Abstract
Energy metabolism is essential for maintaining function and substance metabolism in osteoblasts. However, the role of cyclic stretch in regulating osteoblastic energy metabolism and the underlying mechanisms remain poorly understood. In this study, we found that cyclic stretch (10% elongation at 0.1 Hz) significantly enhanced glucose consumption, lactate levels (determined using a glucose/lactate assay kit), intracellular adenosine triphosphate (ATP) levels (quantified using rLuciferase/Luciferin reagent) and the mRNA expression of energy metabolism-related enzymes [mitochondrial ATP synthase, L-lactate dehydrogenase A (LDHA) and enolase 1; measured by RT-qPCR], and increased the phosphorylation levels of Akt, mammalian target of rapamycin (mTOR) and p70s6k (measured by western blot analysis) in human osteoblast-like MG-63 cells. Furthermore, the inhibition of Akt or mTOR with an antagonist (wortmannin or rapamycin) suppressed the stretch-induced increase in glucose consumption, lactate levels, intracellular ATP levels and the expression of mitochondrial ATP synthase and LDHA, indicating the significance of the Akt/mTOR/p70s6k pathway in regulating osteoblastic energy metabolism in response to mechanical stretch. Thus, we concluded that cyclic stretch regulates energy metabolism in MG-63 cells partially through the Akt/mTOR/p70s6k signaling pathway. The present findings provide novel insight into osteoblastic mechanobiology from the perspective of energy metabolism.
Keywords: mechanical stress, osteoblasts, energy metabolism, mammalian target of rapamycin, signal transduction
Introduction
Bone can continuously remodel its own mass and architecture in response to external mechanical stimulation, which has been described by Wolff's law, a bone adaptation theory that posits bones can adapt and become stronger in response to stress (1,2). Substantial evidence has demonstrated that mechanical loading promotes bone formation (3,4), whereas the loss of weight-bearing activities (e.g., prolonged bed rest or microgravity) results in bone loss in terms of both quantity and quality (5,6). The processes of mechanical loading-mediated bone modeling and remodeling involve the coordinated regulation of bone-resorbing osteoclasts and bone-forming osteoblasts (7,8). Studies have demonstrated the ability of osteoblasts to sense and transduce various forms of mechanical stimuli, such as fluid shear stress (9–11) and hydrostatic pressure (12,13). Studies have also shown that mechanical stimuli regulate the biological activities of osteoblasts, such as proliferation, differentiation and apoptosis (14–16). In all cellular biochemical reactions, energy metabolism plays an essential role in sustaining normal cellular functions (e.g., ion transport, biosynthesis and signal transduction) (17). However, the effects of cyclic stretch on energy metabolism and the underlying mechanisms in osteoblasts remain poorly understood.
The mammalian target of rapamycin (mTOR) protein is a highly conserved serine/threonine (Ser/Thr) protein kinase. mTOR is phosphorylated by activated Akt and subsequently phosphorylates ribosomal protein S6 kinase (p70s6k, 70 kDa), a downstream effector of mTOR, to regulate cell survival by balancing translation, transcription, lipogenesis and autophagy (18,19). In addition, mTOR is regarded as an essential sensor and controller of cellular energy metabolism (20–22). It has been demonstrated that sufficient nutrition, e.g., from amino acids, activates mTOR and stimulates p70s6k phosphorylation in rat hepatoma cells, which is regulated by phosphorylated (p-)Akt, whereas p70s6k phosphorylation through the activation of mTOR is halted in the absence of amino acids (20). Moreover, studies have further revealed the potential correlation between mitochondrial function and mTOR/p70s6k signaling: the blockade of mitochondrial function (23) and the depletion of adenosine triphosphate (ATP) levels (24) in hepatocytes inhibit p70s6k phosphorylation, which is dependent on the activation of mTOR. Furthermore, mTOR may be associated with the mitochondrial outer membrane (23). Although the aforementioned research has revealed the potential correlation between mTOR signals and cellular energy metabolism, it remains unknown as to whether mTOR signals are activated in osteoblasts under cyclic stretch. It also remains unclear as to whether the Akt/mTOR/p70s6k pathway plays an essential role in modulating stretch-induced energy metabolism in osteoblasts.
Therefore, in this study, the effects and potential molecular mechanisms of cyclic stretch in regulating energy metabolism were systematically investigated in human osteoblast-like MG-63 cells, which are derived from osteosarcomas and exhibit various osteoblast-like characteristics (25). First, we observed the effects of cyclic stretch on energy metabolism and the Akt/mTOR/p70s6k pathway in MG-63 cells. We then investigated the role of the Akt/mTOR/p70s6k pathway in energy metabolism in MG-63 cells which were subjected to cyclic stretch. Our findings provide a novel molecular mechanism through which cyclic stretch regulates osteoblastic energy metabolism through activation of the Akt/mTOR/p70s6k pathway.
