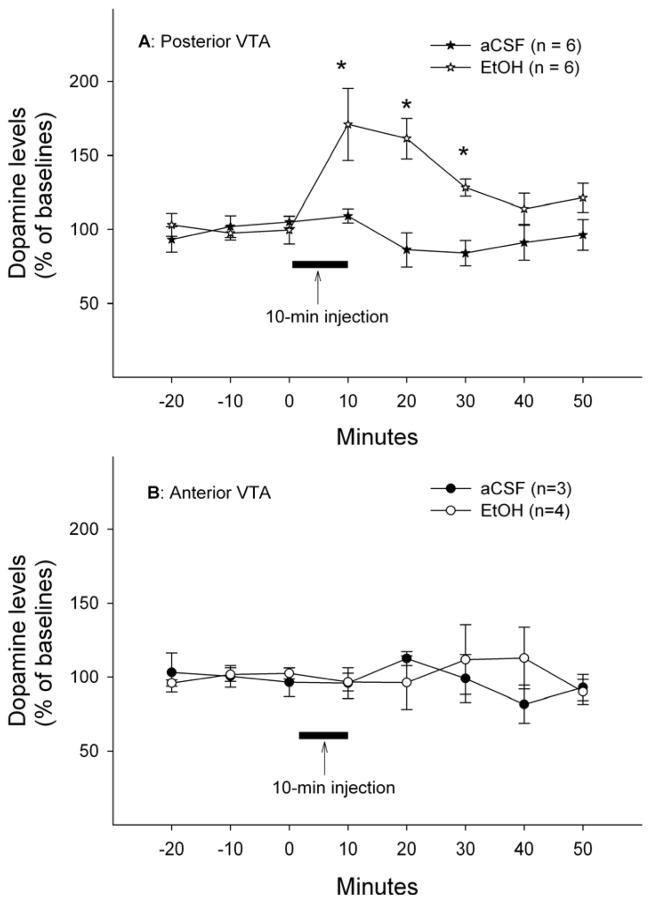
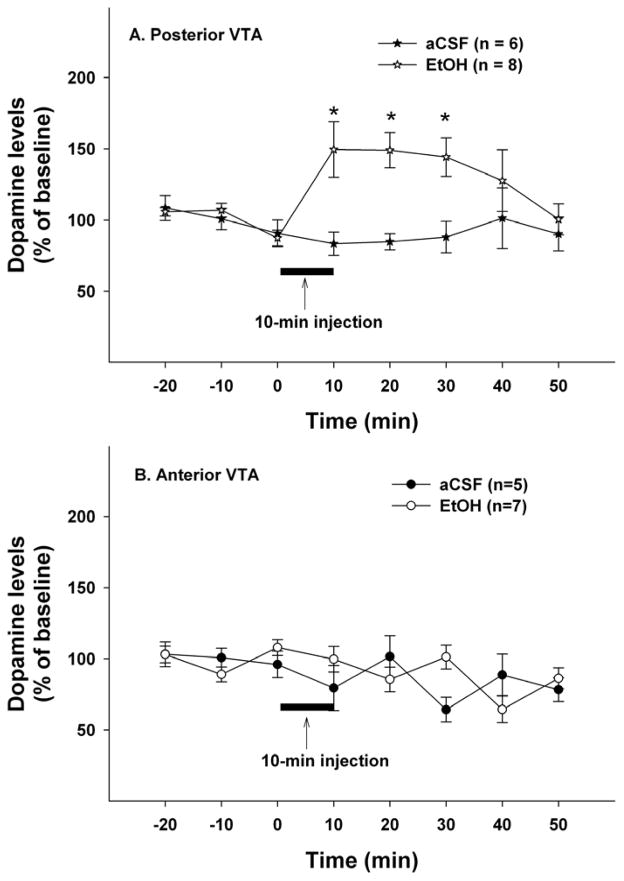
Figure 3 shows time-course effects of microinjections of aCSF or EtOH (200mg%; 44 mM) in different sub-regions of the VTA on extracellular DA levels in the VP. The average basal extracellular DA levels in each group were shown in Table 1. Mixed repeated-measures ANOVA with ‘time’ as within factor and ‘region’ and ‘treatment’ as between factors revealed significant ‘time’ x ‘region’ x ‘treatment’ interaction (F5, 11 = 3.78, p = 0.031). The significant interaction term allowed further analyses to be conducted in each individual sub-region of the VTA. In the pVTA, significant effects of ‘time’, ‘treatment’ and ‘time’ x ‘treatment’ interaction were found (all F values > 3.87, all p values < 0.05). Microinjections of 200 mg% EtOH in the pVTA significantly increased extracellular DA levels in the VP to approximately 160% of baseline levels, whereas microinjections of aCSF had no effect on extracellular DA levels (Fig. 3A). In the aVTA, there was no significant effect of ‘time’, ‘treatment’ or ‘time’ x ‘treatment’ interaction (all F values < 0.41, all p values > 0.5). Microinjections of neither 200 mg% EtOH nor aCSF in the aVTA significantly altered extracellular DA levels in the VP (Fig. 3B). Furthermore, EtOH produced a significantly greater effect in the pVTA than aVTA (F1, 8 = 6.43, p = 0.035).
Figure 3.
