Abstract
OBJECTIVES
To determine if sleep benefits motor memory in healthy elderly subjects, and, if so, whether the observed sleep-related benefits are comparable to those observed in healthy young subjects.
DESIGN
Repeated measures cross-over design.
SETTING
Boston, Massachusetts (general community) and Harvard University.
PARTICIPANTS
Sixteen healthy elderly and 15 healthy young subjects.
MEASUREMENTS
Motor Sequence Task (MST) performance was assessed at training and at the beginning and end of the retest session; polysomnographic sleep studies were recorded for the elderly subjects.
RESULTS
After 12hrs of daytime wakefulness, elderly subjects showed a dramatic decline in MST performance at the beginning of retest, relative to training, and only a non-significant improvement by the end of retest. In contrast, when the same subjects trained in the morning, but were retested 24hr after training, after a day of wake plus a night of sleep, they maintained their performance at the beginning of retest, and demonstrated a highly significant 17.4% improvement by the end of the retest session, essentially identical to the 17.3% improvement seen in young subjects. These strikingly similar improvements occurred despite the presence of other age-related differences, including overall slower motor speed, a lag in the appearance of sleep-dependent improvement, and an absence of correlations between overnight improvement and either sleep architecture or sleep spindle density in the elderly subjects.
CONCLUSION
These findings provide compelling evidence that sleep retains the capacity to optimize motor skill performance across the adult life span.
Keywords: sleep, motor skill, memory consolidation
INTRODUCTION
Sleep and Motor Skill in Young Healthy Subjects
Research on sleep-dependent memory processing in healthy young subjects has produced unequivocal evidence confirming the unique role sleep plays in memory processing. There is now a large literature demonstrating the relationship of various sleep stages, as well as several EEG signatures of specific sleep stages, with sleep-dependent memory consolidation. Different forms of learning and memory (e.g., word pairs, emotional stories, spatial navigation, visual discrimination, motor adaptation and motor sequence learning) as well as different stages in the consolidation process (e.g., stabilization, enhancement, and integration) correlate with different stages of sleep and different EEG signatures.1, 2 Some of the clearest evidence of sleep’s mnemonic power has been observed within the procedural memory domain. One task that has yielded consistent evidence of an active role of sleep in memory processing is the finger-tapping motor sequence task (MST).3 The MST is a simple motor task that requires subjects to repeatedly type a sequence of digits (e.g., 4-1-3-2-4) as quickly and accurately as possible across a number of timed trials.4 Performance of the MST requires the development and execution of a repetitive motor routine that is analogous to a number of activities, such as typing, playing a musical instrument, data entry, and myriad other tasks requiring fine, coordinated finger movements. Following a normal night of sleep, healthy college students typically show a highly significant 17–20% enhancement of motor skill speed across 3 retest trials, whereas performance following a period of wakefulness increases by only a non-significant 3–4%.4–6 Remarkably, extra training trials across a day of wakefulness only produce small incremental increases in motor speed, on the order of 1% for each additional trial, whereas a night of sleep that follows this extra daytime training imparts an additional 12% jump in performance, a clear demonstration of the potent and unique impact of sleep on motor skill processing.6
The enhancements of motor skill that emerge in young subjects as a function of sleep appear to depend on specific physiological components of sleep, namely the amount of stage 2 non-rapid eye movement (Non-REM) sleep, and sleep spindles, which are a defining signature of stage 2 sleep. Sleep spindles are intermittent, synchronous, thalamocortical bursts (12–16hz) that occur between 3–7 times per minute.7 The amount of stage 2 sleep obtained over a night of sleep4, 8 or over a daytime nap,5 as well as the number and density (number of spindles per minute) of stage 2 sleep spindles9, 10 have been shown to correlate with sleep-dependent improvement on motor memory tasks. Spectral power in the spindle frequency range (12–16hz) also correlates with this improvement,5, 8 strongly suggesting that stage 2 sleep physiology is important for motor memory processing.
Motor Skill and Sleep in Aging
It is well known that motor skills generally decline with age,11 and that typing skills in particular are susceptible to deterioration in an age-dependent fashion. However, not all aspects of typing performance are found to suffer in the elderly. For example, inter-keystroke interval does not vary with age when subjects are asked to transcribe a printed text. However, on a choice reaction time task, requiring subjects to respond to computer presentation of the letters ‘L’ and ‘R’ as quickly as possible, performance is highly correlated with age, with average reaction times increasing 2ms per year from age 20 to 70.12 This age-related decline in performance is also observed in the serial reaction time task (SRTT), which requires subjects to respond to elements in a 10-item repeating sequence that appear, one at time, at 1 of 4 different spatial locations on a computer screen. Recent research demonstrates that reaction times during SRTT training in healthy older subjects (mean age=59 years) are about twice those seen in young subjects.13, 14 These results suggest that while general typing speed is preserved for tasks that are well-learned (text transcription), speed monotonically decreases with age when subjects are required to respond quickly to novel, or unpredictable, stimuli.