Materials and methods
Reagents
Rapamycin (#9904), wortmannin (#9951) and rabbit monoclonal antibodies against Akt (#4691), p-Akt (Thr308; #2965), p-Akt (Ser473; #9271), mTOR (#2983), p-mTOR (Ser2448; #5536), p70s6k (#2708), p-p70s6k (Thr389; #9234) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; #2118) were obtained from Cell Signaling Technology (Danvers, MA, USA). Mouse anti-ATP5B monoclonal antibody (sc-135903) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit anti-ATP5J polyclonal antibody (ab65841) was purchased from Abcam (Cambridge, MA, USA). HRP-conjugated goat secondary antibodies (AP307P and AP308P) were obtained from Millipore (Billerica, MA, USA). Alexa Fluor® 488-conjugated secondary antibodies (A27034 and A28175) were obtained from Invitrogen (Carlsbad, CA, USA). ECL reagent was purchased from GE Healthcare (Pittsburgh, PA, USA), and the BCA Protein assay kit was obtained from Pierce Chemical Co. (Rockford, IL, USA).
Cell culture and mechanical stretch stimulation
Human osteoblast-like MG-63 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The MG-63 cells were maintained in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) (both from HyClone, Logan, UT, USA) in a water-saturated atmosphere with 5% CO2, at 37°C. For the mechanical stretch experiment, the MG-63 cells were implanted at 2×105 cells/well in 2 ml medium on 6-well BioFlex culture plates (Flexcell International, Hillsborough, NC, USA) and incubated for 24 h. The cells were then cultured in serum-free MEM for 24 h in order for them to be synchronized prior to mechanical stimulation. The medium was then changed to fresh MEM containing 10% FBS, and the cells were subjected to cyclic stretch [10% elongation at 0.1 Hz (5 sec stretch/5 sec relaxation) using the FX-4000 Tension System (Flexcell International) and harvested for biochemical assay at 1, 4, 8 or 12 h after the application of mechanical stretch. The control groups were seeded on the same plates and maintained under the same experimental conditions without applying mechanical stretch.
Pharmacological drugs and cytotoxicity assay
Rapamycin, an mTOR inhibitor (55 nM in DMSO), wortmannin (500 nM in DMSO), a phosphoinositide 3-kinase (PI3K) antagonist that blocks Akt phosphorylation, or DMSO (0.025%) as the vehicle control, were added to the MG-63 cells 30 min prior to subjection to mechanical stretch. The cells were then subjected to cyclic stretch for 8 h and harvested for the following assay. Cells in the control group were maintained under the same conditions without mechanical stimulation.
Cytotoxicity assays were performed using a Cell Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) to assess cell survival following the addition of the inhibitors. Briefly, the MG-63 cells were maintained in culture medium in the presence of 500 nM wortmannin, 55 nM rapamycin or 0.025% DMSO for 24 h. Cytotoxicity was subsequently measured by CCK-8 assay according to the manufacturer's instructions.
Immunofluorescence staining
The MG-63 cells in the BioFlex culture plates were fixed with 4% paraformaldehyde for 30 min and subsequently permeabilized with 0.1% Triton X-100 for 5 min. After blocking with 2% goat serum in phosphate-buffered saline (PBS) at 37°C for 1 h, the cells were incubated overnight at 4°C with primary antibodies to Akt (1:400), p-Akt (Thr308; 1:400), mTOR (1:50), p-mTOR (Ser2448; 1:50) and ATP5B (1:50). The cells were then incubated with Alexa Fluor® 488-conjugated goat anti-mouse (A28175) or anti-rabbit (A27034) antibodies (1:400) at 37°C for 1 h, counterstained with 4′,6-diamidino-2-phe-nylindole (DAPI) for 5 min at room temperature and then photographed using a confocal laser scanning microscope (FV1000; Olympus, Tokyo, Japan). The negative controls were incubated with non-immune mouse or rabbit immunoglobulin G (IgG; A7016 and A7028; Beyotime Institute of Biotechnology, Haimen, China) instead of the primary antibodies.