Time-course effects of microinjections of EtOH (200 mg%; 44 mM) or aCSF in the posterior (A) or anterior (B) ventral tegmental area on extracellular dopamine levels in the ventral pallidum. * p < 0.05, significantly different from baselines and aCSF group.
Table 1.
Basal extracellular dopamine levels in the ventral pallidum or the medial prefrontal cortex in each group of rats
| Group | Ventral Pallidum (nM) | medial Prefrontal Cortex (nM) |
|---|---|---|
| Posterior VTA-aCSF | 0.5 ± 0.1 | 0.2 ± 0.1 |
| Anterior VTA-aCSF | 0.7 ± 0.1 | 0.3 ± 0.1 |
| Posterior VTA-200 mg% EtOH | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Anterior VTA-200 mg% EtOH | 0.9 ± 0.4 | 0.3 ± 0.1 |
| 200 mg% EtOH + 100 μM ICS | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 200 mg% EtOH + 200 μM ICS | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 200 μM ICS | 0.3 ± 0.1 | 0.3 ± 0.1 |
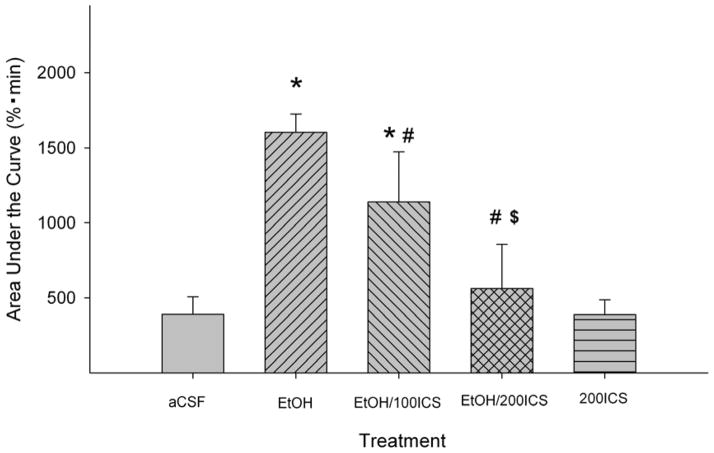
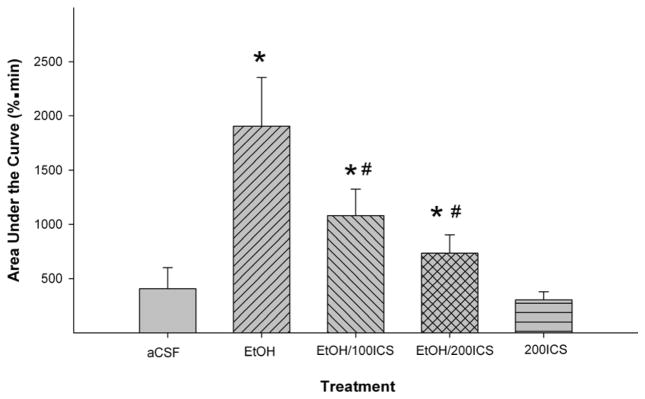
Figure 4 shows the effects of co-injections of the 5HT3 receptor antagonist ICS-205,930 (100 or 200 μM) with EtOH in the pVTA. The basal DA levels in each group were shown in Table 1. AUC values were calculated from each group and were analyzed with one-way ANOVA (Fig. 4). The analysis indicated a significant effect of treatment (F4, 23 = 6.10, p = 0.002). Post-hoc analysis indicated that ICS-205,930 significantly reduced the effect of 200 mg% EtOH in a concentration-dependent manner (p < 0.05). In addition, 200 μM ICS-205,930 alone did not significantly alter extracellular DA levels in the VP.
