Abstract
Psychosomatic disorders are comprised of an array of psychological, biological, and environmental features. The existing evidence points to a role for genetic factors in explaining individual differences in the development and maintenance of a variety of disorders, but studies to date have not shown consistent and replicable effects. As such, the attempt to uncover individual differences in the expression of psychosomatic disorders as a function of genetic architecture requires careful attention to their phenotypic architecture, or the various intermediate phenotypes that make up a heterogeneous disorder. Ambulatory monitoring offers a novel approach to measuring time-variant and situation-dependent intermediate phenotypes. Recent examples of the use of ambulatory monitoring in genetic studies of stress reactivity, chronic pain, alcohol use disorders, and psychosocial resilience are reviewed in an effort to highlight the benefits of ambulatory monitoring for genetic study designs.
Ambulatory monitoring offers a unique approach to capturing time-variant processes that may inform genetic models of human behavior and psychosomatic disorders. In this article, we provide a rationale for the application of ambulatory monitoring methods to psychosomatic research involving molecular genetics. In so doing, we introduce some of the pitfalls of molecular genetic methods, and develop a working framework through which ambulatory monitoring may improve upon past efforts to characterize gene-phenotype relations in complex psychosomatic disorders. Although ambulatory monitoring can be applied to both subjective self-report and objective psychophysiological data collection, our focus in this article is primarily on the former. We have chosen this direction because such a sizable portion of the common symptoms and modulatory factors associated with psychosomatic disorders - including stress, cognition, emotion, pain, and well-being-- require assessment of individual subjective impressions, and unreliable measurement of these subjective variables can affect the validity of genetic associations. As such, it is necessary to evaluate the utility of ambulatory monitoring in the assessment of complex phenotypes that rely on subjective self-report. That being said, we recognize the importance of ambulatory psychophysiological measurement and will comment on its utility in the genetics of psychosomatic medicine later in the article.
After initially promising results in twin-based studies signaled the involvement of genetic factors in the development of such varied disorders as depression (1), cardiovascular disease (2), alcohol dependence (3, 4), and chronic pain (5), recent years have seen a proliferation of studies that have attempted to identify specific molecular genetic associations with these disorders and others that sit at the crossection of biology and behavior. Despite the considerable investment of resources to improve the precision and scope of genomic analysis, little has been definitively learned about the genetic basis of complex behavioral disorders. Valid and replicable effects remain the holy grail of molecular genetic research, and there continues to be controversy about the best path forward, as well as the best vehicle to take us there. Much of this controversy concerns modeling on the genetic side of the gene-phenotype equation. Will it be more fruitful to focus on common or rare alleles? Do copy number variants hold more promise than single nucleotide polymorphisms (SNPs)? These and countless other gene-oriented questions are beyond the scope of this article. Rather, here we turn to the phenotypic side of the gene-phenotype equation, and make a case for the role of ambulatory monitoring in this uncertain calculus.
Psychosomatic disorders are by definition multifaceted and characterized by interactions of factors of the body and mind that are constantly evolving and responding to shifting environmental demands. As such, the attempt to uncover individual differences in the expression of these disorders as a function of genetic architecture requires careful attention to the “phenotypic architecture,” or the various intermediate phenotypes1 that make up any complex disorder with both biological and behavioral inputs (6, 7). Ambulatory monitoring is primed to play a leading role in this effort. To support this notion, we first describe the principle problems with molecular genetic applications to psychosomatic research to date. We then will argue for how ambulatory monitoring, in general, can address each of these methodological shortcomings. Table 1 summarizes the “threats” to the validity of molecular gene-phenotype associations, along with the “solutions” offered by ambulatory monitoring. These “solutions” by no means solve the dilemmas facing genetic research, but, rather, offer methodological strategies that, we believe, may improve our understanding of the genetic basis of psychomatic illnesses. Finally, we will highlight several specific psychosomatic research domains in which the marriage of ambulatory monitoring and molecular genetic methods holds promise and, in some cases, has already begun to advance our understanding of common illnesses.
Table 1. Threats to Detecting True Gene-Phenotype Associations and Solutions Offered by Ambulatory Monitoring.
| Threats | Ambulatory Monitoring Solutions |
|---|---|
| 1. Low phenotype reliability | 1. Improved reliability through real-time assessment in a natural environment; reduced bias from recall errors |
| 2. Low power due to small effects and small samples | 2. Improved power through repeated measurements |
| 3. Poor phenotypic specification | 3. Flexible, ecologically valid phenotyping |
Threats to the Validity of Genetic Associations in Psychosomatic Research
Threat 1: Reliability and Validity
Perhaps the greatest problem with molecular genetic studies of complex illness to date has been poor replicability of reported effects (8, 9) and, consequently, widespread skepticism about the validity of these effects. Although a number of factors could influence the replicability of an observed effect, an unreliably measured intermediate phenotype will weigh heavily on this likelihood. When elucidating the genetic basis for psychosomatic disorders, which are heterogeneous and comprised of a variety of independent and interacting intermediate phenotypes, reliable and ecologically valid characterization of the genetic association with the disorder may require measurement of intermediate phenotypes in both simulated and natural environments. As such, gene × environment designs have become attractive tools for genetic inquiry (10). In such models, exposure to an environmental factor, such as life stress, is expected to interact with a genetic variant of interest in the prediction of disease development, maintenance, or severity. As a principle advantage of ambulatory monitoring is its contribution to enhanced ecological validity, this approach is a natural fit for gene × environment designs.