Total RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the MG-63 cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions and quantified using spectrophotometry (NanoDrop 2000c spectrophotometer; Thermo Fisher Scientific, Rochester, NY, USA). A total of 1 µg RNA was reverse transcribed into cDNA [20 µl] with oligo(dT)18 as a primer, using the First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Pittsburgh, PA, USA) according to the manufacturer's instructions. qPCR was performed on 1 µl cDNA in a reaction of 20 µl with SYBR Premix Ex Taq II (Takara Bio Inc., Shiga, Japan) using the Bio-Rad CFX96 real-time PCR detection system (Bio-Rad, Philadelphia, PA, USA). The primer sequences utilized in RT-qPCR are listed in Table I. The protocol for the RT-qPCR reactions consisted of an initial denaturation step at 95°C for 30 sec followed by 45 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 15 sec, and a final extension at 72°C for 15 sec. GAPDH was used as an internal control for normalization. The relative quantity of mRNA was calculated using the 2−ΔΔCt method.
Table I.
Primer sequences utilized in RT-qPCR.
| mRNA | GenBank accession no. | Forward primer (5→3′) | Reverse primer (5′→3′) | Product size (bp) |
|---|---|---|---|---|
| GAPDH | NM_002046 | AGAAGGCTGGGGCTCATTTG | AGGGGCCATCCACAGTCTTC | 258 |
| ATP5B | NM_001686 | CAGCAGATTTTGGCAGGTG | CTTCAATGGGTCCCACCATA | 70 |
| ATP5J | NM_001003703 | GTTCTCCTCTGTCATTCGGTCA | TCCAGATGTCTGTCGCTTAGAT | 151 |
| ATP5F1 | NM_001688 | ATTAGCGCAGAGACCTTCACT | CGCCTCTTCTAGTTGGGCAAG | 132 |
| F1-ATPase α | X59066.1 | TGCTGCCACTCAACAACT | TGATAGTGCCCAACAAGG | 218 |
| Enolase 1 | NM_001428 | GCCGTGAACGAGAAGTCCTG | ACGCCTGAAGAGACTCGGT | 79 |
| LDHA | NM_005566.3 | ATGGCAACTCTAAAGGATCAGC | CCAACCCCAACAACTGTAATCT | 86 |
LDHA, L-lactate dehydrogenase A; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Western blot analysis
The cells were washed with ice-cold PBS and lysed to release the whole proteins using RIPA buffer with 1 mM phenylmethanesulfonylfluoride (PMSF). The cell lysates were transferred into a pre-cooled microfuge tube and constant agitation was maintained for 30 min at 4°C. The protein extracts were then centrifuged at 4°C for 20 min at 13,500 × g. The protein content of the supernatant was collected, and the protein concentration was determined by BCA assay. The protein extracts (30 µg/sample) were subjected to electro-phoretic separation by 8 and 10% Tris-glycine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Millipore) after being mixed with 5X loading buffer and boiled for 8 min. The PVDF membranes were blocked in TBST (Tris-buffered saline, 0.5% Tween-20) containing 5% BSA for 2 h and incubated overnight at 4°C with primary antibodies to Akt (1:1,000), p-Akt (Thr308; 1:1,000), p-Akt (Ser473; 1:1,000), mTOR (1:1,000), p-mTOR (Ser2448; 1:1,000), p70s6k (1:1,000), p-p70s6k (Thr389; 1:1,000), GAPDH (1:1,000), ATP5B (1:500) and ATP5J (1:500) in TBST containing 5% BSA. The membranes were then incubated with HRP-conjugated goat anti-rabbit or anti-mouse secondary antibody (1:5,000 dilution) for 1 h at room temperature, and then visualized using an ECL system (GE ImageQuant 350; GE Healthcare). Semi-quantitative analysis was performed using Quantity One software (Bio-Rad). GAPDH was used as an internal control for normalization.
Measurement of glucose consumption and lactate levels
Glucose and lactate levels in the cell culture medium were determined using a glucose/lactate assay kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. Glucose consumption was calculated as the difference in glucose concentration as compared with the control (0 h). Glucose consumption and lactate levels were normalized against the cell numbers.
Measurement of ATP concentration
The ATP concentration was quantified using rLuciferase/Luciferin reagent (Promega Corp., Madison, WI, USA) in a luminometer according to the manufacturer's instructions. Briefly, 100 µl protein lysate (1:100) were added to 100 µl rL/L reagent for 3 sec before the intensity of the emitted light was measured for 10 sec using the GloMax 20/20 luminometer (Promega Corp.). The relative light units (RLU) of the samples were corrected by subtracting the RLU of the blank samples containing the lysate buffer instead of the protein sample. The ATP concentration was determined by plotting an ATP standard curve and was normalized to protein concentration.