Figure 4.
Effects of co-injections of the 5-HT3 receptor antagonist ICS-205,930 with EtOH (200 mg%; 44 mM) in the posterior ventral tegmental area on EtOH-induced dopamine increase in the ventral pallidum (n = 5–8/group). ‘EtOH’ = ‘200 mg% EtOH’; ‘EtOH/100ICS’ = ‘200 mg% EtOH + 100 μM ICS’; ‘EtOH/200ICS’ = ‘200 mg% EtOH + 200 μM ICS’; ‘200ICS = ‘200 μM ICS’. * p < 0.05, significantly different from the ‘aCSF’ group; # p < 0.05, significantly different from the ‘EtOH’ group; $ p < 0.05, significantly different from the ‘EtOH/100ICS’ group.
Effects on extracellular DA levels in the mPFC
Figure 5 shows the time-course effects of microinjections of aCSF or 200 mg% EtOH into different sub-regions of the VTA on extracellular DA levels in the mPFC. Basal extracellular DA levels in each group were shown in Table 1. Mixed repeated-measures ANOVA with ‘time’ as within factor and ‘region’ and ‘treatment’ as between factors revealed a significant ‘time’ x ‘region’ x ‘treatment’ interaction (F7,16 = 3.07, p = 0.03). Further statistical analyses followed the same procedure as outlined above for data shown in Fig. 3. Microinjections of 200 mg% EtOH into the pVTA significantly increased extracellular DA levels in the mPFC to approximately 150% of baseline levels (F1, 12 = 10.14, p < 0.05); microinjections of aCSF, on the other hand, had no effect (Fig. 5A). In the aVTA, however, neither aCSF nor 200 mg% EtOH significantly altered mPFC DA levels (all F values <1.98, all p values > 0.05, Fig. 5B). Similarly, EtOH produced greater effects in the pVTA compared to the aVTA (F1, 13 = 14.98, p = 0.002).
Figure 5.
Time-course effects of microinjections of EtOH (200 mg%; 44 mM) or aCSF in the posterior (A) or anterior (B) ventral tegmental area on extracellular dopamine levels in the medial prefrontal cortex. * p < 0.05, significantly different from baselines and aCSF group.
Figure 6 shows the effects of co-injection of the 5HT3 receptor antagonist ICS-205,930 (100 or 200 μM) on the actions of EtOH in the pVTA. The basal DA levels in each group were shown in Table 1. AUC analysis indicated a significant effect of treatment (Fig. 6, F4, 25 = 4.35, p = 0.008). Post-hoc analysis indicated that ICS-205,930, at both 100 and 200 μM concentrations, produced similar reduction on EtOH-induced DA increase in the mPFC (p < 0.05). In addition, microinjections of 200 μM ICS-205,930 alone did not significantly alter extracellular DA levels in the mPFC.
Figure 6.