Such changes in motor skill performance across the life span develop in parallel with changes in sleep patterns. Age-related decreases in total sleep time and, more dramatically, in slow wave sleep (SWS), along with increases in sleep fragmentation, are consistently observed in polysomnographic sleep studies.15–17 However, there is often the misperception that these age-related changes in sleep are an indication of impaired sleep quality. In fact, in a recent survey of 248 elderly, community-dwelling individuals reporting no other medical complaints, 90% rated their sleep quality as “Excellent,” “Very Good,” or “Good,” whereas those with medical conditions were up to four times as likely to rate their sleep as “Fair/Poor”.18 This finding is corroborated in an excellent review by Ancoli-Israel et al.,19 who conclude that compromised sleep quality in the elderly is by and large a result of underlying comorbidity, including changes in circadian rhythmicity, medication effects, or sleep disorders such as obstructive sleep apnea and REM sleep behavior disorder.
While research findings are scant, a handful of studies suggest that aging may also be associated with decrements in sleep-dependent memory consolidation. Middle-aged subjects (mean age=50) improve less following an interval of nocturnal sleep than young subjects on a word pair recall task, and this recall difference is associated with decreased SWS in the older subjects.20 The two studies that have examined the effect of sleep on motor skill memory in the elderly present a mixed picture. In one study, young subjects became faster on the SRTT following a night of sleep, while reaction times in older subjects (mean age=59) actually slowed following sleep.14 In contrast, a study using a simple pursuit rotor task found that while young subjects learn the task with greater facility than elderly subjects, percentage improvement in performance (relative to training) seen one week later did not differ between the two groups.9 These findings suggest that there may be qualitative differences between tasks (e.g., task difficulty or complexity) that influence sleep’s impact on motor memory processing.
Given our extensive knowledge of the sleep-dependent enhancements of memory for the finger-tapping motor sequence task (MST) in healthy young subjects, we examined whether sleep also enhances MST performance in healthy elderly individuals.
Using a repeated-measures cross-over design, the present study assessed MST performance in a group of healthy elderly subjects across a 12hr period of wakefulness and across a 24hr period containing a day of wakefulness and a night of sleep. We also trained and tested college students across an identical 24hr interval to assess age-related differences in MST performance both at training and following sleep-dependent consolidation.
METHODS
Subjects
The study was approved by the Beth Israel Deaconess Medical Center IRB, and all subjects provided written consent prior to participation.
Elderly Subjects
Sixteen healthy, right-handed elderly subjects (11 females, mean age=68.0 ± 6.6yrs, age range, 60–79), with an average of 3.1 ± 3.1 (SD) yrs of college education, participated in the study. An additional three subjects completed the study, but were excluded from data analysis due to a failure to consistently type the correct 5-digit sequence, producing an average accuracy (percent correctly typed keystrokes) of only 50%, more than 5 SDs below the lowest accuracy rate (80%) of the other 16 subjects. Subjects with psychiatric disease, dementia, motor or vision impairment, drug/alcohol dependence in the past 15 years, or diagnosed current sleep disorders were excluded from participation. Subjects were also excluded if they consumed more than two 12oz caffeinated beverages per day, were taking medications known to affect sleep, reported difficulty initiating sleep, or reported an average total sleep time of less than 6hrs per night. Subjects reported no sleep complaints prior to participation, and no previous diagnoses of sleep disorders. Subjects were not allowed to nap during the course of the study. A daily sleep log was completed to monitor the regularity of each subject’s sleep/wake cycle. Ambulatory polysomnographic recordings were conducted in each subject’s home on the night across which MST performance was tested.
Young (Control) Subjects
Fifteen healthy, right-handed university students (11 females, mean age=20.1 ± 1.2yrs) participated in the study. Subjects were instructed to abstain from caffeine and alcohol for 24hrs prior to and during the study. All young subjects were medication free (except for birth control, n=1), and all reported habitual total sleep times greater than 6hrs per night. Regularity of subjects’ sleep schedules was assessed by a 3-day retrospective sleep log.