Statistical analysis
All data presented are expressed as the means ± standard deviation (SD). Statistical analyses were performed using Microsoft SPSS version 13.0 for Windows (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) with Tukey's post hoc analysis were used to determine the differences between 2 groups. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
Cyclic stretch promotes energy metabolism in osteoblast-like MG-63 cells
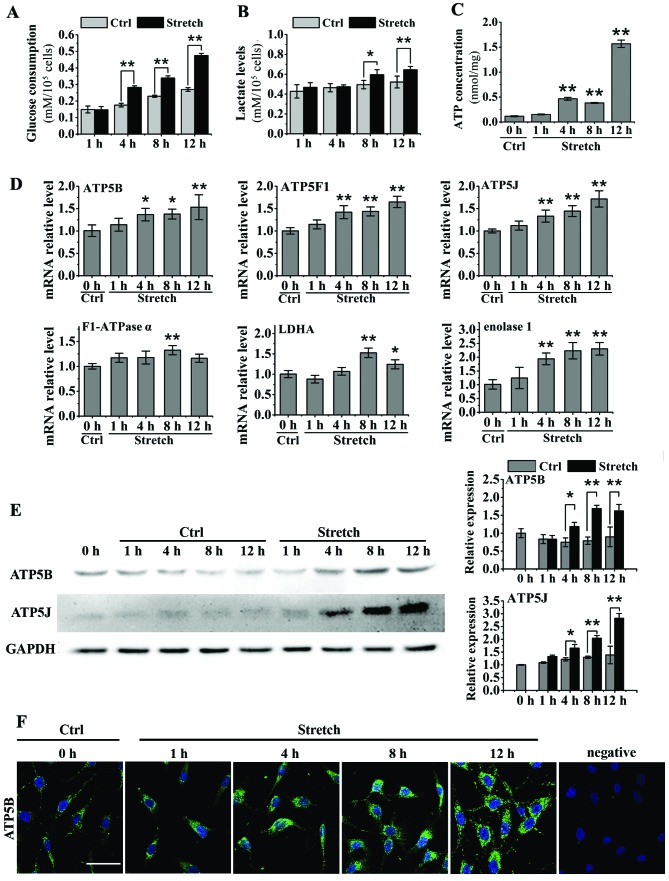
Cyclic stretch for 4, 8 and 12 h significantly promoted glucose consumption in MG-63 cells (P<0.05; Fig. 1A). The lactate levels were also significantly upregulated after the MG-63 cells were subjected to cyclic stretch for 8 and 12 h (P<0.05; Fig. 1B). Cyclic stretch for 4, 8 and 12 h significantly upregulated the ATP concentration in the MG-63 cells (P<0.05), and this was particularly prominent at 12 h ([almost a 12-fold increase vs. the control group (0 h); Fig. 1C]. Cyclic stretch induced an increase in the mRNA expression of ATP5B, ATP5F1, ATP5J, F1-ATPase α, enolase 1 and L-lactate dehydrogenase A (LDHA) in the MG-63 cells (Fig. 1D). Furthermore, the results from western blot analyses revealed that 4, 8 and 12 h of cyclic stretch significantly promoted the protein expression of ATP5B and ATP5J in the MG-63 cells (P<0.05; Fig. 1E). The increase in ATP5B protein expression induced by cyclic stretch was further confirmed by immunofluorescence staining, which revealed an obvious increase in the ATP5B protein levels after 4, 8 and 12 h of cyclic stretch (Fig. 1F).
Figure 1.
Effects of cyclic mechanical stretch (10% elongation, 0.1 Hz) on the energy metabolism of osteoblast-like MG-63 cells. (A and B) Effects of cyclic stretch for 1, 4, 8 and 12 h on glucose consumption and lactate levels in the medium in MG-63 cells (n=6). (C) Effects of cyclic stretch on adenosine tri-phosphate (ATP) concentrations in MG-63 cells determined using a luminometer and rLuciferase/Luciferin reagent (n=3). (D) Measurement of the mRNA expression of ATP5B, ATP5F1, ATP5J, F1-ATPase α, enolase 1 and L-lactate dehydrogenase A (LDHA) in the cells after being subjected to cyclic stretch by RT-qPCR (n=5). (E) Western blot analysis was used to measure the protein levels of ATP5B and ATP5J in the MG-63 cells after being subjected to cyclic stretch (n=3). (F) Immunofluorescence staining of ATP5B protein in MG-63 cells after being subjected to cyclic stretch (scale bar, 50 µm). Data are presented as the means ± SD. *P<0.05 and **P<0.01. Ctrl, control.