Effects of co-injections of the 5-HT3 receptor antagonist ICS-205,930 with EtOH (200 mg%; 44 mM) in the posterior ventral tegmental area on EtOH-induced dopamine increase in the medial prefrontal cortex (n = 4–8/group). ‘EtOH’ = ‘200 mg% EtOH’; ‘EtOH/100ICS’ = ‘200 mg% EtOH + 100 μM ICS’; ‘EtOH/200ICS’ = ‘200 mg% EtOH + 200 μM ICS’; ‘200ICS = ‘200 μM ICS’. * p < 0.05, significantly different from the ‘aCSF’ group; # p < 0.05, significantly different from the ‘EtOH’ group.
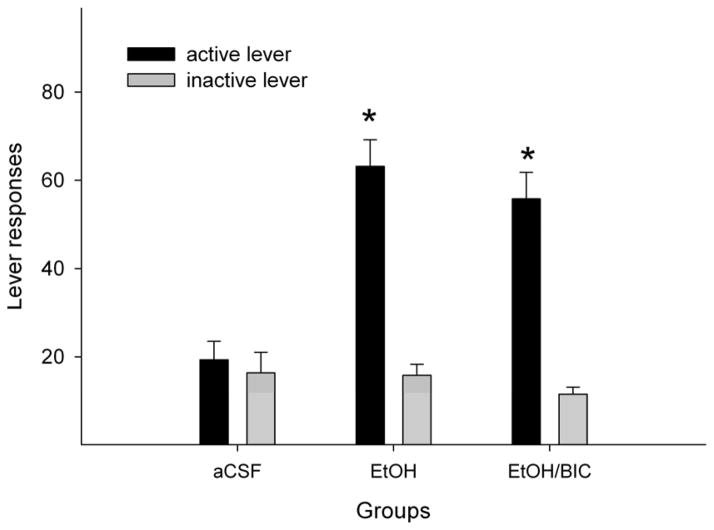
Since ICS-205,930 also interacts with the GABAA receptor at high micro-molar ranges (Klein et al. 1994), a separate preliminary experiment was carried out to assess the involvement of GABAA receptors in the local reinforcing actions of EtOH on DA neurons within the pVTA. In a intracranial self-administration paradigm (Ding et al. 2009c; Rodd-Henricks et al. 2000), Wistar rats readily responded for the self-infusion of 200 mg% EtOH alone into the pVTA and discriminated the active from the inactive lever (Fig. 7, Rodd-Henricks et al. 2000, 2003). Co-administration of 250 μM bicuculline, a GABAA receptor antagonist, with 200 mg% EtOH did not significantly alter responding on the EtOH lever (Fig. 7), suggesting that activation of GABAA receptors are not involved in the local stimulating effects of EtOH within the pVTA.
Figure 7.
Effects of co-infusion of the GABAA receptor antagonist bicuculline with EtOH on self-administration of 200 mg% EtOH into the posterior ventral tegmental area (VTA) of female Wistar rats. ‘aCSF’ = self-administration of aCSF in the posterior VTA (n = 4); ‘EtOH’ = self-administration of 200 mg% EtOH alone (n = 5); ‘EtOH/BIC’ = co-infusion of 200 mg% EtOH and 250 μM bicuculline (n = 5). Intracranial self-administration experiments were conducted as previously described (Rodd-Henricks et al. 2003). * p < 0.05, significantly higher responses on the active than inactive lever, and significantly higher responses on the active lever compared to responses by the ‘aCSF’ group. Responses on the active lever with 200 mg% EtOH alone were not significantly different than responses with 200 mg% EtOH plus 250 μM bicuculline.
Discussion
The current study demonstrated that local administration of EtOH into the pVTA but not aVTA increased DA release in both the VP and mPFC (Figs. 3 & 5), and that co-injections of the 5-HT3 receptor antagonist ICS-205,030 attenuated the local stimulating effects of EtOH on pVTA DA neurons projecting to the VP and mPFC (Figs. 4 and 6). These results suggest that DA neurons in the pVTA, but not the aVTA, are stimulated by EtOH and the stimulating effects of EtOH are mediated, at least in part, by activation of local 5-HT3 receptors.