Motor Sequence Task (MST)
The MST has been used in a number of previous studies.3 Subjects were instructed to repeatedly type a 5-digit series of numbers (4-1-3-2-4 or 2-3-1-4-2) with their left (non-dominant) hand “as quickly and accurately as possible” for each of twelve 30sec trials. The 5-digit number was always displayed at the top of the screen, and each keystroke was represented by a dot on the screen starting from the left edge of the monitor. Subjects were given a 30sec rest period between trials. They completed 12 trials during the training session and 12 trials at retest. As in previous research, the average number of correct sequences across the last 3 training trials was used as the measure of training performance (Figure 1, dashed line), while the mean of the first 3 retest trials (Figure 1, trials 1–3) was the measure of immediate retest performance, consistent with earlier studies in which the retest session consisted of only 2 or 3 retest trials.4–6 In the present study we extended the retest session for all groups to 12 trials to examine how performance might change across a longer retest session, as we have previously found that healthy midlife subjects (44 ± 6 years of age) require 2–3 retest trials before performance reaches a stable plateau.21 We therefore also examined the average of the last 3 retest trials as a measure of plateau performance (Figure 1 trials 10–12).
Figure 1.
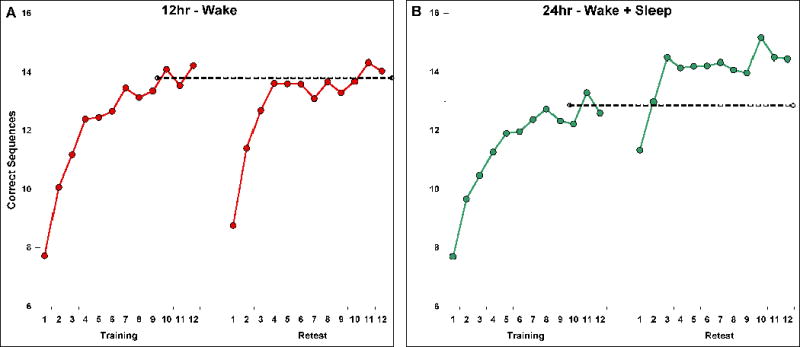
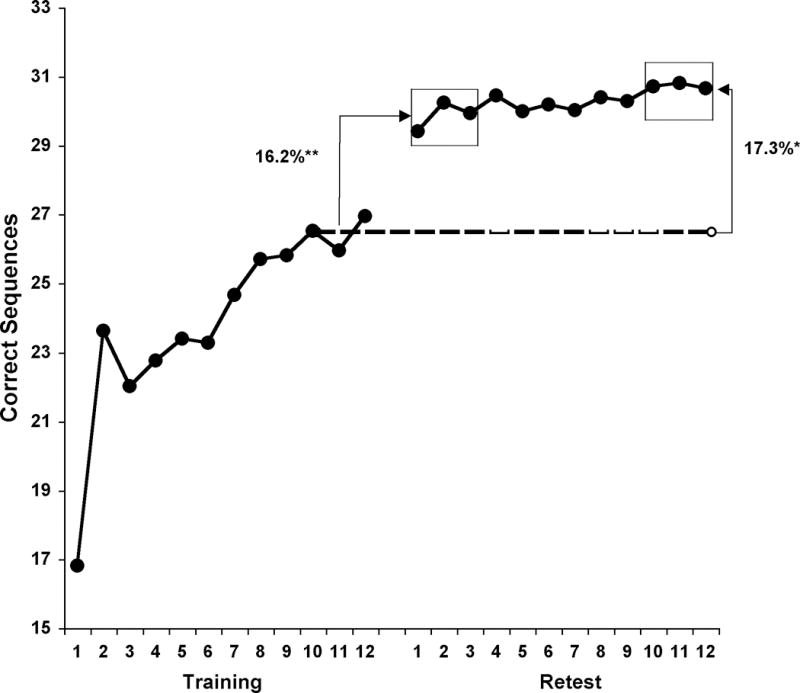
MST performance in elderly (A & B) and young (C) subjects. The dashed lines represent training performance (average of the last 3 training trials). Retest trials 1–3 represent “immediate retest”; retest trials 10–12 represent “plateau retest” trials. The y-axis represents the number of correct sequences per 30s trial.
Analyses
The primary dependent measures are immediate improvement (first 3 retest trials – last 3 training trials), and plateau improvement (last 3 retest trials – last 3 training trials) as well as percent improvement ([first 3 retest trials – last 3 training trials / last 3 training trials]*100, and [last 3 retest trials – last 3 training trials / last 3 training trials]*100).
Procedure
Elderly Subjects
MST testing and sleep recordings were done in the subjects’ homes. Each subject completed two training-retest sessions. During one session they were trained on a sequence (e.g., 4-1-3-2-4) in the morning (9am) and retested after 12hr of wakefulness (9pm); in the other session they were trained on a second sequence (e.g., 2-3-1-4-2) in the morning (9am) and tested 24hr later (9am), after a day of wakefulness and a night of sleep. Session and sequence orders were counterbalanced across subjects. The Stanford Sleepiness Scale22 was administered prior to each training and retest session to assess level of alertness/sleepiness.
