Abstract
Introduction
Intradermal bacille Calmette-Guérin (BCG) vaccination by needle-free, disposable-syringe jet injectors (DSJI) is an alternative to the Mantoux method using needle and syringe (NS). We compared the safety and immunogenicity of BCG administration via the DSJI and NS techniques in adults and newborn infants at the South African Tuberculosis Vaccine Initiative (SATVI) research site in South Africa.
Method
Thirty adults and 66 newborn infants were randomized 1:1 to receive intradermal BCG vaccine (0.1 mL in adults; 0.05 mL in infants) via DSJI or NS. Wheal diameter (mm) and skin fluid deposition at the site of injection (SOI) were measured immediately post-vaccination. Adverse events and SOI reactogenicity data were collected 30 min and 1, 2, 4, and 12 weeks after vaccination for adults and at 30 min and 4, 10, and 14 weeks for infants. Blood was collected in infants at 10 and 14 weeks to assess BCG-specific T-cell immune responses.
Results
More infant BCG vaccinations by DSJI deposited >5 μL fluid on the skin surface, compared to NS (49% versus 9%, p = 0.001). However, all 12 infant vaccinations that did not produce any SOI wheal occurred in the NS group (36%, p < 0.001). Median wheal diameter, in participants for which an SOI wheal formed, did not differ significantly between groups in infants (combined 3.0 mm IQR 2.0 to 4.0, p = 0.59) or in adults (combined 9.0 mm IQR 7.0 to 10.0, p = 0.13). Adverse events were similar between study arms. Proportion of participants with BCG scars after three months did not differ in adults (combined 97%, p = 0.67) or infants (combined 62%, p = 0.13). Frequencies of BCG-specific clusters of differentiation 4 (CD4) and clusters of differentiation 8 (CD8) T-cells co-expressing IFN-γ, TNF-α, IL-2, and/or IL-17 were not different in the DSJI and NS groups.
Conclusion
BCG vaccination of newborn infants via DSJI was more likely to deliver an appropriate intradermal wheal at the SOI as compared to NS, despite leaving more fluid on the surface of the skin. Safety, reactogenicity, and antigen-specific T-cell immune responses did not differ between DSJI and NS techniques.
Keywords: BCG, Jet injector, Intradermal vaccine, Mantoux, Vaccine administration
Abbreviations: AE, adverse event; BCG, bacille Calmette-Guérin; CD4/CD8, cluster of differentiation 4/8; CFU, colony-forming units; DSJI, disposable-syringe jet injector; ICS, intracellular cytokine staining; IQR, interquartile range; NS, needle and syringe; SATVI, South African Tuberculosis Vaccine Initiative; SOI, site of injection; TB, tuberculosis; Th1, T helper type 1 cells
1. Introduction
Disposable-syringe jet injectors (DSJI) are needle-free administration devices for parenteral vaccines. DSJIs are designed to be rugged and easy for health care workers to learn to use; they may contribute to more consistent vaccine administration in the field as compared to conventional administration by needle and syringe (NS) [1,2]. These factors may be particularly useful for intradermal vaccines, such as bacille Calmette-Guérin (BCG), for which the intradermal administration by the Mantoux delivery method with NS (termed Mantoux) is technically challenging to successfully perform [3].
BCG, the only licensed vaccine against tuberculosis (TB), is the most widely administered intradermal vaccine in the world. It is given shortly after birth in many TB-endemic countries. Global BCG coverage is high (80% to 90%) and is part of the routine immunization program in more than 169 countries [4,5]. BCG is known to induce a robust T helper type 1 (Th1) immune response against a number of TB antigens present in Mycobacterium tuberculosis [6]. TB remains a huge global health problem. In 2012, more than 8.6 million people developed the disease and 1.3 million died of it [7]. BCG and future novel TB vaccines represent an important strategy in combating this major disease [8].
BCG vaccine administration must be optimal to achieve maximum effectiveness. The conventional technique for BCG administration is intradermal vaccination with NS by the Mantoux technique. This technique requires exact placement and the correct angle of insertion and orientation of the needle point into the intradermal layer of the skin; otherwise, the deposition of vaccine fluid is either too deep (subcutaneous) or too superficial (potentially leaking fluid on the skin surface). The Mantoux technique is technically complex and requires training, experience, and optimal physical conditions to perform correctly [3].
Intradermal vaccine administration via DSJI may be simpler and more consistent than Mantoux NS techniques. DSJI devices employ needle-free technology to deliver injectable fluid into the intradermal, subcutaneous, or intramuscular layers of tissue under high pressure through a small nozzle. Although the DSJI hand piece is designed to be reusable and robust for use in the field, the component that comes into contact with the patient (designated the needle-free syringe or cartridge) is single-use and disposable. The advantages of this technology include a simplified administration technique that may be less dependent on user skill and requires simpler user training and experience. It eliminates the risk of needle reuse or needlestick injuries and potentially offers a less variable deposition of vaccine fluid into the intended layer of skin [3]. Much of the work with DSJIs has focused on the potential for dose-sparing by intradermal administration of vaccines traditionally administered intramuscularly, such as influenza and inactivated poliovirus vaccines [9–11]. Although previous generations of multi-use nozzle jet injectors were widely used for BCG administration, the new generation of improved DSJI devices has not yet been evaluated for BCG vaccination [12]. Given the potential advantages of DSJI, our objective was to test whether BCG vaccination via DSJI was safe and immunogenic when compared to BCG vaccination via the conventional Mantoux NS method.
2. Methods
2.1. Study design and setting
We employed a partially blinded, randomized clinical trial design to compare BCG vaccination via the experimental DSJI to standard-of-care NS. The trial was conducted in two stages: an adult stage, to exclude a major safety signal, before proceeding to an infant stage, during which newborns were vaccinated. BCG vaccination shortly after birth is routine in this TB-endemic study population, which represents the target population for the intervention. In both stages, a random 1:1 allocation was performed into standard-dose, intradermal BCG vaccination via DSJI or via NS. The DSJI device used in this study was the Bioject ID Pen (Bioject, USA). The ID Pen is a small, compact spring-powered device that uses an autodisable, single-use disposable syringe with a spacer to limit fluid deposition to the intradermal tissue. The BCG Danish strain 1331 (Staten Serum Institut, Denmark) was administered at a standard dose of 0.1 mL or 2–8 × 105 colony forming units (CFU) in adults and 0.05 mL or 1–4 × 105 CFU in infants. The trial was conducted in the Worcester region of the Western Cape Province in South Africa, at the research site of the South African Tuberculosis Vaccine Initiative (SATVI) [13].