In addition to the edge in ecological validity provided by ambulatory monitoring, measurement reliability may also be improved. Research has consistently indicated that repeated measures of self-report variables in close temporal proximity to that which is being reported are more accurate and reliable than single-occasion assessments requiring recall (for a review, see: 11). The replicability of observed associations between genes and phenotypes based on self-report also hinges on the reliability of the measure, and the occasion(s) in which it is measured. For intermediate phenotypes that would be expected to vary over time, such as mood, pain, or other time-varying expressions of self-reference, an ambulatory monitoring approach provides the flexibility to assess these variables in close to real-time.
Cognitive and social psychological research has shown that self-reports of past experiences are influenced by the past frequency of occurrence of the experience (12). Further, the recency and peak intensity of stimuli such as stress or pain have been shown to influence retrospective reports (13). Ambulatory monitoring of self-reports minimizes the bias introduced by recall errors by assessing self-report in close to real-time. Further, ambulatory monitoring affords investigators the opportunity to analyze data prospectively, a technique that facilitates causal inference and decreases error variance (14). Thus, the close-to-real-time measurement provided by ambulatory monitoring techniques should improve the reliability and validity of measured self-report phenotypes, a critical step in the path toward valid and replicable genetic effects.
Threat 2: Low Power to Detect Small Effects
The increased reliability of repeated measurements through the intensive longitudinal study designs inherent in ambulatory monitoring of self-reported experience also enhances statistical power. Underpowered studies will not be able to reliably detect effects of complex illnesses. In fact, meta-analyses (15, 16) of the classic 5-HTTLPR × life stress interaction predicting risk for depression (17) have argued that most studies of this phenomenon have been underpowered, resulting in mixed findings and diminished confidence in the validity of positive findings (for a counter argument, see: 18). Effect sizes, which partly determine power, have typically been very small in molecular genetic studies of complex psychiatric and behavioral disorders (19, 20). Cohen (21) explained that unreliability attenuates observed effect sizes and, hence, statistical power. In the absence of consistently well-powered designs, an effect is unlikely to be consistently replicated. Therefore, the increased measurement reliability realized through ambulatory monitoring may improve power.
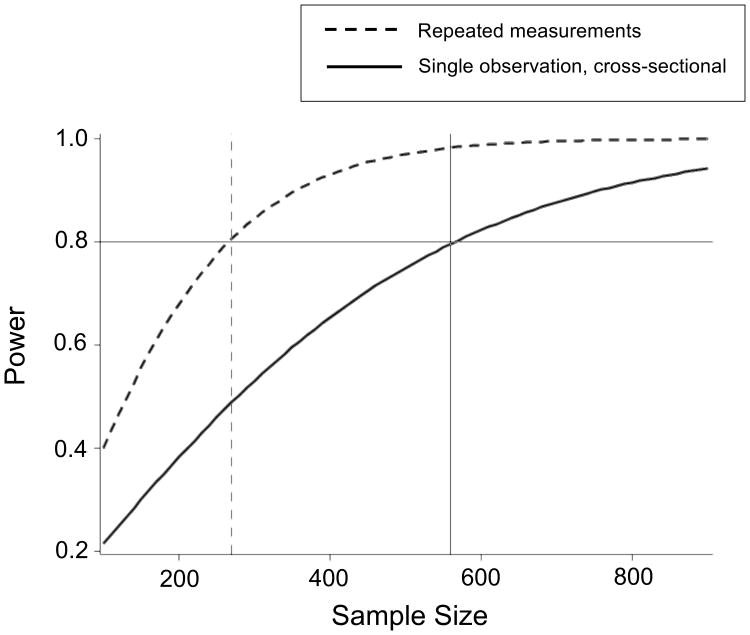
When effect sizes are expected to be small, sample size demands increase quickly in order to achieve appropriate statistical power. Ambulatory monitoring, however, can reduce the burden of sample size through repeated measurements. Figure 1 illustrates the statistical power advantage of repeated measurements over single observation, cross-sectional data collection by plotting needed sample size and statistical power to detect a small effect of size .15, on the metric of a biserial correlation coefficient (measuring the association between a categorical variable, the gene, and a continuous phenotypic measure). For this illustration we assume a reliability of .6 for a single observation, cross-sectional design that includes 200 subjects, and a reliability of .8 for a repeated measure design with 100 repeated measurements (a number that is now relatively easily obtainable through digital technology). It should be noted that the autocorrelation of repeated measurements needs to be considered in the estimation of power as well, and can be controlled for, in developing these designs; power diminishes as autocorrelation increases. Figure 1 was simulated while using an autocorrelation parameter of .6, which is consistent with what we have historically found in our daily diary data. The simulation shows that under these specific parameters, between 250-300 subjects are needed to obtain power of .8 in the repeated-measures design (dotted line), whereas nearly 600 subjects are needed for similar power in the single observation, cross-sectional design (solid line). Thus, ambulatory monitoring may be an appropriate phenotypic measurement strategy in studies where recruitment of very large samples is not feasible (e,g., clinical samples).