On the night of the 24hr session, polysomnographic (PSG) sleep recordings were acquired using an Embla A-10 ambulatory PSG system, with Somnologica software. Central (C3/C4) and occipital (O1/O2) electroencephalography (EEG), left and right eye movements (electro-oculography; EOG), and chin muscle activity (electromyography; EMG) were recorded. Sleep stages 1–4 and REM sleep were scored in accordance with standard criteria.23 Spectral analysis was conducted to assess EEG power (μV2) within the spindle frequency range. Prior to spectral analysis, EEG data were filtered between 0.5 and 35 Hz. EEG power in fast (12–14 Hz) and slow (14–16 Hz) spindle frequency bands was analyzed using Hanning windowing on all artifact-free 30s epochs, and was then averaged across central EEG channels C3 and C4.
Young Subjects
Subjects arrived at the computer laboratory at 9am. They completed the sleep log and the Stanford Sleepiness Scale, and then trained on the MST. As with the elderly subjects, young subjects performed 12 trials at training and then another 12 trials at retest 24 hr later, at 9am the following morning.
RESULTS
Sleep Log and PSG Data
Prior to each training session subjects reported a number of subjective sleep variables by sleep log, including the amount of time spent in bed, the amount of sleep they obtained, how long it took to fall asleep, and sleep quality. The measure of sleep quality was based on a 1–4 scale (1=Excellent, 2=Good, 3=Fair, 4=Poor). Young and elderly subjects reported similar values for all of these subjective measures (all p values > .10).
MST Performance – Elderly Subjects
We observed no evidence of carryover effects from the first to the second MST sequence (paired samples t-test for number of correct sequences at training (Sequence 1: 13.1 ± 1.1, Sequence 2: 13.6 ± 1.2, t(15)=0.97, p=.35). Nor were there differences between the Wake and Wake + Sleep conditions in subjective sleepiness (Stanford Sleepiness Scale, p>.5 at both training and retest), improvement during training (percentage improvement from the first 3 to the last 3 training trials: 55.7 ± 12.3% v. 50.7 ± 10.2, t(15)=.46, p=.65) or training accuracy (93.2 ± 1.4% v. 93.2 ± 1.3%, t(15)=.00, p>.9). However, we did observe a difference in training performance between the Wake and Wake + Sleep conditions (14.0 ± 1.2 v. 12.7 ± 1.0, t(15)=2.53, p=.02), presumably a sampling artifact, given the identical training conditions and counterbalancing of order and sequence.
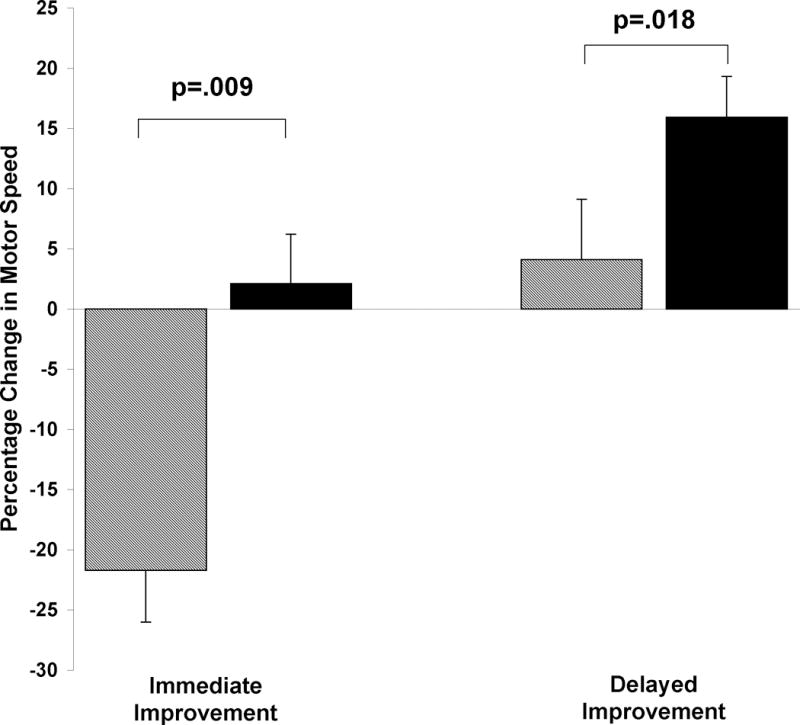
Performance at immediate retest after 12hr of wakefulness dropped by 3.0 correct sequences compared to training performance (Figure 1A, dashed line), from 14.0 to 11.0 correct sequences (−22%, p<.001; Figure 1A, and Figure 2 left, striped bar). In contrast, following a 24hr period containing a day of wake and a night of sleep, performance was maintained relative to training, increasing nominally from 12.7 to 12.9 correct sequences (+2%, ns; Figure 1B, and Fig 2 left, filled bar). Overall, subjects demonstrated significantly better immediate improvement after 24hr containing a night of sleep than after just 12hr without sleep (paired samples t-tests: numeric improvement, t(15)=3.7, p=.002; percent improvement, t(15)=4.2, p=.001).