Cyclic stretch stimulates the activation of the Akt/mTOR/p70s6k pathway in osteoblast-like cells
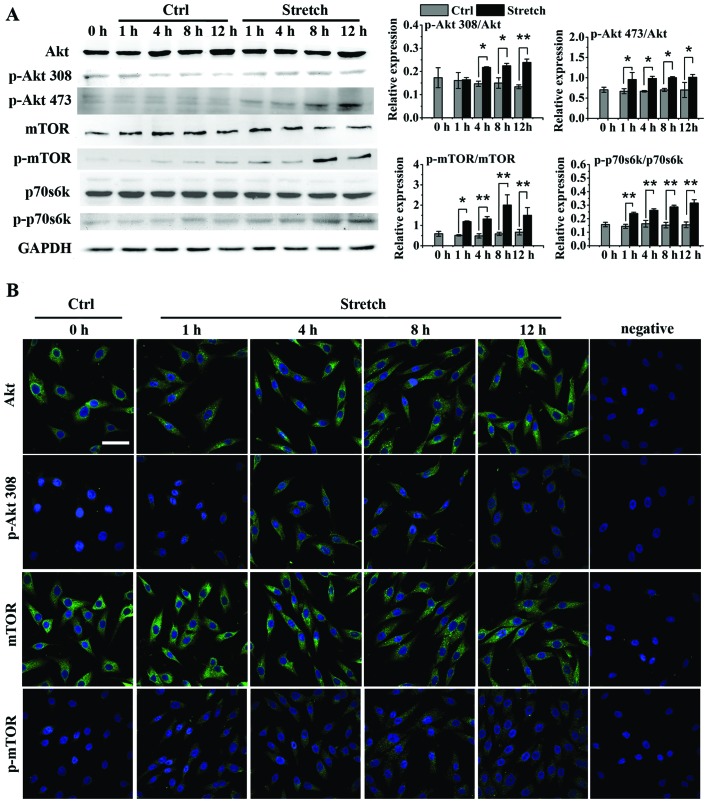
The basal protein levels of Akt, mTOR and p70s6k and their phosphorylation levels were not significantly altered in the MG-63 cells in the control group throughout the 12 h of the experiment (Fig. 2A). Cyclic stretch did not have a marked impact on the protein expression of Akt, mTOR or p70s6k (P>0.05 for all time points), but induced a significant increase in the phosphorylation of Akt, mTOR and p70s6k [P<0.05 for all time points except p-Akt (Thr308) at 1 h] compared to the control. These findings were further confirmed by immunofluorescence staining, which revealed relatively unaltered Akt and mTOR levels, but higher expression levels of the phosphorylated proteins in the MG-63 cells after being subjected to cyclic stretch (Fig. 2B).
Figure 2.
Cyclic mechanical stretch activates the Akt/mammalian target of rapamycin (mTOR)/p70s6k pathway in osteoblast-like MG-63 cells. (A) Western blot analysis of the stretch-induced protein expression of Akt, phosphorylated Akt (p-Akt on Thr308 and Ser473), mTOR, phosphorylated mTOR (p-mTOR, Ser2448), p70s6k, and phosphorylated p70s6k (p-p70s6k, Thr389). (B) Immunofluorescence staining of stretch-induced protein expression of Akt, phosphorylated Akt (p-Akt, Thr308), mTOR, and phosphorylated mTOR (p-mTOR, Ser2448) (scale bar, 50 µm). Data are presented as the means ± SD (n=3). *P<0.05 and **P<0.01. Ctrl, control.
Inhibition of Akt and mTOR halts the stretch-induced activation of the Akt/mTOR/p70s6k pathway in osteoblast-like cells
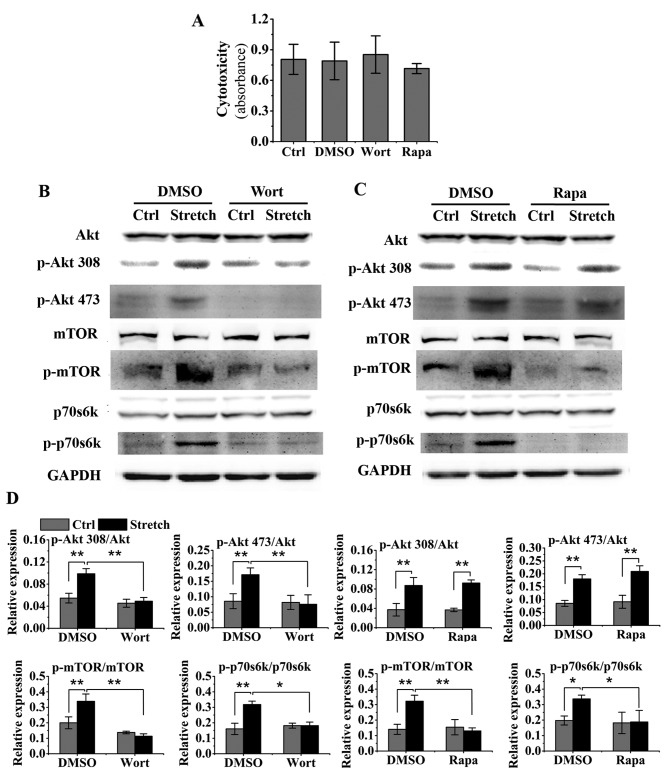
As shown in Fig. 3A, treatment with 0.025% DMSO, 55 nM rapamycin and 500 nM wortmannin had no marked effect on the survival of MG-63 cells, as determined using CCK-8 cytotoxicity assays. As shown in Fig. 3B-D, wortmannin abrogated the stretch-induced phosphorylation of Akt, mTOR and p70s6k, whereas rapamycin only suppressed the stretch-induced phosphorylation of mTOR and p70s6k, but not that of Akt.