Figure 1.
Statistical power as a function of sample size to detect a bi-serial correlation coefficient of size .15 in a repeated-measure design with 100 repeated measures, autocorrelation of .6, and reliability of .8, versus single observation, cross-sectional designs with reliability of .6.
We recognize that increasing sample size is a stronger approach to increasing power than adding within-person units. However, as our simulation demonstrates, the addition of within-person units can relieve a sizable sample recruitment burden without affecting power, assuming that reliability of the measured construct is increased through the repeated measurement occasions. Recent technological developments have made the collection of such a volume of repeated measurements more palatable to the participant through multiple within-day measurements on personal digital devices. A recent study, for example, assessed nicotine craving and other self-report variables 8 times daily for 21 consecutive days (22). Further, passive ambulatory psychophysiological techniques, such as actigraphy, can reach hundreds of data points with even less participant burden.
Threat 3: Poor Phenotypic Specification
Until recently, molecular genetic research has been primarily concerned with genetic predictability of the presence or absence of disease. For psychosomatic disorders, diagnostic criteria can be unreliable, and do not always accurately characterize the various factors that may account for individual differences in the population (e.g., 23). It is for this reason that scholars have argued for the measurement of intermediate phenotypes, which are designed to shorten the ‘predictive distance’ between gene and disease by defining and measuring a phenotype that is descriptive of the processes engendered by the function of the gene (7).
Ambulatory monitoring is poised to improve phenotypic specification by permitting greater flexibility in the choice of phenotypic markers that may be pertinent to disease development and maintenance. For example, people with major depressive disorder have been shown to be more stress-reactive than the general population (24). Stress reactivity, then, could be considered an intermediate phenotype that addresses a key feature of that disorder (25). Stress reactivity and other process-oriented variables may be more accurately measured through daily self-report than is possible in laboratory-based simulations (26). When the goal is to determine genetic association with an intermediate phenotype, the specificity of that phenotypic marker to the disease process is essential; subtle differences in how a phenotype is measured could produce variable results. Reactivity to an interpersonal stress challenge in a laboratory setting (e.g., Trier Social Stress Test, 27), for example, may produce a more acute, amplified, and truncated physiological and psychological response than that which is observed through a participant's report of ongoing negative interpersonal events and his or her reaction to them, thereby presenting a threat to criterion validity (28). In fact, one review of cardiovascular reactivity found only 20-25% concordance between hypothesized relations in the laboratory and the field (28). A recent empirical study found that heart rate reactivity to several different types of laboratory stressors did not reliably predict ambulatory heart rate variation (29). Further, ambulatory blood pressure monitoring has been shown to be a better predictor of cardiovascular disease than a laboratory assessment (30). Thus, laboratory simulations of processes thought to be descriptive of an intermediate phenotype may not always be good proxies of the process in the flow of daily life. In such instances, ambulatory monitoring may provide an advantage to laboratory assessments in detecting gene-phenotype association. In the following section, we highlight examples from several domains of psychosomatic research in which ambulatory monitoring has been employed to measure putative intermediate phenotypes in the context of genetic investigations.
Ambulatory Monitoring Applications in Psychosomatic Research
Stress Reactivity
The ability of ambulatory monitoring to capture daily dynamic stress-distress sequences as phenotypes was initially demonstrated in twin designs. Jacobs et al. (31) utilized momentary assessment to develop an index of stress reactivity in a population-based sample of twin pairs. Whereas most correlational studies investigating the gene-environment interplay in stress-based disorders had focused on ‘macro-level’ stressors (i.e., major life events), Jacobs et al. reasoned that vulnerability-stress processes also occur in the context of ‘micro-level’ daily stressors, which may also be partially explained by genetic factors. Stress reactivity in this study was gauged with ecological momentary assessment (EMA; 11) and defined as momentary changes in negative affect in response to negative event-induced stress. Structural equation modeling revealed that 12% of the individual differences in stress reactivity could be explained by genetic factors. An additional study found that monozygotic twins whose co-twin had a lifetime history of major depression had greater stress reactivity than dizygotic twins, providing further evidence for the association of genetic factors with this putative intermediate phenotype for the development of depression (25).