Figure 2.
MST immediate and plateau improvement in elderly and young subjects. MST change in performance across a 12hr period of wakefulness (striped bars), and across a 24hr interval containing a day of wake and a night of sleep in elderly subjects (black bars) and young subjects (gray bars). Bars represent means ± SEMs. ** p<.001.
Across the retest session, there was a significant increase in correct sequences from the first 3 to the last 3 retest trials in both conditions (Wake: 3.1 ± 0.5 sequences, t(15)=5.90, p<.001; Wake + Sleep: 1.8 ± 0.3, t(15)=6.05, p<.001), which resulted in significant plateau improvement in the Wake + Sleep condition (+17.4%, t(15)=4.49, p<.001), but not in the Wake condition (+4.0%, t(15)=.81, p=.43; Figure 1A and B, and Figure 2 right). The difference between conditions for plateau improvement was also significant (t(15)=2.66, p=.018), indicating that subjects in the wake condition failed to exhibit improvement comparable to that expressed following a night of sleep.
Correlation of Overnight Improvement with Sleep Measures – Elderly Subjects
Polysomnographic sleep data for the elderly sample are presented in Table 1. Average total sleep time was 5.7hrs, with an average sleep efficiency of 77%, values quite similar to normative values obtained for a comparably aged cohort from the Sleep Heart Health Study (6.0hrs and 81.8%, respectively).16
Table 1.
Polysomnographic Data – Elderly Subjects.
| Minutes ± SD | % of TST ± SD | |
|---|---|---|
| Time In Bed | 445.4 ± 67.9 | |
| Sleep Onset Latency | 28.5 ± 39.6 | |
| Total Sleep Time | 341.6 ± 75.4 | |
| Sleep Efficiency (%) | 76.5 ± 11.9 | |
| Wake After Sleep Onset | 131.4 ± 71.5 | |
| Stage 1 | 41.8 ± 19.2 | 12.5 ± 5.2 |
| Stage 2 | 204.5 ± 63.7 | 59.2 ± 9.3 |
| Stage 3 | 25.4 ± 14.1 | 7.9 ± 5.3 |
| Stage 4 | 1.5 ± 3.8 | 0.5 ± 1.3 |
| Slow Wave Sleep | 26.9 ± 17.2 | 8.4 ± 6.5 |
| REM Sleep | 68.5 ± 24.7 | 19.9 ± 6.9 |
Note: Sleep data in the 24hr Wake + Sleep condition. Time in Bed represents the interval from the time the subject got into bed in the evening to the time the subject got out of bed in the morning. Sleep Onset Latency is the time between lights out and the first epoch of Stage 1 sleep. Wake After Sleep Onset is the amount of wake time during the night after sleep onset. Slow Wave Sleep = amount of Stage 3 + Stage 4 sleep. Sleep efficiency = (total sleep time/time in bed)*100. Values are Means ± SD.
A number of studies have established a link between PSG-recorded sleep parameters and overnight motor skill improvement in young subjects, especially between improvement and amount of either stage 2 sleep4, 8 or sleep spindle activity during stage 2 sleep.5, 9 In one study using a similar motor sequence task, overnight improvement correlated with amount of REM sleep.24 However, no correlations were seen in a prior study of healthy middle-aged subjects.21 Similar to that study, we found no significant relationships between sleep and sleep-related motor skill enhancement in our healthy elderly sample. Nor did we observe any significant relationships between slow or fast spindle spectral power and immediate or plateau retest performance (Table 2).
Table 2.
Correlations between Sleep Parameters and Motor Task Performance.
| Sleep Stage Data
|
||||
|---|---|---|---|---|
| % Change Immediate Improvement | % Change Plateau Improvement | |||
|
| ||||
| r | p | r | p | |
| Stage 1% | −.07 | .80 | −.33 | .22 |
| Stage 2% | −.15 | .59 | −.32 | .23 |
| SWS% | −.15 | .58 | .28 | .29 |
| REM% | .38 | .14 | .42 | .11 |
| Slow (12–14hz) and Fast (14–16hz) Spindle Power
|
||||
|---|---|---|---|---|
| % Change Immediate Improvement | % Change Plateau Improvement | |||
|
| ||||
| r | p | r | p | |
| C3 Slow | −.07 | .83 | .03 | .92 |
| C3 Fast | .06 | .85 | .11 | .73 |
| C4 Slow | −.05 | .87 | .01 | .97 |
| C4 Fast | .05 | .88 | .07 | .81 |
Note: Correlations of sleep stage parameters and spindle frequency (12–16hz) power (μV2) with 24hr MST improvement, measured as percent change in number of correctly typed sequences from training to retest. ‘Immediate Improvement’ indicates performance on the first 3 retest trials relative to the last 3 training trials, while ‘Plateau Improvement’ indicates performance on the last 3 retest trials relative to the last 3 training trials.