Figure 3.
Wortmannin and rapamycin inhibit the stretch-induced activation of the Akt/mammalian target of rapamycin (mTOR)/p70s6k pathway in osteoblast-like MG-63 cells. (A) Evaluation of the effects of wortmannin, rapamycin and DMSO on cell survival using cell counting kit-8 (CCK-8) cytotoxicity assays (n=4). Cells were pre-treated with wortmannin (500 nM, inhibitor of Akt), rapamycin (55 nM, inhibitor of mTOR), and DMSO (0.025%, vehicle control) for 30 min and then subjected to cyclic stretch for 8 h. (B-D) Western blot analysis was used to examine the effects of wortmannin and rapamycin on stretch-induced protein expression: Akt, phosphorylated Akt (p-Akt, Thr308 and Ser473), mTOR, phosphorylated mTOR (p-mTOR, Ser2448), p70s6k and phosphorylated p70s6k (p-p70s6k, Thr389). *P<0.05 and **P<0.01. Wort, wortmannin; rapa, rapamycin; ctrl, control.
Inhibition of Akt and mTOR abrogates the stretch-induced increase in energy metabolism in osteoblast-like cells
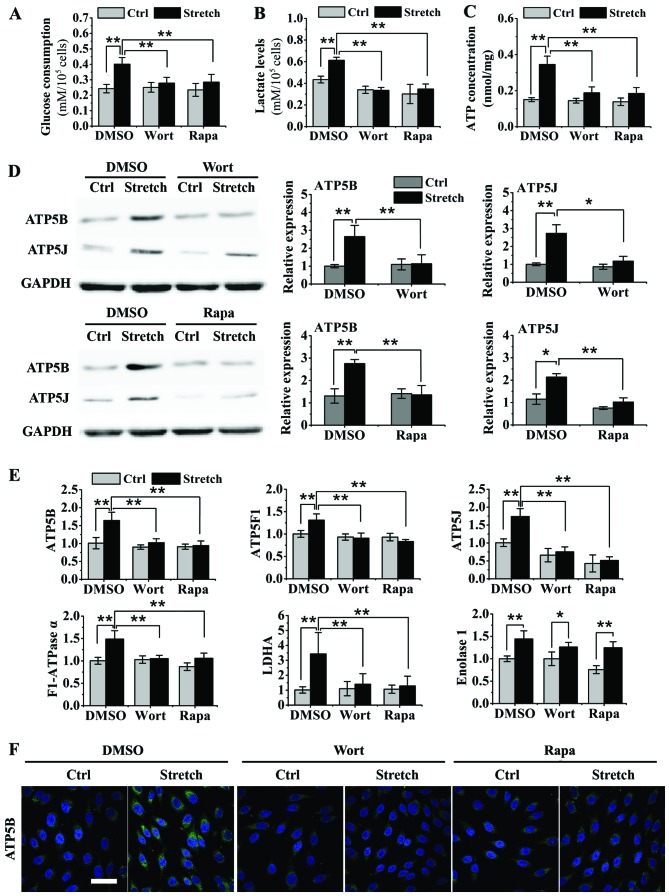
The upregulation of glucose consumption and lactate concentrations induced by 8 h of cyclic stretch was suppressed by treatment with wortmannin and rapamycin (P<0.05; Fig. 4A and B). The upregulation in the ATP concentration induced by 8 h of cyclic stretch was also suppressed by treatment with wortmannin and rapamycin (P<0.05; Fig. 4C). The cyclic stretch-induced upregulation in the mRNA levels of ATP5B, ATP5F1, ATP5J, F1-ATPase α and LDHA was significantly inhibited by treatment with wortmannin and rapamycin; however, the levels of enolase 1 were not markedly inhibited (P<0.05; Fig. 4E). Furthermore, the results form western blot analysis revealed that treatment with wortmannin and rapamycin abrogated the stretch-induced increase in the protein levels of ATP5B and ATP5J (Fig. 4D), which was further verified by immunofluorescence staining of ATP5B protein in the osteoblast-like cells (Fig. 4F).