These promising twin-based findings were complemented by work of Gunthert and colleagues (32) in their molecular genetic study exploring stress reactivity as an intermediate phenotype for anxiety disorders. These authors noted that, although numerous studies have attempted to identify specific genes that predispose people to anxiety-related outcomes, studies of the relation between the 5-HTTLPR polymorphism and anxiety-related personality traits have produced mixed results. One explanation for the mixed results is that genetic vulnerability might be revealed in reports of anxiety, but only when activated by stress. Indeed, animal research and neuroimaging studies in humans indicate that 5-HTTLPR differences in anxiety may be observed under conditions of immediate threat (33-36). Results indicated that variation in 5-HTTLPR acts as a genetic diathesis for the experience of anxious mood in response to daily stressors. On days when college students experienced more intense stressors, short allele carriers reported elevated feelings of anxiety, compared to long allele carriers. This gene gene × stress interaction was not replicated when measures of trait anxiety and neuroticism were substituted for the marker of daily stress reactivity.
A more recent investigation by Simons et al. (37) utilized ambulatory monitoring of self-report to capture genetic variation in stress-paranoia sequences, which could have implications for the development of psychotic disorders. In light of research showing that paranoid responses to daily stressors may be moderated by brain-based differences in mesolimbic dopaminergic and nerve growth factor signaling, ambulatory monitoring provided an opportunity to assess individual differences in daily reactivity as a function of variation in candidate genes known to affect these pathways. Indeed, the authors found that carriers of vulnerability alleles in the catechol-O-methyltransferase (COMT) and brain-derived neurotrophic factor (BDNF) genes evidenced greater paranoid responses to daily negative events and social stressors, respectively, than those without the vulnerability alleles.
These findings complement emerging research regarding the role of environmental circumstances in the expression of genetic differences (10). By virtue of their ability to capture affective and cognitive states in close temporal proximity to daily stressors, they also highlight the utility of ambulatory phenotypic markers for answering research questions dependent on the characterization of time- and situation-variant processes.
Cognitive and Affective Modulation of Pain
Similar to the role of dynamic stress and mood sequences in differentiating people with and without psychopathology, cognitive and affective reactivity styles have been shown to contribute to individual differences in chronic pain outcomes (38). Therefore, ambulatory monitoring may also be an important phenotypic measurement tool to assess the cognitive and affective antecedents and sequelae of pain experiences among. Our group has recently published two studies that, to our knowledge, are the first to employ ambulatory monitoring in assessing genetic associations with chronic pain (39, 40). The first study (39) found that variation in the COMT gene was associated with daily positive affective reactivity to pain among fibromyalgia (FM) patients. Here, ambulatory monitoring of self-report with electronic daily diaries helped specify an intermediate phenotype that was both relevant to the function of neurotransmitter pathways affected by variation in COMT, and was also a highly specific process shown to be dysregulated in FM patients: positive affective reactivity to daily pain (41, 42). The relation of COMT genotype to positive affect was not direct, but rather was contingent on daily pain experience.
In a separate report, Finan and colleagues (40) extended earlier cross-sectional findings by George et al. (43) which showed that variation in COMT interacted with pain catastrophizing to predict pain-related outcomes post-shoulder surgery. Our study extended those findings to FM patients by using measurements of pain and pain catastrophizing obtained through an electronic daily diary. We hypothesized that pain and the extent to which people catastrophized about its meaning would vary both between people with FM and within individuals from day to day. Results indicated that COMT genotype did not differentiate pain reports on average, but FM patients homozygous for the met allele evidenced a greater spike in pain on low catastrophizing days relative to high catastrophizing days than those with other COMT genotypes. In both studies, ambulatory monitoring was critical in showing that COMT is related to FM through daily contingent subjective experiences, rather than through direct relations with common symptoms.
Situational Determinants of Alcohol Abuse
Alcohol use disorders are another intriguing class of phenotypes for which research has attempted to identify a genetic basis (44, 45). Alcohol abuse is known to be influenced by a variety of situational factors that change daily and may be influenced by genetic factors, including stress exposure, chemical exposure, social context, motivation, and incentive salience (46). Therefore, there is a need for studies to measure intermediate phenotypes that account for the substantial array of environmental and psychological predictors of alcohol abuse.