MST Performance Data – Young Subjects
Young subjects averaged 27.5 correct sequences at training (Figure 1C, dashed line). Twenty-four hours later, on the first 3 retest trials, performance jumped to 31.7 ± 6.4 sequences (Figure 1C), which represented a significant immediate improvement of 16.2% relative to training (paired samples t-test, t(14)=6.93, p<.001; Figure 2 left, gray bar). Unlike the elderly subjects, who demonstrated a highly significant increase from the first 3 to the last 3 retest trials (+1.8 sequences, p<.001), the younger cohort showed no such change (+0.3 sequences, 1.7%; paired samples t-test, t(14)=.43, p=.68; Figure 1C), although plateau improvement was still highly significant (+4.5 sequences, 17.3 ± 2.2%; paired samples t-test, t(14)=7.83, p<.001; Figure 2 right, gray bar), demonstrating that while MST speed jumps dramatically from training to the first 3 retest trials, performance thereafter remains unchanged.
Age Differences – 24hr Wake + Sleep
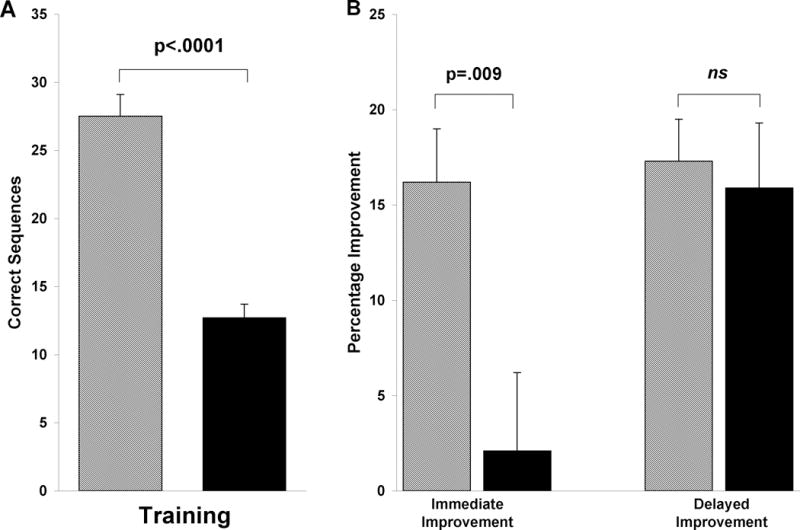
In the 24hr Wake + Sleep protocol, young and elderly subjects produced similar learning curves across the training session, with young subjects improving by 5.4 sequences, versus a 3.4 sequence increase in the elderly subjects, from the first 3 to the last 3 training trials. Accuracy across the 12 training trials was also very similar (Young: 93.0 ± 1.2%, Elderly: 93.2 ± 1.3%, t(29)=.10, p=.92). However, there was a notable difference in overall MST speed, with young subjects typing more than double the number of correct sequences by the end of training (27.5 ± 1.6 sequences; Figure 1C) as the elderly sample (12.7 ± 1.0, t(29)=7.95, p<.0001).
While the two age groups showed a similar degree of learning during the training session, the young subjects demonstrated a 16.2% immediate improvement after 24 hr, while the elderly subjects only maintained their performance across the 24hr interval, improving by a non-significant 2% (t(29)=2.81, p=.009; Figure 2, left). However, by the end of the retest session, the elderly subjects attained a level of motor skill improvement strikingly similar to that of the young subjects, increasing their performance by 17.4 ± 3.9%, compared to 17.3 ± 2.2% for young subjects (t(29)=.03, p=.98; Figure 2, right). This finding clearly shows that elderly subjects show robust post-sleep MST improvements that parallel those observed in healthy young subjects, although full expression of these improvements is not seen until the third retest trial (Figure 1B).