Local application of 200 mg% EtOH in the pVTA significantly increased extracellular DA levels in the VP to approximately 160% of baseline (Fig. 3), indicating EtOH stimulation of mesopallidal DA neurons in the pVTA. These findings suggest that local stimulation of pVTA mesopallidal DA neurons may underlie the neurochemical effects of systemically administered EtOH on the mesopallidal DA system (Melendez et al. 2003, 2004). However, the results of the present study do not eliminate the possibility that systemic EtOH could also have an effect directly within the VP. The elevation of extracellular DA levels in the VP may be involved in some important functions, such as locomotor activation (Klitenick et al. 1992). Two studies have also linked VP DA to EtOH self-administration in alcohol-preferring P rats (Melendez et al. 2004, 2005).
Direct application of EtOH into the pVTA also stimulates mesocortical DA neurons, as indicated by elevated DA release in the mPFC (Fig. 5). These findings are in contrast to previous reports that systemic administration of EtOH did not alter extracellular levels of DA in the mPFC (Engleman et al. 2006; Hegarty and Vogel 1993). Reasons for this apparent disagreement between the i.p. injection and microinjection studies may be due to local administration of ethanol only acting on DA cell bodies within the VTA, whereas systemic administration of ethanol acts not only on the cell bodies in the VTA, but also in terminal areas within the mPFC. It is possible that systemically-administered EtOH may produce certain inhibitory effects on DA terminals within the mPFC, which overrides the stimulatory effects of ethanol in the cell body area. In the mPFC, there appears to be tonic GABA-mediated inhibition of DA release (Santiago et al. 1993). EtOH has been shown to increase both GABAA and GABAB receptor-mediated inhibition (Allan et al. 1987; Federici et al. 2009). Therefore, a tentative hypothesis can be proposed that EtOH in the mPFC could inhibit DA release locally by enhancing GABAergic inhibition, which could counteract the stimulating effects of EtOH in the VTA. Another possibility to explain why systemic EtOH did not alter mPFC DA levels may be due to EtOH activating inhibitory projections from other brain regions to the mPFC, which inhibits DA release within the mPFC. Further studies will be needed to test these possibilities.
The current study suggested that activation of local 5-HT3 receptors may be involved in the stimulating effects of EtOH on DA neurons in the pVTA, as demonstrated by the findings that co-infusion of the 5-HT3 receptor antagonist ICS-205,930 significantly attenuated EtOH-induced DA release in the VP and mPFC (Figs. 4 & 6). These findings are consistent with findings showing co-infusion of 5-HT3 receptor antagonists inhibit the local reinforcing actions of EtOH in the pVTA (Rodd-Henricks et al. 2003), as well as findings demonstrating that local perfusion of ICS-205,930 attenuated systemic EtOH-induced DA increase in both the VTA and NAc (Campbell et al. 1996; Campbell and McBride 1995).
Activation of 5-HT3 receptors within the pVTA can increase DA neuronal activity and produce reinforcing effects. The 5-HT3 receptor agonist, 1-(m-chlorophenyl)-biguanide (CPBG), can be self-administered into the pVTA (Rodd et al. 2007) and increase local extracellular DA levels (Campbell et al. 1996; Liu et al. 2006). Furthermore, EtOH can increase the 5-HT3 receptor-mediated current in vitro (Lovinger and White 1991; Machu and Harris 1994) and local application of CPBG can enhance the systemic EtOH-induced increase of extracellular DA levels in the VTA (Campbell et al. 1996). Therefore, research with both 5-HT3 agonists and antagonists discussed above suggests that EtOH can stimulate DA neurons in the pVTA via enhancing the activity of 5-HT3 receptors and that the inhibition of 5-HT3 receptors would attenuate the stimulating effects of EtOH.