Several candidate gene studies have utilized ambulatory monitoring to strengthen the reliability of phenotypic measurement of self-reported alcohol use via prospective assessment (47-51). Two studies, however, stand out for their use of ambulatory monitoring to more thoroughly assess the phenotypic architecture of alcohol use disorders by combining the assessment of consumption quantity and frequency with time-variant behavioral pharmacological variables. Tidey et al. (51) employed EMA to determine if individual differences in the dopamine receptor d4 (DRD4) and OPRM1 genes moderated the blunting effect of the opioid antagonist naltrexone on craving for alcohol in a natural environment for heavy drinkers. Ambulatory monitoring was particularly pertinent in this study because prior results in laboratory studies of genetic moderation of the naltrexone-craving effect had been mixed (52, 53). The findings indicated that DRD4 genotype moderated naltrexone's effect on percent of heavy drinking days but not on self-reported craving, whereas OPRM1 genotype did not moderate heavy drinking frequency or craving. These findings provided concurrent validity for those observed in a lab-based study (52), and suggest that the ecological validity of earlier lab-based results may need to be revisited.
In a separate report, the same polymorphisms on DRD4 and OPRM1 were proposed to moderate the subjective effects of alcohol among heavy drinkers (50). EMA was used in this case to measure the momentary contingencies of alcohol consumption on mood and urge to drink. Across measurement occasions, consumption of alcohol resulted in higher levels of vigor and lower levels of negative mood among carriers of the asp allele on OPRM1 relative to those without the asp allele, and a higher urge to drink among long allele carriers relative to carriers of the short allele on DRD4. However, momentary analyses revealed that as estimated blood alcohol content increased, these effects were attenuated. The authors suggest that these unique findings, uncovered for the first time in a natural environment, reveal that the subjective effects resulting from the initial stimulatory versus long-term depressive properties of alcohol may differ by genotype profiles in the μ-opioid system (50). Further, the fact that DRD4 long allele carriers reported a higher urge to drink overall, but lower urge to drink in the context of an increasing estimated blood alcohol content, suggests that alcohol cues among long allele carriers may affect drinking behavior to a greater degree than alcohol content (50). Such subtle ‘when-then’ contingencies begin to unravel the role of genetic factors in alcohol dependence, describing highly specific intermediate phenotypes in a naturalistic environment that both plays host to and directly influences the interaction of behavior and biology on a moment-to-moment basis.
Psychosocial Resilience
Genetic studies that have employed ambulatory monitoring have not been solely focused on phenotypes of vulnerability. Rather, researchers have begun to examine the potential role of genetic factors in resilience to stress and psychopathology. Twin-based research using EMA has shown that momentary positive affect buffers the negative affective reactivity to stress and decreases the genetic vulnerability for depression (54). In an EMA study designed to assess the experience of reward via momentary positive affective reactivity to positive event appraisals, variation in COMT, which confers greater tonic dopamine activity in the prefrontal cortex, predicted higher positive affect at increasing levels of positive event appraisal (55). Importantly, a main effect of COMT on positive affect was not observed. In a separate study, Wichers and colleagues (56) demonstrated the role of BDNF genotype on the positive affective buffering of momentary negative affective reactivity to social stress. Again with these studies, ambulatory monitoring was essential to the elucidation of subtle dynamic sequences.
Psychophsyiological Measurement
Just as we have trumpeted the utility of ambulatory monitoring for complex psychological phenotypes measured through self-report, it is important to acknowledge the value of ambulatory monitoring to molecular genetic inquiries involving psychophysiological variables. In fact, the majority of published ambulatory monitoring studies in general involve psychophysiological, as opposed to psychological, measurements (57). A variety of psychophysiological parameters, including blood pressure, heart rate, cortisol, blood glucose, and physical activity, may be considered intermediate phenotypes for a variety of psychosomatic disorders. Accurate and reliable ambulatory psychophysiological devices provide an advantage in ecological validity relative to laboratory measurements of psychophysiological processes and, according to some theorists, increase the heritability of intermediate phenotypes. This has been explicitly demonstrated in twin studies of heart rate variability (58, 59). Further, ambulatory monitoring has long been a routine method for assessing blood pressure over daytime and nighttime intervals, and so it is no surprise that some genetic studies have employed this strategy for phenotypic measurement (e.g., 60).
Disorders for which psychophysiological ambulatory monitoring is especially pertinent, such as hypertension, have accrued mixed findings among molecular genetic studies. The relative paucity of thorough phenotyping has been cited as a principle source of culpability for those inconsistencies (61). For example, heart rate variability has been proposed as an intermediate phenotype for a variety of psychopathological disorders (62), and a twin study has established ambulatory heart rate variability as a highly heritable phenotype, with heritability estimates ranging from 35-48% (59). However, recent twin-based work has indicated that heart rate variability in response to a stressor is no more heritable than resting heart rate variability and may be predicted by approximately 60-81% of the same genetic factors as resting heart rate variability (63). This finding, consequently, raises the question of whether heart rate variability measured in the flow of daily life will a better choice than resting heart rate variability in molecular genetic studies. This example highlights the broader need for more work to focus on ‘deep phenotyping,’ with synergistic models of candidate genetic information, ambulatory psychophysiological variables, time-variant environmental exposures, and subjectively reported psychological and somatic experiences.