Approval was obtained from the Human Research Ethics Committee of the University of Cape Town, the PATH Research Ethics Committee, and the World Health Organization Ethics Review Committee. A local medical monitor and a data safety monitoring board oversaw participant safety, which was required per protocol to approve progression from the adult stage to the infant stage and the continuation of enrollment after the first 20 vaccinated infants. The trial was registered on ClinicalTrials.gov (NCT01742364) and South African National Clinical Trials Register (DOH-27-1112-4239) [14].
2.2. Screening, randomization, and vaccination
For the adult stage, 30 healthy adults from 18 to 50 years of age were enrolled after undergoing screening, which occurred after signed informed consent. Participants were excluded if they had major concomitant medical conditions; were HIV positive; or had a household TB contact, a history of TB disease, a chest X-ray suggestive of previous TB disease, or a positive test for TB infection (Quantiferon TB-Gold test; Cellestis, Australia).
For the newborn vaccination stage, 66 infants were enrolled. Informed consent was obtained from the mothers of potential infant participants during the later stages of pregnancy. Study inclusion required mothers to have a documented negative HIV test and an uncomplicated pregnancy and delivery. Caesarian sections for maternal indications were allowed. Newborns needed to have an Apgar score ≥7 at 5 min, birth weight ≥2500 g, estimated gestation ≥38 weeks, and be in good general health to be eligible for enrollment. Gestation age was determined using the best estimate combination of last menstrual period, clinical assessment, and pregnancy ultrasound where available.
The vaccinating nurse was unblinded, but did not take part in follow-up assessments. The remainder of the study team remained blinded to study group allocation until database lock. Parents were not present during infant vaccination, and were therefore blinded. However, adult participants could not be blinded. Vaccinations were performed by three research nurses who received hands-on training for the DSJI device and Mantoux NS technique, including proficiency testing. Vaccination of adults occurred at the clinical trial site. Vaccination of infants occurred at community birthing units within a maximum of 48 h after birth. In the adults and infants, random study group allocation by participant study number was pre-determined by a randomization list prepared by the blinded data manager using a random number generator. After enrollment and study number assignment, and immediately before vaccination, the vaccinator opened sequential sealed individual envelopes labeled with the participant number and marked inside with the pre-assigned group allocation. The envelopes were then immediately destroyed to prevent inadvertent unblinding.
2.3. Post-vaccination follow-up
Adults were seen 1, 2, 4, and 12 weeks after vaccination. Infants attended study visits 4, 10, and 14 weeks after vaccination, and parents were contacted by telephone 1, 7, and 14 days after vaccination. Phlebotomy for immunogenicity was performed at 10 and 14 weeks in infants only.
2.4. Clinical endpoint data collection
Three groups of clinical data were collected: injection performance data, including wheal diameter (measured in millimeters) and skin fluid deposition at the site of injection (SOI) immediately post-vaccination; safety data (adverse events [AEs], including systemic reactions); and specific characteristics of the BCG SOI lesion (ulcer and scar formation).
Wheal diameter was measured with a transparent ruler immediately after vaccination. Skin fluid deposition at the injection site was estimated in adults using an observational scale immediately after vaccination (skin damp, flow of fluid on skin, fluid spray in air, fluid runs out of injection site, “wet shot”). A vaccination was considered a “wet shot” when all the administered fluid was observed to be located on the skin surface and not properly injected at the desired intradermal depth. Skin fluid deposition at the injection site was measured in infants using an objective filter paper technique (PATH, unpublished data). The filter paper was applied to the skin immediately after vaccination. Fluid absorbed by the paper caused a demarcated patch, the diameter of which was measured. Patch diameters had been correlated pre-trial with standardized fluid volumes and reported in six categories (≤2.5 μL, ≤5 μL, ≤10 μL, ≤20 μL, ≤40 μL, >40 μL).
Adverse events were collected 30 min following vaccination and during follow-up visits through history and examination and participant diaries. The AEs were graded for severity and causality by an investigator. All AEs were recorded, including characteristic BCG site of injection reactions such as erythema, induration, ulcer, and scar formation. AEs were classified as “injection site reactions” if they occurred at the site of vaccine administration; all other AEs were classified as “systemic.”
2.5. Immunogenicity endpoint data collection
BCG-specific immunogenicity was tested in infants only, since they are the target study population and BCG immunogenicity in adults is known to be different from that in infants. Currently there are no known immune-correlates of protection against TB; therefore, utilizing a whole blood intracellular cytokine staining (ICS) assay, we analyzed cytokine co-expression patterns by BCG-specific CD4 and CD8 T-cells [15]. Briefly, 0.5 mL heparinized whole blood was incubated for 12 h with BCG (1.2 × 106 CFU/mL, Statens Serum Institut), no antigen or phytohemagglutinin (PHA) (10 μg/mL, Sigma-Aldrich, USA) in the presence of anti-CD28 and anti-CD49d (0.5 μg/mL each, BD Biosciences, USA), with the last 5 h including Brefeldin A (10 μg/mL, Sigma-Aldrich) prior to treating with BD FACS™ Lysing Solution (BD Biosciences) and cryopreservation. Cells were batch-thawed, permeabilized with BD Perm/Wash™ buffer (BD Biosciences) and stained with fluorescent antibodies as follows: CD3-BV421 (clone UCHT1), CD8-PerCPCy5.5 (SK1), CCR7-PE (150503), IFN-γ-AlexaFluor700 (B27), IL-17-AlexaFluor647 (SCPL1362), IL-2-FITC (5344.111, all from BD Biosciences), TNF-α-PECy7 (MAb11, eBiosciences, USA), CD45RA-BV570 (HI100, BioLegend, USA), and CD4-QDot605 (S3.5, Life Technologies, USA). At least 120,000 CD3+CD4+ T-cells were acquired for the no-antigen and BCG samples on a BD™ LSR II flow cytometer (BD Biosciences).