Low to moderate densities of 5-HT3 receptor binding sites were detected in the VTA with receptor autoradiography (Ge et al. 1997). Although the cellular localization of 5-HT3 receptors is not known, the VTA DA neuron is a strong candidate because of the demonstrated synaptic connectivity of 5-HT terminals on VTA DA neurons (Van Bockstaele et al. 1994). The 5-HT3 receptors on DA neurons could be involved in the effects of EtOH, as activation of these 5-HT3 receptors would directly stimulate DA neurons. However, 5-HT3 receptors on GABA inter-neurons and/or GABA terminals may not be involved in the EtOH effect, as activation of these receptors would increase GABA release onto DA neurons.
At micro-molar concentrations, ICS-205,930 has been shown to inhibit the GABAA receptor-mediated inhibition (Klein et al. 1994). Therefore, it is possible that ICS-205,930 could be acting at GABAA receptors to produce its inhibitory effects on EtOH stimulated DA release. GABAA receptors form different circuits in regulating DA neuron activity within different sub-regions of the VTA (Arnt and Scheel-Kruger 1979; Ding et al. 2009a; Ikemoto et al. 1997b; 1998), with little tonic GABAA receptor-mediated inhibition in the pVTA. A preliminary study ( Fig. 7) was conducted to examine the effects of a GABAA antagonist on the reinforcing stimulating effects of EtOH on DA neurons in the pVTA. The results of this study indicated that bicuculline had no effect on the self-infusion of EtOH in the pVTA, suggesting that the reinforcing stimulating effects of EtOH within the pVTA are not mediated via activation of local GABAA receptors. These latter results support the idea that the effects of ICS-205,930 observed in the present study may be occuring through inhibition of 5-HT3 receptors. This conclusion is consistent with a previous study (Rodd-Henricks et al. 2003) indicating that zacropride, another 5-HT3 receptor antagonist without an interaction at the GABAA receptor (Smith et al. 1988), was more potent than ICS-205,930 in inhibiting EtOH self-administration in the pVTA, suggesting that inhibition of local 5-HT3 receptors alone was sufficient to prevent the stimulating actions of EtOH within the pVTA.
In addition, ICS-205,930 functions as a partial agonist at the α7 nicotinic receptor (Macor et al. 2001). In the VTA, these receptors are located pre-synaptically on glutamatergic terminals (Jones and Wonnacott 2004) and are involved in the stimulation of DA neurons (Schilstrom et al. 1998). However, several lines of evidence suggest these receptors may not be involved in the effects of ICS-205,930 observed in the present study (Figs. 4 & 6). First, α7 nicotinic receptors may not mediate the stimulating effects of EtOH as demonstrated by findings that EtOH can inhibit the activity of the α7 nicotinic receptor (Narahashi et al. 1999). Second, the findings that microinjection of 200 μM ICS-205,930 alone did not significantly alter extracellular DA levels in the VP and mPFC suggest that the action of ICS-205,930 on α7 nicotinic receptors did not produce general inhibition of DA neurons. Therefore, the effects of ICS-205,930 observed in the present study are not likely due to an action on local α7 nicotinic receptors.
The stimulating effects of EtOH within the VTA appear to be sub-region dependent. Local application of 200 mg% (44 mM) EtOH in the pVTA, but not aVTA, induced a comparable increase (50–60 %) of extracellular DA levels in the NAC, VP and mPFC (Figs. 3 & 5) (Ding et al. 2009b). These findings are consistent with behavioral studies demonstrating that EtOH is reinforcing in the pVTA but not aVTA (Rodd-Henricks et al. 2000), and suggest that activation of mesopallidal and mesocortical systems, in addition to the mesolimbic system, may contribute to the reinforcing properties of EtOH in the pVTA. Together, these studies suggest that the pVTA is more sensitive than the aVTA to the stimulating effects of EtOH. The mechanisms for this difference are unknown. One possibility may be related to possible differential projections to the target regions from the two VTA sub-regions. However, neuro-anatomical evidence does not support this idea because both the aVTA and pVTA appear to project to the VP and mPFC (Klitenick et al. 1992; Swanson 1982). Another possibility may be differences in receptors and/or neuronal circuits between the two subregions, e.g. 5-HT3 receptors. The pVTA appeared to be more sensitive to 5-HT3 receptor activation than the aVTA (Liu et al. 2006; Rodd et al. 2007), which may contribute to the differential effects of EtOH between the two sub-regions of the VTA. The differential functions of 5-HT3 receptors may be due to differential expression of 5-HT3 receptors between the aVTA and pVTA; however, further neuro-anatomical studies need to be conducted to test this hypothesis.