Figure 4.
Inhibition of Akt and mammalian target of rapamycin (mTOR) suppresses the stretch-induced increase in energy metabolism in osteoblast-like MG-63 cells. (A and B) Effects of wortmannin and rapamycin on glucose consumption and lactate levels in the medium in stretch-stimulated MG-63 cells (n=9). (C) Effects of wortmannin and rapamycin on adenosine triphosphate (ATP) concentrations in stretch-stimulated MG-63 cells were determined using a luminometer and rLu-ciferase/Luciferin reagent (n=6). (D) Evaluation of the effects of wortmannin and rapamycin on the protein expression of ATP5B and ATP5J in stretch-stimulated MG-63 cells by western blot analysis (n=3). (E) Evaluation of the effects of wortmannin and rapamycin on the gene expression of ATP5B, ATP5F1, ATP5J, F1-ATPase α, L-lactate dehydrogenase A (LDHA) and enolase 1 in stretch-stimulated MG-63 cells by RT-qPCR (n=5). (F) Immunofluorescence staining for the determination of the effects of wortmannin and rapamycin on ATP5B protein expression in stretch-stimulated MG-63 cells (scale bar, 50 µm). Data are presented as the means ± SD. *P<0.05 and **P<0.01. Wort, wortmannin; rapa, rapamycin; Ctrl, control.
Discussion
Studies have demonstrated the role mechanical stress plays in regulating osteoblastic functions, such as cellular proliferation, osteogenic differentiation, signal transduction and apoptosis (14–16,26). Energy metabolism is essential to maintaining the biological activities of osteoblasts (17). In the present study, we revealed a novel mechanism in osteoblastic mechanobiology, through which cyclic stretch promotes osteoblastic energy metabolism by increasing the expression of enzymes associated with energy metabolism, partially through the Akt/mTOR/p70s6k signaling pathway. Firstly, cyclic mechanical stretch promoted energy metabolism in the MG-63 cells, which was evidenced by the increased glucose consumption, and the increased levels of lactate, intracellular ATP, and energy metabolism-related genes (ATP5B, ATP5F1, ATP5J, F1-ATPase α, LDHA and enolase 1), and ATP5B and ATP5J proteins. Secondly, cyclic mechanical stretch stimulated the activation of the Akt/mTOR/p70s6k pathway by prompting the continuous phosphorylation of Akt, mTOR and p70s6k in the MG-63 cells. Finally, the stretch-induced phosphorylation of all the signal molecules was suppressed by wortmannin, and the phosphorylation of mTOR and p70s6k was blocked by rapamycin. In addition, wortmannin and rapamycin also inhibited the stretch-induced responses of glucose consumption, lactate levels, intracellular ATP levels, ATP5B and ATP5J proteins and energy metabolism-related enzymes genes (apart from enolase 1).
Energy metabolism is essential for maintaining the biological functions and material metabolism in both organs and cells (17). In the present study, we revealed that glucose consumption and ATP levels were significantly increased in the MG-63 cells after being subjected to cyclic stretch. These results indicate that glucose may be the major fuel source for the increase in ATP levels in mechanically stretched osteoblasts. Furthermore, we demonstrated that cyclic stretch promoted the mRNA and protein synthesis of mitochondrial ATP synthase subunits, which have been demonstrated to control the synthesis of ATP via oxidative phosphorylation in the presence of transmembrane electroosmotic proton gradient generated by electron transport chain (27,28). As demonstrated in the present study, cyclic stretch accelerated the mRNA transcription of LDHA, catalyzing the transformation of pyruvate into lactate via aerobic glycolysis (29). Moreover, our results demonstrated that long periods of cyclic stretch (8 and 12 h) increased lactate levels in MG-63 cells, suggesting that longer-period mechanical loads promote the activation of the aerobic glycolytic metabolic pathway and increase lactate levels. The reason is likely that longer-period mechanical loads induce more cellular energy and increase glucose consumption, but the glucose cannot be effectively utilized via oxidative phosphorylation. As a result, aerobic glycolysis is increased to supplement the cellular energy needs. Our results also demonstrated that cyclic mechanical stretch induced an increase in the gene transcription of enolase 1, which has been shown to be a key glycolytic enzyme catalyzing the formation of phosphoenolpyruvate (30). In addition, protein expression (ATP5B and ATP5F1) showed a greater increase than the qPCR data in response to cyclic stretch. This indicated that mechanical stretch may exert greater effects at the translational level than the transcriptional level. A possible reason is that proteins act as essential functional molecules in fulfilling the requirements for stretch-induced osteoblastic energy metabolism.