2.6. Sample size and analysis
The sample size of 30 for the adult group was selected as adequate to demonstrate safety before progressing to infants, allowing detection of severe AEs occurring in 6% of the study population, bound on the upper 95% confidence interval.
Calculation of sample size for the infant group was based on the primary immunogenicity endpoint. A sample size of 66 participants (33 per study group) would allow demonstration of an effect size of 33% for difference in frequency of CD4+ cytokine-producing cells with a power of 80% and alpha 0.05 and attrition of 10%, based on expected mean response and variation in previously published data [16].
Site of injection reactogenicity and injection performance were analyzed using Stata data analysis and statistical software (StataCorp, USA). Frequency of AEs and measurement of injection performance and BCG lesion parameters were compared between study groups using the Kruskal–Wallis test, Fisher's exact test, and the Chi-square test for trend. A p-value of ≤0.05 was considered statistically significant.
Immunogenicity data analysis was performed with FlowJo cytometry data analysis software version 9.0 (TreeStar, USA) with Supplementary Fig. 1 illustrating hierarchical gating strategy. The Boolean gate platform was used with individual cytokine gates to create all possible response pattern combinations. The data analysis programs PESTLE (version 1.7) and SPICE (Simplified Presentation of Incredibly Complex Evaluations, version 5.32) were used to subtract background responses (unstimulated control) from antigen-specific responses and to analyze flow cytometry data (both provided by Mario Roederer; Vaccine Research Center, US National Institute of Allergies and Infectious Diseases, US National Institutes of Health, 2013) [17]. Statistical analysis and graphs were performed using Prism software version 6 (GraphPad, USA). T-cell responses between the DSJI and NS groups were compared using Mann–Whitney U tests; a p-value of ≤0.05 was considered significant. To account for the multiple testing, the Bonferroni adjustment was applied where applicable. Adjusted p-values considered to be significant when comparing multiple cell subsets and/or time points are indicated in the figure legends.
3. Results
3.1. Participant allocation and baseline
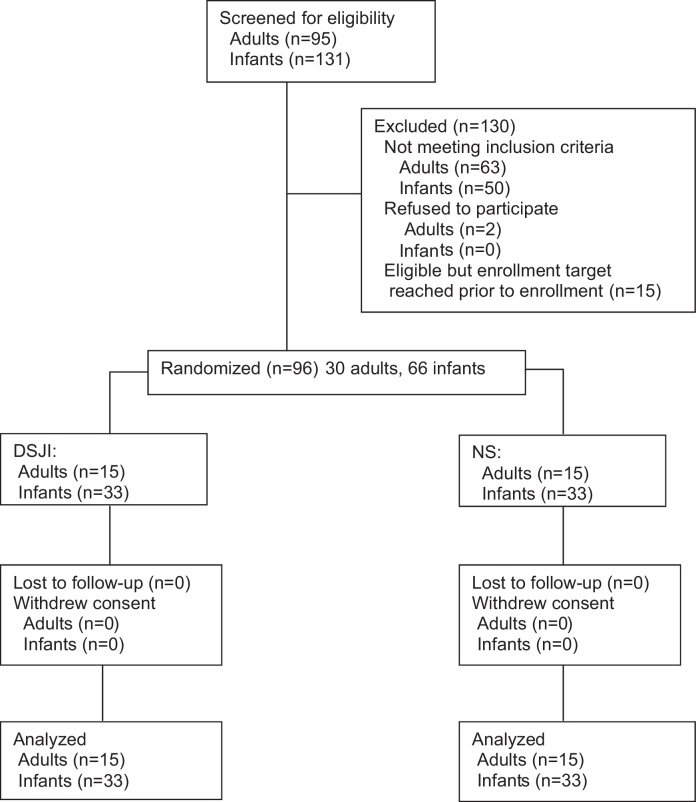
At baseline, age and gender distributions were similar by study arm allocation in adults, and gestation period, birth weight, and gender distributions were similar by study arm allocation in infants (Table 1). All infants were vaccinated within 24 h of birth. No participants were lost to follow-up (Fig. 1).
Table 1.
Baseline characteristics of adult and infant participants. There were no differences between the study groups.
| Combined | Jet injector (DSJI) | Needle and syringe (NS) | |
|---|---|---|---|
| Adults | n = 30 | n = 15 | n = 15 |
| Median age in years (IQR) | 34.5 (22.0–41.0) | 29.0 (21.0–43.0) | 35.0 (22.0–41.0) |
| Female gender, n (%) | 23 (76.7%) | 13 (86.7%) | 10 (66.7%) |
| Vaccinator | |||
| Nurse 1 | 13 (43.3%)* | 7 (53.8%) | 6 (46.2%) |
| Nurse 2 | 12 (40.0%) | 6 (50.0%) | 6 (50.0%) |
| Nurse 3 | 5 (16.7%) | 2 (40.0%) | 3 (60.0%) |
| Infants | n = 66 | n = 33 | n = 33 |
| Female gender, n (%) | 34 (51.5%) | 18 (54.5%) | 16 (48.5%) |
| Median birth weight in grams (IQR) | 3140 (2940–3420) | 3060 (2960–3510) | 3155 (2940–3360) |
| Median gestation in weeks (IQR) | 39 (38–40) | 39 (38–40) | 40 (38–40) |
| Type of delivery, n (%) | |||
| Normal delivery | 50 (75.8%) | 28 (84.8%) | 24 (72.7%) |
| Assisted | 1 (1.5%) | 0 (0.0%) | 1 (3.0%) |
| Caesarean section | 15 (22.7%) | 5 (15.2%) | 8 (24.2%) |
| Vaccinator | |||
| Nurse 1 | 31 (46.9%)* | 14 (45.2%)* | 17 (54.8%)* |
| Nurse 2 | 24 (36.4%) | 14 (58.3%) | 10 (41.7%) |
| Nurse 3 | 11 (16.7%) | 5 (45.5%) | 6 (54.5%) |
IQR: interquartile range.
Proportions of vaccinations performed by each nurse are shown as percentages of the total number of vaccinations and administration method.