Summary and Conclusions
In this article, we have presented a rationale for the inclusion of ambulatory monitoring techniques in genetic applications to psychosomatic research. Molecular genetic studies have suffered from low reliability, low power, and poor phenotypic specification and measurement. Ambulatory monitoring, particularly for intermediate phenotypes requiring self-report, is uniquely poised to address each of these pitfalls, and its utility has been displayed in research on the genetics of stress reactivity, chronic pain, alcohol abuse, and affective resilience.
The importance of replication in genetic studies cannot be overstated. Replication of findings discovered through ambulatory monitoring studies may be a more burdensome task than replication of a case-control association study in which diagnosis may be the only phenotypic measure. For studies measuring intermediate phenotypes, measurement choice will be critical. For example, use of the two most widely used depression measures, the Hamilton Rating Scale for Depression (64) and the Beck Depression Inventory (65) varies significantly between basic science and treatment outcome disciplines. A genetic association with such a complex phenotype as depression may vary significantly between scales purported to measure the same construct. As an example, a recent review of the efforts made to replicate the classic Caspi et al. (17) 5HTTLPR × life stress interaction highlights that affirmative replications have tended to use objective indicators or in-depth interviews to assess stress exposure, whereas nonreplications have tended to use brief self-report measures (18). As more rich and varied forms of phenotypic measurement are introduced, careful thought must be given to what a valid replication means in the context of genetic association studies with intermediate phenotypes in psychosomatic research.
Clinicians and clinical researchers may be interested in how molecular genetic findings, particularly in gene × environment research, pertain to clinical outcomes and the propensity for change through behavioral treatment. For example, if risk genotypes are treated as diatheses for illness, should they be considered to be immutable, and their carriers impervious to change through clinical intervention? Alternatively, can individuals be selected by their genetic profiles for a personalized medical regimen? Although ambulatory monitoring may improve the flexibility of phenotypic specification and reliability of measurement, the expectation is still that effect sizes will be relatively small, and that multiple genetic variants, or perhaps multiple systems of genes and their products will contribute to variance in an illness. Therefore, clinicians should proceed with caution in interpreting an individual's likelihood for behavioral treatment success based on genotype. Valid genetic associations with intermediate phenotypes measured through ambulatory monitoring may enhance our understanding of the mechanisms contributing to the development and maintenance of psychosomatic disorders. The extent to which these associations inform personalized medical and behavioral treatment decisions will depend on the consistency of the findings across studies, and the effect sizes generated through meta-analysis. Future clinical research should investigate whether genetic factors explain individual differences in the modification of daily when-then contingencies (i.e., coping with daily stressors) by psychological interventions, such as cognitive-behavioral therapy.
Despite the theoretical and statistical considerations we have offered in this article, an empirical study has not yet been conducted to directly test whether gene-phenotype associations, in particular, are more reliably captured through repeated versus single-occasion measurements. Although empirical data does support the increase in reliability afforded by ambulatory monitoring in general psychological designs (66), a direct test of this hypothesis with genetic data is needed.
The application of genetic association designs to psychosomatic research is still in its infancy. While genetic methods in psychiatry have rapidly evolved to allow for massive genome-wide investigations with large-scale, multi-site databases, methods for assessing the phenotypic processes that mediate and moderate gene-disease associations have lagged behind. Ambulatory monitoring, by permitting the investigation of genetic associations with daily processes measured in close to real-time, is a promising strategy for assessing intermediate phenotypes.
Acknowledgments
The authors would like to acknowledge the work of Joseph Schacht, Ph.D., and Kent Hutchison, Ph.D., who provided expert insight on earlier versions of this manuscript. Dr. Tennen would like to acknowledge funding support during the development of this manuscript from grants 5P60-AA003510 and 1R21- AA017584 from the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
Footnotes
To clarify, a phenotype is the observed expression of the function of a gene, and can be as broadly conceptualized as the presence of disease, or as narrowly conceptualized as a symptom or state that is characteristic of a disease. Symptoms or states that are considered intermediate between the function of a gene and the development of a disease are considered ‘intermediate phenotypes.’ For example, if alcoholism is the target disease phenotype, researchers may be interested in measuring the effect of a target genetic variant on alcohol sensitivity as an intermediate phenotype.