DISCUSSION
The primary objectives of the present study were to examine motor skill proficiency in healthy elderly subjects and, more importantly, to determine whether motor skill performance is subject to sleep-dependent enhancement, as has been shown numerous times in healthy young subjects.4, 6, 24 Using a within-subjects design, we examined MST performance following a 12hr period of wake and a 24hr period containing a full day of wakefulness followed by a full night of sleep. Compared to a 22% performance decrease at immediate retest following 12hr of wakefulness, performance after a full night of sleep was maintained (+2% change). Across the first 3 retest trials wake subjects showed rapid improvement (Figure 1B), but by the last 3 retest trials still had only improved by a non-significant 4% above training performance. This modest over-wake improvement stands in stark contrast to the significant 17% improvement observed at the retest plateau following 24hr containing a full night of sleep. It is noteworthy that after 12hr of wakefulness, retest performance in this group did not cross the training performance threshold until trial 11 of the retest session (Figure 1A). However, following a 24hr interval that included sleep, performance surpassed training performance on the second retest trial, and was 14% above training levels by the third retest trial (Figure 1B), remaining essentially constant across the remainder of the retest session. These findings strongly suggest that sleep makes a unique and powerful contribution to the enhancement of motor skill performance in the elderly, although the fullest expression of this enhancement is only expressed after the first few retest trials.
It might be suggested that the performance advantage observed following a 24hr period including sleep, compared to a briefer 12hr period that contains wake only, is a result of the mere passage of time. However, if time were related to performance in a linear fashion, we would expect two outcomes: 1) that immediate retest performance 24hrs after training would decrease by twice the 22% seen at the 12hr time point, or 2) that the plateau 4% improvement following 12hr of wake would increase another 4% after an additional 12hr interval. Our findings, however, suggest a non-linear improvement over time, similar to previous research,6 such that an additional 12hr interval that includes a night of sleep produces immediate performance maintenance (i.e., 24% above immediate retest performance after 12hr of wakefulness), and a 4-fold increase in plateau improvement.
Previous research has shown that aspects of motor skill ability decline with age.25, 26 Here we found that elderly subjects performed a procedural motor sequence task much slower than healthy college subjects. While this observed difference in training performance is dramatic, with young subjects completing more than two times as many sequences as the elderly subjects at both the start and end of training (Figure 1), both groups produced similar learning curves and similar accuracy over the 12-trial training session, suggesting that both groups executed and learned the task similarly, with elderly subjects simply performing at a slower rate.
The age differences observed at training were also evident in immediate retest performance. In contrast to young subjects who showed a robust 16% improvement at immediate retest, no performance enhancement was expressed in elderly subjects, whose performance was virtually unchanged (+2%) from training to immediate retest. This nominal change in performance is also considerably diminished compared to a 12% improvement in one study of MST performance in healthy middle-aged subjects,21 although comparable to the non-significant 4.2% improvement seen in a second such study.27
When we examined plateau improvement across the last 3 retest trials in our elderly subjects, we observed a significant 17.4% increase in performance over training levels, which is essentially identical to the 17.3% enhancement achieved at the end of retest in the young subjects. Thus, while young subjects start the retest session well above training levels, their performance is unchanged across the remaining retest trials, increasing only 1% by the last 3 retest trials. The immediate retest performance lag in the elderly suggests that the task may have been more difficult for the elderly subjects, and that the first few retest trials were necessary to permit complete expression of the overnight improvement. The idea that this delayed expression of overnight improvement is related to task difficulty, rather than to age per se, is supported by previous research showing that when young subjects perform a difficult, bi-manual 9-digit finger tapping sequence, they show a similar retest performance lag on the first retest trial, followed by a substantial speed increase across trials 2 and 3.28
The finding that MST performance is enhanced in healthy elderly subjects following a time interval containing a night of sleep is consistent with previous research examining sleep-dependent motor skill performance in the elderly. For example, Peters, et al. (2008) showed that elderly subjects’ performance on a pursuit rotor task, when retested after a week’s delay, showed the same proportional degree of improvement observed in their sample of young subjects. However, using a more complex motor task, the SRTT, Spencer, et al. (2007) found that the reaction times produced by elderly subjects actually slowed relative to training, after a night of sleep, while reaction times were significantly faster in young subjects. The difference in outcomes across this small sampling of studies may relate to the fact that the MST and pursuit rotor task are considerably simpler to execute and acquire than the SRTT, a task that requires greater mental agility and attentional resources to execute, as the 10-element sequences in this task require the processing of spatial as well as contextual information embedded within each sequence. It may be that the elderly subjects in that study did not attain the level of task proficiency required to trigger sleep-dependent enhancement processes, or that sleep in the elderly loses its capacity to robustly process these more complex tasks.