Fig. 1.
Consort flow diagram summarizing participant recruitment, allocation, and retention.
3.2. Injection performance
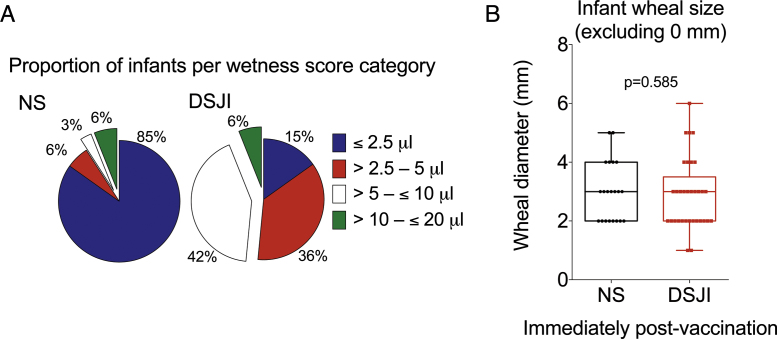
Among adults, a site of injection (SOI) wheal formed in all vaccinations, and median wheal diameter did not differ between DSJI and NS (p = 0.13). Among the 66 infants who were vaccinated, 12 (18.2%) had no visible wheal at the SOI. All 12 infants without a visible wheal were in the NS group (Table 2, p = 0.001). In the 54 infants on whom an SOI wheal was observed (i.e., wheal diameter >0 mm), the median wheal diameter did not differ between study arms (p = 0.588) (Fig. 2B). In a sensitivity analysis, including those infants without a visible wheal (i.e., wheal diameter = 0 mm) in the comparison, median wheal diameter was significantly lower in the NS group (2.0 mm; IQR 0.0 to 3.0 in NS versus 3.0 mm; IQR 2.0 to 3.0 in DSJI; p = 0.032).
Table 2.
Injection performance parameters (wheal diameter and skin fluid deposition immediately post-vaccination) by study group.
| Combined | Jet injector (DSJI) | Needle and syringe (NS) | p-Value | |
|---|---|---|---|---|
| Adults | n = 30 | n = 15 | n = 15 | |
| Wheal diameter (mm) | ||||
| No wheal | 0 | 0 | 0 | |
| Wheal > 0 mm, n (%) | 30 (100%) | 15 (100%) | 15 (100%) | |
| Median (IQR) mm* | 9.0 (7.0–10.0) | 7.0 (5.0–10.0) | 9.0 (8.0–10.0) | p = 0.130 |
| Skin fluid deposition category, n (%) | ||||
| No wetness | 7 (23.3%) | 0 | 7 (46.6%) | |
| Damp skin | 23 (76.7%) | 15 (100%) | 8 (53.3%) | p = 0.003 |
| Flow on skin | 0 | 0 | 0 | |
| Spray in air | 0 | 0 | 0 | |
| Fluid runs out inj. site | 0 | 0 | 0 | |
| Complete wet shot | 0 | 0 | 0 | |
| Infants | n = 66 | n = 33 | n = 33 | |
| Wheal Diameter (mm) | ||||
| No wheal | 12 (18.2%) | 0 | 12 (36.4%) | |
| Wheal > 0 mm, n (%) | 54 (81.8%) | 33 (100%) | 21 (63.6%) | p = 0.001 |
| Median (IQR) (mm)* | 3.0 (2.0–4.0) | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | p = 0.588 |
| Skin fluid deposition category, n (%) | ||||
| A: ≤2.5 μL | 33 (50.0%) | 5 (15.2%) | 28 (84.8%) | |
| B: >2.5–≤5 μL | 14 (21.2%) | 12 (36.3%) | 2 (6.1%) | |
| C: >5–≤10 μL | 15 (22.7%) | 14 (42.4%) | 1 (3.0%) | p = 0.001 |
| D: >10–≤20 μL | 4 (6.1%) | 2 (6.1%) | 2 (6.1%) | |
| E: >20–≤40 μL | 0 | 0 | 0 | |
| F: >40 μL | 0 | 0 | 0 | |
| >5 μL cumulative** (C + D + E + F combined) | 19 (28.8%) | 16 (48.5%) | 3 (9.1%) | p = 0.001 |
| >10 μL cumulative** (D + E + F combined) | 4 (6.1%) | 2 (6.1%) | 2 (6.1%) | |
IQR: Interquartile range. Wheal diameter (in mm) was measured by ruler. For adults, the skin fluid deposition was estimated and categorized by the vaccinator; for infants, volume was measured objectively and categorized by the filter paper technique. p-Values for fluid deposition categories calculated using Chi-square test for trend, wheal diameters using Kruskal–Wallis test, and difference in proportions using Fisher's exact test.
Includes only wheal sizes >0 mm in infants. All wheal sizes were >0 mm in adults.
5 μL = 10% of injected volume of 0.05-ml infant dose. 10 μL = 20% of injected volume.
Fig. 2.
Site of injection (SOI) skin fluid deposition and wheal diameter (in millimeters) measured immediately post-vaccination of BCG in infants. (A) The proportion of infants with measured SOI skin fluid deposition scores that fall into the categories ≤2.5 μL, >2.5–5 μL, >5–≤10 μL, >10–≤20 μL are represented in pie charts. There were no cases of fluid deposition in the 20–40 μL or >40 μL categories. (B) Wheals that were undetectable in infants are not included (zero mm: 12 needle and syringe [NS], 0 jet injector [DSJI]). Horizontal lines represent medians, boxes represent interquartile range (IQR), and whiskers the range. p-Values were calculated using the Kruskal–Wallis test, comparing the NS group versus the DSJI group.
In adults, all 15 (100%) DSJI injections resulted in dampness on the skin, compared to 8 (53.3%) in the NS group. No “wet shots,” running or flowing fluid on the skin, or fluid spray above the surface of the skin were observed in either group. Significantly more infant vaccinations by DSJI resulted in skin fluid deposition at the site of injection (p = 0.001 by Chi-square test for trend, Table 2). More infant DSJI vaccinations resulted in skin fluid deposition ≥5 μL, compared with NS (n = 16, 48.5% versus n = 3, 9.0%; p = 0.001; represented as proportions in Fig. 2A). However, the frequency of skin fluid deposition ≥10 μL was equal in the two groups (n = 2 versus n = 2, 6.1%) (Table 2).