References
- 1.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–62. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 2.Luft FC. Twins in cardiovascular research. Hypertension. 2001;37:350–6. doi: 10.1161/01.hyp.37.2.350. [DOI] [PubMed] [Google Scholar]
- 3.Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–96. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 4.Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Sullivan PF, Evengard B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis & Rheumatism. 2006;54:1682–6. doi: 10.1002/art.21798. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 7.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis J. Non-replication and inconsistency in the genome-wide association setting. Human Heredity. 2007;64:203–13. doi: 10.1159/000103512. [DOI] [PubMed] [Google Scholar]
- 9.Ioannidis J, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29:306–9. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 10.Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 11.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 12.Tversky A, Kahneman D. Availability: a heuristic for judging frequency and probability. Cognitive Psychology. 1973;5:207–32. [Google Scholar]
- 13.Stone AA, Broderick JE, Kaell AT, DelesPaul PA, Porter LE. Does peak-end phenomenon observed in laboratory pain studies apply to real-world pain in rheumatoid arthritics? Journal of Pain. 2000;1:212–7. doi: 10.1054/jpai.2000.7568. [DOI] [PubMed] [Google Scholar]
- 14.Affleck G, Zautra A, Tennen H, Armeli S. Multilevel daily process designs for consulting and clinical psychology: A preface for the perplexed. Journal of Consulting and Clinical Psychology. 1999;67:746–54. doi: 10.1037//0022-006x.67.5.746. [DOI] [PubMed] [Google Scholar]
- 15.Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–9. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, McClay J, Mill J, Martin J, Braithwaite A. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 18.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010:1–19. doi: 10.1176/appi.ajp.2010.09101452. AiA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannidis JP, Trikalinos TA, Khoury MJ. Implications of small effect sizes of individual genetic variants on the design and interpretation of genetic association studies of complex diseases. American Journal of Epidemiology. 2006;165:609–14. doi: 10.1093/aje/kwj259. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS. “A gene for…”: The nature of gene action in psychiatric disorders. American Journal of Psychiatry. 2005;162:1243–52. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1977. [Google Scholar]
- 22.Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: Neuroal correlates of breaking the link between craving and smoking. Psychological Science. 2011;22:498–506. doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract Res Clin Rheumatol. 2003;17:685–701. doi: 10.1016/s1521-6942(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 24.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–51. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wichers M, Myin-Germeys I, Jacobs N, Peeters F, Kenis G, Derom C, Vlietinck R, DelesPaul PA, Van Os J. Genetic risk of depression and stress-induced negative affect in daily life. British Journal of Psychiatry. 2007;191:218–23. doi: 10.1192/bjp.bp.106.032201. [DOI] [PubMed] [Google Scholar]
- 26.Moskowitz DS, Young SN. Ecological momentary assessment: What it is and why it is a method of the future in clinical psychopharmacology. Journal of Psychiatry and Neuroscience. 2006;31:13–20. [PMC free article] [PubMed] [Google Scholar]
- 27.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 28.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosomatic Medicine. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 29.Johnston DW, Tuomisto M, Patching GR. The relationship between cardiac reactivity in the laboratory and in real life. Heatlh Psychology. 2008;27:34–42. doi: 10.1037/0278-6133.27.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. American Journal of Hypertension. 2008;4:443–50. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs N, Rijsdijk F, Derom C, Vlietinck R, DelesPaul PA, Van Os J, Myin-Germeys I. Genes making one feel blue in the flow of daily life: A momentary assessment study of gene-stress interaction. Psychosomatic Medicine. 2006;68:201–6. doi: 10.1097/01.psy.0000204919.15727.43. [DOI] [PubMed] [Google Scholar]
- 32.Abeles M, Pillinger MH, Solitar BM, Abeles M. Narrative review: The pathophysiology of fibromyalgia. Ann Intern Med. 2007;146:726–34. doi: 10.7326/0003-4819-146-10-200705150-00006. [DOI] [PubMed] [Google Scholar]
- 33.Hariri A. The neurobiology of individual differences in complex behavioral traits. Annual Review of Neuroscience. 2009;32:225–47. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hariri AR, Drabant EM, Munoz KE, Kolachana B, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 35.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 36.Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biological Psychiatry. 2003;54:953–9. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Simons C, Wichers M, Derom C, Thiery E, Myin-Germeys I, Krabbendam L, Van Os J. Subtle gene-environment interactions driving paranoia in daily life. Genes, Brain, & Behavior. 2009;8:5–12. doi: 10.1111/j.1601-183X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 38.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 39.Finan PH, Zautra A, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. Genetic influences on the dynamics of pain and affect in fibromyalgia. Health Psychology. 2010;29:134–42. doi: 10.1037/a0018647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain. 2011;152:300–7. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosomatic Medicine. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 42.Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, Davis MC. Fibromyalgia: Evidence for deficits in positive affect regulation. Psychosomatic Medicine. 2005;67:147–55. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, Iii, Sack BK, Herbstman DM, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: Pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- 45.Tyndale RF. Genetics of alcohol and tobacco use in humans. Annals of Medicine. 2003;35:94–121. doi: 10.1080/07853890310010014. [DOI] [PubMed] [Google Scholar]