In addition to the 5-HT3 receptor, other mechanisms may also contribute to the differential effects of EtOH. Evidence has shown that EtOH can directly activate VTA DA neurons (Brodie et al. 1999), which may be through the actions of EtOH on certain voltage dependent ion channels (Brodie and Appel 1998; Okamoto et al. 2006) and/or α4β2 nicotinic receptors in VTA DA neurons (Narahashi et al. 1999). On the other hand, EtOH could indirectly stimulate DA neurons via suppression of GABA inter-neurons through either increasing opioid receptor neurotransmission to GABA inter-neurons (Johnson and North 1992) and/or decreasing NMDA receptor functions on these neurons (Stobbs et al. 2004). It is possible that some or all of these mechanisms may exist in the pVTA but not in the aVTA, thus contributing to the differential effects of EtOH between these two sub-regions. Further studies will be needed to address the mechanisms of differential effects of EtOH within the VTA.
In summary, the present study indicated that direct application of EtOH in the pVTA but not aVTA stimulates local mesopallidal and mesocortical DA neurons. These findings suggest that activation of these systems may be involved in mediating the local effects of EtOH. Furthermore, the stimulating effects of EtOH within the pVTA appeared to be mediated, at least in part, by activation of local 5-HT3 receptors, as indicated by the reduction of EtOH effects by co-infusions of the 5-HT3 receptor antagonist ICS-205,930.
Acknowledgments
This study was supported by research grants AA07462, AA 10717, AA 10721 and AA 12262. We thank Amanda Moran and Brandon Dewell for their technical help.
References
- Allan AM, Huidobro-Toro JP, Bleck V, Harris RA. Alcohol and the GABA receptor-chloride channel complex of brain. Alcohol Alcohol Suppl. 1987;1:643–646. [PubMed] [Google Scholar]
- Arnt J, Scheel-Kruger J. GABA in the ventral tegmental area: differential regional effects on locomotion, aggression and food intake after microinjection of GABA agonists and antagonists. Life Sci. 1979;25:1351–1360. doi: 10.1016/0024-3205(79)90402-8. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–244. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav. 1995;51:835–842. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Liu W, Engleman EA, Rodd ZA, McBride WJ. Differential effects of dopamine D2 and GABAA receptor antagonists on dopamine neurons between the anterior and posterior ventral tegmental area of female Wistar rats. Pharmacol Biochem Behav. 2009a;92:404–412. doi: 10.1016/j.pbb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009b;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Toalston JE, Oster SM, McBride WJ, Rodd ZA. Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology. 2009c;204:381–390. doi: 10.1007/s00213-009-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Federici M, Nistico R, Giustizieri M, Bernardi G, Mercuri NB. Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. Eur J Neurosci. 2009;29:1369–77. doi: 10.1111/j.1460-9568.2009.06700.x. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Ge J, Barnes JM, Towers P, Barnes NM. Distribution of S(−)-zacopride-insensitive [125I]R(+)-zacopride binding sites in the rat brain and peripheral tissues. Eur J Pharmacol. 1997;332:307–312. doi: 10.1016/s0014-2999(97)01091-1. [DOI] [PubMed] [Google Scholar]
- Gessa G, Muntoni AL, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Hegarty AA, Vogel WH. Modulation of the stress response by ethanol in the rat frontal cortex. Pharmacol Biochem Behav. 1993;45:327–34. doi: 10.1016/0091-3057(93)90247-q. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappell AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABAA receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997a;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusions of GABAA antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997b;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-administrations. Pharmacol Biochem Behav. 