There was no association between wheal diameter and skin fluid deposition score (r = 0.1, p = 0.610), date of vaccination (r = 0.2, p = 0.144), or vaccinating study team member (r = 0.3, p = 0.200). The 12 vaccinations in the NS group with a 0.0-mm wheal diameter were not associated with a particular time period or vaccinator and did not result in more or fewer AEs or altered immunogenicity (data not shown).
3.3. Adverse events and BCG lesions
No differences in frequencies of AEs between the DSJI and NS study arms were observed in either infants or adults. The majority of AEs were characteristic injection-site, BCG-associated lesions that did not differ in frequency between the study groups (Table 3). At 12 and 14 weeks post-vaccination, 96.7% of adults, but only 62.1% of infants, had developed a visible BCG scar at the site of injection. No difference was detected in proportion of scar formation by 14 weeks between infants in the DSJI group (n = 24, 72.7%) and NS group (n = 17, 51.5%) (p = 0.127).
Table 3.
Summary of adverse events and BCG lesion by study arm.
| Combined | Jet injector (DSJI) | Needle and syringe (NS) | p-Value | |
|---|---|---|---|---|
| Adults | n = 30 | n = 15 | n = 15 | |
| Total adverse events | 272 | 126 (46.3%) | 146 (53.7%) | p = 0.187 |
| Type of AEs, n (%) | ||||
| Injection site* | 227 (83.5%) | 108 (85.7%) | 119 (81.5%) | p = 0.414 |
| Systemic | 45 (16.5%) | 18 (14.3%) | 27 (18.5%) | |
| Moderate and severe AEs, n (% of total AEs) | 26 (9.6%) | 11 (8.7%) | 15 (10.2%) | p = 0.098 |
| Pain with injection, n (% of total AEs) | 5 (1.8%) | 2 (1.6%) | 3 (2.1%) | p = 0.564 |
| Bleeding at injection site, n (% of total AEs) | 7** (2.6%) | 3 (2.4%) | 4 (2.7%) | p = 0.454 |
| BCG lesion at 12 weeks | ||||
| Ulcer present, n (%) | 25 (83.3) | 12 (80.0%) | 13 (86.7%) | p = 0.690 |
| Scarring present, n (%) | 29 (96.7%) | 15 (100%) | 14 (93.3%) | p = 0.670 |
| Infants | n = 66 | n = 33 | n = 33 | |
| Total adverse events, n | 319 | 158 (49.5%) | 161 (50.5%) | p = 0.475 |
| Type of AEs, n (%) | ||||
| Injection site* | 279 (87.5%) | 138 (87.3%) | 141 (87.6%) | p = 0.949 |
| Systemic | 40 (12.5%) | 20 (12.7%) | 20 (12.4%) | |
| Moderate and severe AEs, n (%) | 24 (7.5%) | 11 (7.0%) | 13 (8.1%) | p = 0.325 |
| Bleeding at injection site, n (%) | 13 (4.0%)** | 7 (4.4%) | 6 (3.7%) | p = 0.665 |
| BCG lesion at 12 weeks | ||||
| Ulcer present, n (%) | 18 (27.3%) | 6 (18.2%) | 12 (36.4%) | p = 0.166 |
| Scarring present, n (%) | 41 (62.1%) | 24 (72.7%) | 17 (51.5%) | p = 0.127 |
p-Values were calculated using Fisher's exact test. % proportions are shown per column category (combined, DSJI, NS).
AEs were classified as “injection site reactions” if they occurred at the site of vaccine administration; all other AEs were classified as “systemic.” AEs were recorded 30 min after vaccination and throughout all study visits.
All cases of bleeding were mild with drops of blood only.
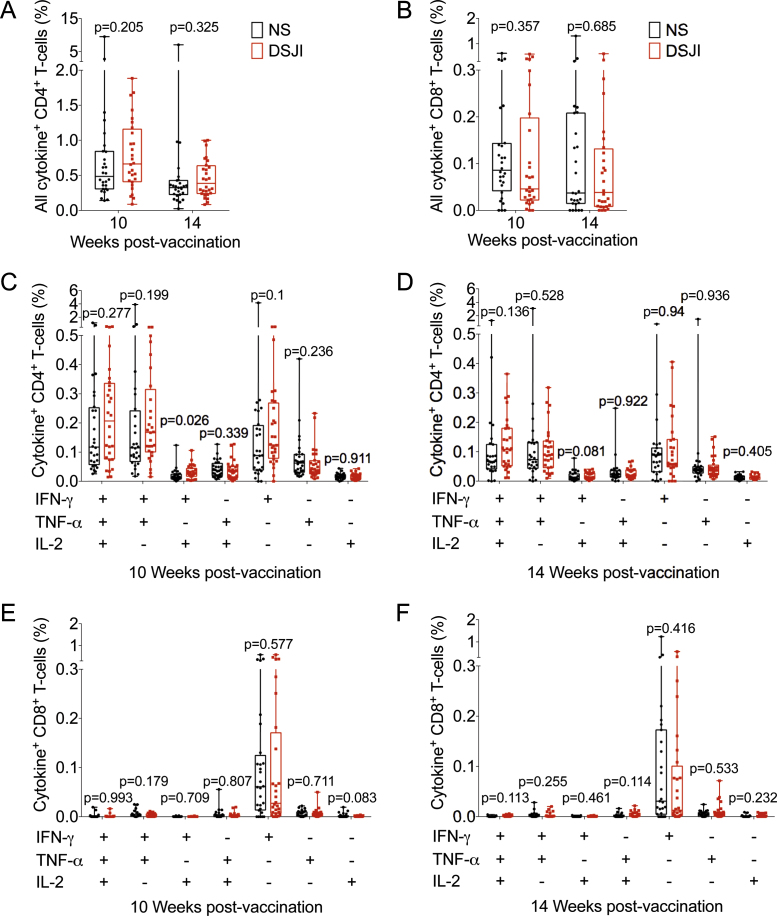
3.4. Immunogenicity
Antigen-specific CD4 and CD8 T-cells expressing IFN-γ, TNF-α, IL-2, and/or IL-17 were measured by whole blood ICS assay and multi-parameter flow cytometry (Supplementary Fig. 1). Both administration methods induced BCG-specific Th1 responses, which were readily detectable in all infants 10 and 14 weeks after vaccination (Fig. 3A). The most prominent CD4 cytokine-producing subsets expressed IFN-γ alone, or co-expressed IFN-γ, TNF-α and IL-2 or IFN-γ and TNF-α (bifunctional cells) (Fig. 3C and D). Frequencies of these Th1 subsets were not different in the DSJI and NS groups, either at 10 or at 14 weeks (Fig. 3A). IL-17-expressing BCG-specific CD4 T-cells occurred at a very low frequency and were mostly not detected in both groups (data not shown).