In young subjects, multiple studies have revealed a relationship between overnight MST enhancement, the amount of stage 2 sleep across the night,4, 8 and sleep spindle activity.5, 9, 10 However, in our healthy elderly subjects there was a distinct lack of correspondence between sleep parameters and overnight MST performance. One possible explanation of this difference pertains to the study design employed here. The subjects in the present study were trained in the morning and retested 24hrs later, with the sleep episode occurring at least 12hr following training on the task. Previous studies with young subjects have only examined the effect of PSG-recorded sleep when it closely followed training, suggesting that long periods of post-training wakefulness may introduce additional factors which reduce the amount of variance explained by specific sleep correlates, such as the amount of stage 2 sleep. But the similar lack of sleep stage correlations in a midlife sample that was trained shortly before sleep27 suggests that the lack of correlation may simply result from changes in sleep with age. Indeed, yet unidentified age-related changes in the sleep-wake cycle may limit our ability to observe the impact of specific sleep characteristics on motor skill enhancement. Further research examining the relationship between recorded sleep stages and overnight performance will be necessary to confirm whether this is a consistent finding in elderly samples.
Although both general memory function and sleep quality change in an age-related fashion, we demonstrate here that sleep in the elderly retains a robust capacity to optimize motor skill performance to levels proportional to those seen in healthy young subjects, even though elderly subjects perform at a markedly slower pace. We hope these findings will pave the way for further exploration of the benefits of sleep on cognition in the elderly.
Acknowledgments
Dr. Stickgold has received research support from Merck & Co., Sepracor Inc., Jazz Pharmaceuticals, and Actelion Pharmaceuticals Ltd. He has received educational grants from Takeda Inc. He has consulted to these companies, and has received honoraria from Eli Lilly & Co. and Epix Pharmaceuticals.
Sponsor’s Role: None
Footnotes
Author Contributions: Stickgold: Study conception. McKinley: Data acquisition; Tucker, McKinley, and Stickgold: Study design, data analysis, manuscript preparation, and interpretation of results.
Matthew Tucker and Sophia McKinley have no conflicts of interest, actual or potential, with any of the work presented in this manuscript.
References
- 1.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 2.Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci. 2010;11(3):218. doi: 10.1038/nrn2762-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker MP. A refined model of sleep and the time course of memory formation. Behav Brain Sci. 2005;28(1):51–64. doi: 10.1017/s0140525x05000026. [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Brakefield T, Morgan A, et al. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 5.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker MP, Brakefield T, Seidman J, et al. Sleep and the time course of motor skill learning. Learn Mem. 2003;10(4):275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 8.Tucker MA, Fishbein W. The impact of sleep duration and subject intelligence on declarative and motor memory performance: how much is enough? J Sleep Res. 2009;18(3):304–312. doi: 10.1111/j.1365-2869.2009.00740.x. [DOI] [PubMed] [Google Scholar]
- 9.Peters KR, Ray L, Smith V, et al. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res. 2008;17(1):23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 10.Peters KR, Smith V, Smith CT. Changes in sleep architecture following motor learning depend on initial skill level. J Cogn Neurosci. 2007;19(5):817–829. doi: 10.1162/jocn.2007.19.5.817. [DOI] [PubMed] [Google Scholar]
- 11.Krampe RT. Aging, expertise and fine motor movement. Neurosci Biobehav Rev. 2002;26(7):769–776. doi: 10.1016/s0149-7634(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 12.Salthouse TA. Effects of age and skill in typing. J Exp Psychol Gen. 1984;113(3):345–371. doi: 10.1037//0096-3445.113.3.345. [DOI] [PubMed] [Google Scholar]
- 13.Spencer RM, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Curr Biol. 2006;16(10):1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 14.Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14(7):480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- 15.Bliwise DL. Normal Aging. In: Kryger R, Dement, editors. Principles and Practice of Sleep Medicine. 4th. W.B. Saunders Company; 2005. [Google Scholar]
- 16.Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27(2):293–298. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- 17.Ancoli-Israel S, Alessi C. Sleep and aging. American J Geriatr Psychiatry. 2005;13(5):341–343. doi: 10.1176/appi.ajgp.13.5.341. [DOI] [PubMed] [Google Scholar]
- 18.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Ancoli-Israel S, Ayalon L, Salzman C. Sleep in the elderly: normal variations and common sleep disorders. Harv Rev Psychiatry. 2008;16(5):279–286. doi: 10.1080/10673220802432210. [DOI] [PubMed] [Google Scholar]
- 20.Backhaus J, Born J, Hoeckesfeld R, et al. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14(5):336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoach DS, Cain MS, Vangel MG, et al. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56(12):951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Hoddes E, Zarcone V, Smythe H, et al. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. US Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- 24.Fischer S, Hallschmid M, Elsner AL, et al. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99(18):11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 26.Bosman EA. Age-related differences in the motoric aspects of transcription typing skill. Psychol Aging. 1993;8(1):87–102. doi: 10.1037//0882-7974.8.1.87. [DOI] [PubMed] [Google Scholar]
- 27.Manoach DS, Thakkar KN, Stroynowski E, et al. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44(2):112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11(6):705–713. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]