1998;61:87–92. doi: 10.1016/s0091-3057(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–239. [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of α7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–52. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Sanna E, McQuilkin SJ, Whiting PJ, Harris RA. Effects of 5-HT3 receptor antagonists on binding and function of mouse and human GABAA receptors. Eur J Pharmacol. 1994;268:237–246. doi: 10.1016/0922-4106(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50:371–86. doi: 10.1016/0306-4522(92)90430-a. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Pohorecky LA. Zacopride, a 5-HT3 receptor antagonist, reduces voluntary ethanol consumption in rats. Pharmacol Biochem Behav. 1992;41:847–850. doi: 10.1016/0091-3057(92)90237-a. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Thielen RJ, Rodd ZA, McBride WJ. Activation of serotonin-3 receptors increases dopamine release within the ventral tegmental area of Wistar and alcohol-preferring (P) rats. Alcohol. 2006;40:167–176. doi: 10.1016/j.alcohol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001;11:319–21. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Eha RD, Cox R, Stewart RB, Dyr W, Murphy JM, McBride WJ, Lumeng L, Li T-K. Serotonin3 receptor antagonism of alcohol intake: effects of drinking conditions. Alcohol. 1998;15:291–298. doi: 10.1016/s0741-8329(97)00132-8. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, McBride WJ, Murphy JM. Alcohol stimulates the release of dopamine in the ventral pallidum but not in the globus pallidus: a dual-probe microdialysis study. Neuropsychopharmacology. 2003;28:939–946. doi: 10.1038/sj.npp.1300081. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Involvement of the mesopallidal dopamine in ethanol reinforcement. Alcohol. 2004;32:137–144. doi: 10.1016/j.alcohol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Dopamine receptor regulation of ethanol intake and extracellular dopamine levels in the ventral pallidum of alcohol preferring (P) rats. Drug Alcohol Depend. 2005;77:293–301. doi: 10.1016/j.drugalcdep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int. 1999;35:131–141. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academy Press; 1996. [Google Scholar]
- Nielsen DM, Crosley KJ, Keller RW, Jr, Glick SD, Carlson JN. Left and right 6-hydroxydopamine lesions of the medial prefrontal cortex differentially affect voluntary ethanol consumption. Brain Res. 1999;823:59–66. doi: 10.1016/s0006-8993(99)01099-9. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res Rev. 1987;12:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol tartet in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Gryszowka VE, Toalston JE, Oster SM, Ji D, Bell RL, McBride WJ. The reinforcing actions of a serotonin-3 receptor agonist within the ventral tegmental area: evidence for subregional and genetic differences and involvement of dopamine neurons. J Pharmacol Exp Ther. 2007;321:1003–1012. doi: 10.1124/jpet.106.112607. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav. 2003;79:581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Santiago M, Machado A, Cano J. Regulation of the prefrontal cortical dopamine release by GABAA and GABAB receptor agonists and antagonists. Brain Res. 1993;630:28–31. doi: 10.1016/0006-8993(93)90638-4. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–9. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Smith WW, Sancilio LF, Owera-Atepo JB, Naylor RJ, Lambert L. Zacopride, a potent 5-HT3 antagonist. J Pharm Pharmacol. 1988;40:301–2. doi: 10.1111/j.2042-7158.1988.tb05253.x. [DOI] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Cestari DM, Pickel VM. Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: potential sites for modulation of mesolimbic dopamine neurons. Brain Res. 1994;647:307–322. doi: 10.1016/0006-8993(94)91330-7. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–267. [PubMed] [Google Scholar]
- Wozniak KM, Pert A, Linnoila M. Antagonism of 5-HT3 receptors attenuates the effects of ethanol on extracellular dopamine. Eur J Pharmacol. 1990;187:287–289. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]