Fig. 3.
Frequencies of CD45RAlow BCG-specific CD4 and CD8 T-cell responses measured by whole blood ICS in infants (n = 31 jet injector [DSJI], n = 30 needle and syringe [NS]). The hierarchical gating strategy is illustrated in Supplemental Fig. 1. Shown are frequencies of all cytokine-expressing (any cell expressing IFN-γ, TNF-α, IL-2 and/or IL-17) BCG-specific CD4 (A) and CD8 (B) T-cell responses at 10 or 14 weeks after vaccination. (C–F) Frequencies of BCG-specific CD4 (C and D) and CD8 (E and F) T-cells co-expressing different combinations of IFN-γ, TNF-α, and IL-2 measured 10 weeks (C and E) or 14 weeks (D and F) after BCG vaccination; IL-17-expressing cells were extremely low or not detected. Horizontal lines represent medians, boxes represent interquartile range (IQR), and whiskers the range. Each individual vaccinee is represented by a dot. p-Values were calculated using the Mann–Whitney test, comparing the NS group versus the DSJI group per time point or per cytokine-expressing cell subset. In (C–F), following Bonferroni adjustment a p-value of less than 0.007 was considered significant.
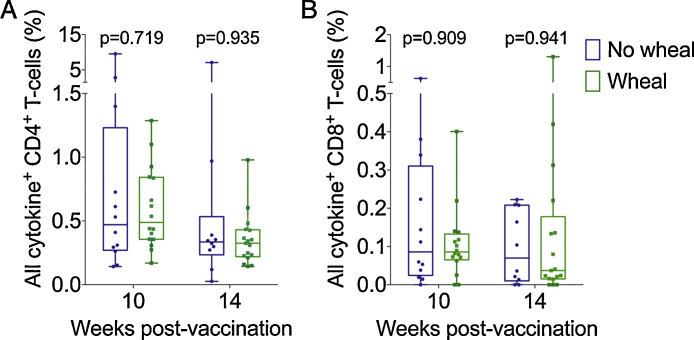
BCG-specific CD8 T-cells were largely restricted to producers of IFN-γ alone at 10 and 14 weeks after vaccination (Fig. 3E and F). As observed for CD4 T-cells, frequencies of cytokine-expressing BCG-specific CD8 T-cells were not different in the DSJI and NS groups (Fig. 3B). BCG-specific CD4 and CD8 T-cells expressing any or all cytokines were also similar in the NS group, irrespective of wheal presence or absence or stratification by wetness of >5 μL or <5 μL (Fig. 4A and B, data not shown). The pattern of CD4 cytokine-producing subsets expressing IFN-γ alone, co-expressing IFN-γ, TNF-α and IL-2 or IFN-γand TNF-α (bifunctional cells) remained the same irrespective of wheal formation or stratification by wetness of >5 μL or <5 μL (data not shown).
Fig. 4.
Frequencies of BCG-specific CD4 and CD8 T-cell responses measured by whole blood ICS in infants separated by the development of a wheal (n = 18) or no wheal (n = 12) in the needle and syringe (NS) group. Shown are frequencies of all cytokine-expressing (any cell expressing IFN-γ, TNF-α, IL-2 and/or IL-17) BCG-specific CD4 (A) and CD8 (B) T-cell responses at 10 or 14 weeks after vaccination. Horizontal lines represent medians, boxes represent interquartile range (IQR), and whiskers the range. Each individual vaccinee is represented by a dot. p Values were calculated using the Mann–Whitney test, comparing the wheal versus the no wheal subgroup per time point or per cytokine-expressing cell subset.
4. Discussion
In this partially blinded, randomized clinical trial, BCG vaccination of adults and infants, either by standard-of-care Mantoux NS technique or DSJI, resulted in safety profiles and BCG-specific T-cell immune responses that did not differ between administration methods. No differences in injection performance were detected in adults. In infants, we observed an increased rate of small-volume skin fluid deposition by DSJI. A number of infant vaccinations with NS did not form a wheal, whereas all DSJI vaccinations formed a visible wheal. To our knowledge, these are the first published data comparing DSJI and NS intradermal injection performance parameters and BCG immunogenicity.
All 12 of the BCG vaccinations that did not form a visible site of injection wheal in infants were performed using NS (36%) whereas all those with DSJI did form wheals. In the vaccinations that formed a wheal, however, the median wheal diameter was similar between administration methods. This suggests a greater variability in successful deposition of vaccine fluid intradermally with NS (observed wheal versus no observed wheal), but equal performance once the fluid is deposited in the correct layer. The difference in wheal formation between DSJI and NS may have been magnified in infants, as compared to adults, by age-specific skin characteristics. The non-formation of a wheal with NS may be associated with Mantoux technique failure. The research nurses in our study were experienced vaccinators with as much or more field experience than nurses doing routine BCG vaccinations in the clinics. Even in those optimal research conditions, it appeared that these nurses were prone to inconsistent Mantoux technique performance. However, any differences in injection performance in infants were not associated with differences in BCG reactogenicity or immunogenicity, which suggests that the BCG dosage deposited by NS was not markedly different in infants with or without wheals.