- 46.Sher KJ, Dick DM, Crabbe JC, Hutchison KE, O'Malley SS, Heath AC. Consilient research approaches in studying gene X environment interactions in alcohol research. Addiction Biology. 2010;15:200–16. doi: 10.1111/j.1369-1600.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AHN, Kranzler HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biological Psychiatry. 2007;61:609–16. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Gacek P, Conner TS, Tennen H, Kranzler HR, Covault J. Tryptophan hydroxylase 2 gene and alcohol use among college students. Addiction Biology. 2008;13:440–8. doi: 10.1111/j.1369-1600.2008.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, Pettinati H, Oncken C. Double-blind, randomized trial of sertraline for alcohol dependence: Moderation by age of onset and 5-HTTLPR genotype. The Journal of Clinical Psychopharmacology. 2011;31:22–30. doi: 10.1097/JCP.0b013e31820465fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray LA, Miranada R, Tidey JW, Mcgeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the mu-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. Journal of Abnormal Psychology. 2010;119:115–25. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranada R, Mcgeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone's effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcoholism: Clinical and Experimental Research. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGeary JE, Monti PM, Rohsenow DJ, Tidey JW, Swift RM, Miranada R. Genetic moderators of naltrexone's effects on alcohol cue reactivity. Alcoholism: Clinical and Experimental Research. 2006;30:1288–96. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 53.Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 54.Wichers M, Myin-Germeys I, Jacobs N, Peeters F, Kenis G, Derom C, Vlietinck R, DelesPaul PA, Van Os J. Evidence that moment-to-moment variation in positive emotions buffer genetic risk for depression: A momentary assessment twin study. Acta Psychiatrica Scandinavica. 2007;115:451–7. doi: 10.1111/j.1600-0447.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 55.Wichers M, Aguilera M, Kenis G, Krabbendam L, Myin-Germeys I, Jacobs N, Peeters F, Derom C, Vlietinck R, Mengelers R, DelesPaul PA, Van Os J. The catechol-O-methyltransferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology. 2008;33:3030–6. doi: 10.1038/sj.npp.1301520. [DOI] [PubMed] [Google Scholar]
- 56.Wichers M, Kenis G, Jacobs N, Myin-Germeys I, Schruers K, Mengelers R, DelesPaul PA, Derom C, Vlietinck R, Van Os J. The psychology of psychiatric genetics: Evidence that positive emotions in females moderate genetic sensitivity to social stress associated with BDNF Val-sup-6-sup-6Met polymorphism. Journal of Abnormal Psychology. 2008;117:699–704. doi: 10.1037/a0012909. [DOI] [PubMed] [Google Scholar]
- 57.Fahrenberg J, Myrtek M, Pawlik K, Perrez M. Ambulatory assessment--monitoring behavior in daily life settings. European Journal of Psychological Assessment. 2007;23:206–13. [Google Scholar]
- 58.Kupper NH, Willemsen G, Posthuma D, de Boer D, Boomsma DI, de Geus EJ. A genetic analysis of ambulatory cardiorespiratory coupling. Psychophysiology. 2005;42:202–12. doi: 10.1111/j.1469-8986.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 59.Kupper NH, Willemsen G, van den Berg M, de Boer D, Posthuma D, Boomsma DI, de Geus EJ. Heritability of ambulatory heart rate variability. Circulation. 2004;110:2792–6. doi: 10.1161/01.CIR.0000146334.96820.6E. [DOI] [PubMed] [Google Scholar]
- 60.Tobin MD, Raleigh SM, Newhouse S, Braund P, Bodycote C, Ogleby J, Cross D, Gracey J, Hayes S, Smith T, Ridge C, Caulfield M, Sheehan N, Munroe PB, Burton PR, Samani NJ. Association of WNK1 gene polymorphisms and haplotypes with ambulatory blood pressure in the general population. Circulation. 2005;112:3423–9. doi: 10.1161/CIRCULATIONAHA.105.555474. [DOI] [PubMed] [Google Scholar]
- 61.Charchar FJ, Zimmerli LU, Tomaszewski M. The pressure of finding human hypertension genes: New tools, old dilemmas. Journal of Human Hypertension. 2008;22:821–8. doi: 10.1038/jhh.2008.67. [DOI] [PubMed] [Google Scholar]
- 62.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Ding X, Su S, Li Z, Riese H, Thayer JF, Treiber F, Snieder H. Genetic influences on heart rate variability at rest and during stress. Psychophysiology. 2009;46:458–65. doi: 10.1111/j.1469-8986.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beck AT, Steer RA, Brown GK. Beck depression inventory. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 66.Affleck G, Tennen H, Keefe FJ, Lefebvre JC, Kashikar-Zuck S, Wright K, Starr K, Caldwell DS. Everyday life with osteoarthritis or rheumatoid arthritis: independent effects of disease and gender on daily pain, mood, and coping. Pain. 1999;83:601–9. doi: 10.1016/S0304-3959(99)00167-0. [DOI] [PubMed] [Google Scholar]