Vaccinations with the DSJI were “wetter” immediately post-vaccination, with all injections in the adults noted as being “damp” and more vaccinations in the infant DSJI group measuring more than 5 μL of fluid on the skin surface (5 μL represents 10% of the injected volume in infants). The implication of small volumes of injected fluid remaining on the skin surface is unclear. We surmise that small-volume skin fluid deposition is characteristic of the DSJI administration technique, but not necessarily indicative of sub-optimal intradermal deposition of BCG vaccine. This suggestion is supported by our findings regarding site of injection wheal diameter, reactogenicity, and immunogenicity. However, we acknowledge that wheal diameter and estimation of skin fluid deposition are proxy measures of clinical injection performance and their validity in determining a successful intradermal vaccination is uncertain. This issue was highlighted by poor correlation between post-vaccination wheal diameter and skin fluid deposition and between immunogenicity readouts and wheal diameter.
In the infants vaccinated with NS in whom no wheal formed and in whom little or no fluid leakage was observed, it is possible that intradermal technique failure resulted in vaccine fluid deposition into the subcutaneous or intramuscular skin layer. The relative “wetness” of wheal-forming DSJI vaccinations may imply some deposition of fluid across more than one layer of the skin. Subcutaneous BCG administration may be a risk factor for BCG complications such as lymphadenopathy and abscess formation [18]. Although we did not observe these complications, it is possible that an association between complications and injections that do not form a wheal may be more evident in a larger sample size. BCG leaked onto the skin during administration may carry a very small risk of environmental exposure to the community, particularly to immunodeficient individuals at greater risk of disseminated BCG.
Although BCG has been in use for decades, there is a paucity of data to inform the expected “benchmark” post-vaccination wheal diameter; the estimation of skin fluid deposition by the Mantoux NS method; or the diameter of characteristic BCG lesion parameters such as erythema, induration, ulceration, and scar formation in infants. The median wheal diameter of 3 mm, in infants receiving the standard 0.05-mL dose of BCG, may serve as a useful reference for future studies.
Although adequate for the analysis of safety and immunogenicity, our trial design was limited by a relatively wide time interval between visits that precluded a detailed analysis of the evolution of the BCG lesion over time. The proportion of BCG lesions that formed characteristic scars at 14 weeks in infants (62%) was lower than expected [18,19]. However, at the 12-week follow-up, 10 infants (2 DSJI, 8 NS) still had active lesions with erythema, induration, or ulceration, which may have developed scarring with longer follow-up. Therefore, ideally, the length of follow-up should have been longer.
As markers of BCG “vaccine take” in infants, we measured BCG-specific CD4 and CD8 T-cell responses. We chose post-vaccination time points at 10 and 14 weeks, as previous experience had identified these as the indicators of peak response and durability of response, respectively [6]. Frequencies and cytokine co-expression patterns of BCG-specific T-cell responses did not differ between the DSJI and NS groups. While these responses do not predict clinical efficacy of the vaccine, they were considered adequate for the study objectives to distinguish a potential difference in immune response between the two vaccination methods [20]. Notably, in a previous study of Japanese BCG administration, in which intradermal NS administration was compared with percutaneous administration, different levels of soluble IFN-γ and IL-2 after BCG stimulation were observed [21]. However, frequencies of IFN-γ-expressing CD4 and CD8 T-cells, measured by whole blood ICS assay, were not different. This may suggest that the short-term ICS assay may not be ideal for detecting administration route-dependent differences in BCG-induced immunity.
Our data demonstrate that BCG administration by DSJI is as safe and immunogenic as conventional Mantoux NS administration. It appears that BCG vaccination of newborn infants via DSJI is more likely to deliver an appropriate intradermal wheal, compared to NS, despite leaving more fluid on the skin surface. The significance of this finding, in terms of injection performance, needs further exploration. Follow-up research is also required to investigate the cost-effectiveness and feasibility of such devices for national vaccination programs considering multiple factors, such as reduced risk of needlestick injuries, reduced waste-management costs, and the potential for fewer staff resources due to a simplified vaccination technique. The potential application for this technology for BCG vaccination is large, given the global coverage of BCG vaccination, and further investigation is justified.
Acknowledgments
The authors would like to thank Bioject for their collaboration and provision of the devices used in this study; the World Health Organization for providing supplementary funding (grant number 2014/412467-1); the study staff from the SATVI field site for all their work; and the participants as well as parents and guardians for their contribution and patience. This work was funded by grant number OPP30451_01 from the Bill and Melinda Gates Foundation. The views expressed herein are solely those of the authors and do not necessarily reflect the views of the Foundation.
Conflict of interest statement
The SATVI research team and authors have no conflict of interest to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.03.074.
Appendix A. Supplementary data
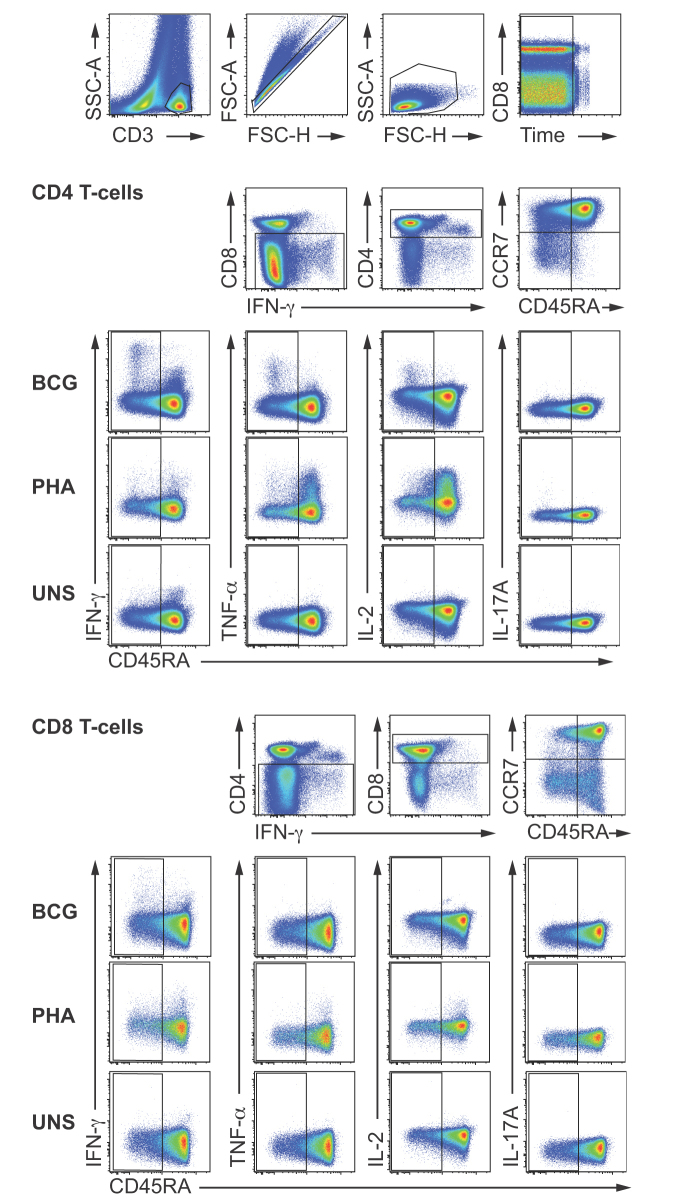
Supplementary Fig. 1 Hierarchical flow cytometry gating strategy employed for whole blood ICS analysis of BCG-induced T-cells expressing cytokines in infants. Representative pseudo-color plots depict the gating strategy in which SSC-AlowCD3+ were selected first, followed by gating on singlets, small lymphocytes, and events with consistent fluorescence levels over sample acquisition time (time gate). These CD3+ lymohocytes were then separated into either CD8−CD4+ or CD4−CD8+ T-cells. Representative cytokine expression by CD4 (upper plots) and CD8 (lower plots) are shown. Specific expression of cytokines by CD45RAhigh T-cells could not be reliably discerned in antigen-stimulated cells because of non-specific background IFN-γ signal in the unstimulated control. As a consequence, only cytokine expression by CD45RAlow memory T-cells is reported. This issue also precluded analysis of memory phenotypes of BCG-specific CD4 and CD8 T-cells. Frequencies of BCG-specific CD4 or CD8 T-cells were calculated by subtracting frequencies of CD45RAlow cells in the unstimulated control from frequencies of CD45RAlow cells in BCG stimulated samples.
The following are the supplementary data to this article:
References
- 1.Weniger B., Papania M. Alternative vaccine delivery methods. In: Plotkin S., Warenstein O., Offit P.A., editors. Vaccines. 5th ed. Saunders Elsevier; Philadelphia, PA: 2008. pp. 1357–1392. [Google Scholar]
- 2.Hickling J., Jones K., Friede M. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Org. 2011;89(3):221–226. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert P.H., Laurent P.E. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26(26):3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) World Health Organization (WHO); 2014. Website for WHO vaccine-preventable diseases: monitoring system 2014 global summary. Available at 〈http://apps.who.int/immunization_monitoring/globalsummary〉 (accessed September 4, 2014) [Google Scholar]
- 5.Zwerling A., Behr M.A., Verma A. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3):e1001012. doi: 10.1371/journal.pmed.1001012. (Epub 2011 Mar 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soares A.P., Kwong Chung C.K., Choice T. Longitudinal changes in CD4+ T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis. 2013;207(7):1084–1094. doi: 10.1093/infdis/jis941. (Epub 2013 Jan 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . WHO; Geneva: 2013. Global tuberculosis report 2013. Available at 〈http://wwwwhoint/tb/publications/global_report/en/〉. [Google Scholar]
- 8.Evans T.G., Brennan M.J., Barker L. Preventive vaccines for tuberculosis. Vaccine. 2013;31(Suppl. 2):B223–B226. doi: 10.1016/j.vaccine.2012.11.081. [DOI] [PubMed] [Google Scholar]
- 9.Zehrung D., Jarrahian C., Wales A. Intradermal delivery for vaccine dose sparing: overview of current issues. Vaccine. 2013;31(34):3392–3395. doi: 10.1016/j.vaccine.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 10.McAllister L., Anderson J., Werth K. Needle-free jet injection for administration of influenza vaccine: a randomised non-inferiority trial. Lancet. 2014;384(9944):674–681. doi: 10.1016/S0140-6736(14)60524-9. [DOI] [PubMed] [Google Scholar]
- 11.Soonawala D., Verdijk P., Wijmenga-Monsuur A.J. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine. 2013;31(36):3688–3694. doi: 10.1016/j.vaccine.2013.05.104. [DOI] [PubMed] [Google Scholar]
- 12.ten Dam H.G., Fillastre C., Conge G. The use of jet injectors in BCG vaccination. Bull World Health Org. 1970;43(5):707–720. [PMC free article] [PubMed] [Google Scholar]
- 13.South African Tuberculosis Vaccine Initiative (SATVI) 2014. SATVI website. Available at 〈http://www.satvi.uct.ac.za/〉 (accessed September 4, 2014) [Google Scholar]
- 14.ClinicalTrials . 2014. Comparative study of Bacille Calmette Guerin (BCG) delivery via disposable syringe jet injector and needle & syringe ( NCT01742364) Available at 〈https://clinicaltrials.gov/ct2/show/ NCT01742364?term= NCT01742364&rank=1〉 (ClinicalTrials.gov website. Accessed September 4, 2014) [Google Scholar]
- 15.Hanekom W.A., Hughes J., Mavinkurve M. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291(1–2):185–195. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Kagina B.M., Abel B., Bowmaker M. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine. 2009;27(40):5488–5495. doi: 10.1016/j.vaccine.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roederer M., Nozzi J.L., Nason M.C. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79(2):167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotte A., Wasz-Hockert O., Poisson N. BCG complications: estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberculosis Res. 1984;21:107–193. [PubMed] [Google Scholar]
- 19.Roth A., Sodemann M., Jensen H. Vaccination technique, PPD reaction and BCG scarring in a cohort of children born in Guinea-Bissau 2000–2002. Vaccine. 2005;23(30):3991–3998. doi: 10.1016/j.vaccine.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Kagina B.M., Abel B., Scriba T.J. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182(8):1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davids V., Hanekom W.A., Mansoor N. The effect of bacille Calmette-Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J Infect Dis. 2006;193(4):531–536. doi: 10.1086/499825. [DOI] [PubMed] [Google Scholar]