Our findings demonstrated that cyclic stretch promoted osteoblastic energy metabolism by modulating the ATP concentrations and enzyme expression associated with energy metabolism. However, the underlying mechanisms of cyclic stretch in regulating osteoblastic energy metabolism remain unclear. The Akt/mTOR/p70s6k pathway has been proven to play an important role in cellular functions, including translation, transcription and autophagy (18,19), as well as in energy metabolism (20–24). Moreover, the following points also imply the close correlation of mechanical stress, mTOR signals and energy metabolism: i) tensile stretch induces the upregulation of transforming growth factor-β1 (TGF-β1) levels in human osteoblast-like cells (31); ii) TGF-β1 modulates overexpression of glucose transporter 1, glucose uptake and metabolism through the positive feedback in glomerular cells (32); and iii) TGF-β1 also regulates the mTOR signals in human cancer cells (33). Nonetheless, it is unclear as to whether cyclic stretch activates the Akt/mTOR/p70s6k pathway and whether this pathway plays a critical role in modulating osteoblastic energy metabolism in response to cyclic stretch.
The present study demonstrated that cyclic stretch induced a significant increase in the phosphorylation levels of Akt, mTOR and p70s6k in MG-63 cells. Moreover, we showed that Akt was upstream of mTOR/p70s6k by studying the differential effects of wortmannin (inhibiting Akt phosphorylation) and rapamycin (inhibiting mTOR phosphorylation) on suppressing the stretch-induced activation of the Akt/mTOR/p70s6k pathway.
Generally, growth factors, such as insulin-like growth factors (IGFs), activate the PI3K pathway and in turn induce the phosphorylation of Akt on Thr308, and then activate mTOR complex 2 (mTORC2) via a series of complex events. The activation of mTORC2 then retroactively induces the phosphorylation of Akt on Ser473. The phosphorylation of Akt on Thr308 and Ser473 together induces the complete activation of Akt (18,34). However, our results revealed that the phosphorylation of Akt on Ser473 preceded the phosphorylation of Akt on Thr308. The reason is likely that mechanical stretch first induces the activation of mTORC2, as was demonstrated in a previous study, which showed that mechanical loads induced the phosphorylation of Akt on the Ser473 via the early activation of mTORC2 in bone marrow-derived stem cells (BMSCs) (35). Furthermore, our results revealed that the phosphorylation of mTOR and p70s6k both preceded the phosphorylation of Akt on Thr308, indicating that cyclic stretch activated the mTOR/p70s6k pathway ahead of Akt. In the skeleto-muscular studies, it has been proven that mechanical loads activate the mTOR/p70s6k pathway via phosphatidic acid (PA) and phospholipase D (PLD) (36,37) in the early phase rather than via the Akt pathway (38,39). Thus, it can be inferred that cyclic stretch induces the early activation of mTOR signaling in MG-63 cells through other pathways (PA/PLD). Growth factors secreted by cells after being subjected to cyclic stretch may also activate Akt signaling, and subsequently activate the mTOR/p70s6k pathway in the late phase of cyclic stretch.
Our findings further demonstrated that the inhibition of Akt and mTOR in the MG-63 cells suppressed the stretch-induced increase in glucose consumption, lactate levels, ATP levels, ATP5B and ATP5J protein expression as well as the increase in the expression of energy metabolism-related enzyme genes, indicating the significance of the Akt/mTOR/p70s6k pathway in regulating osteoblastic energy metabolism. Of note, neither wortmannin nor rapamycin suppressed the stretch-induced enolase 1 gene transcription in the MG-63 cells, revealing that Akt/mTOR/p70s6k is not the only signaling pathway involved in modulating stretch-induced energy metabolism. Thus, we conclude that stretch-induced energy metabolism in MG-63 cells is partially dependent on the activation of the Akt/mTOR/p70s6k pathway. It will also be worthwhile to further explore other potential signaling pathways which may be related to stretch-induced osteoblastic energy metabolism in future studies.
Although the findings of the present study revealed the potential mechanisms of stretch-induced energy metabolism in MG-63 cells, partially through the Akt/mTOR/p70s6k signaling pathway, the main limitation of the present study was that we investigated the effects of cyclic stretch on glucose consumption, lactate levels, ATP levels, and proteins and mRNA expression of energy metabolism-related enzymes, but investigating the role of cyclic stretch in regulating osteoblastic oxidative phosphorylation and tricarboxylic acid cycles fell outside the scope of this study. We aim to investigate these issues in future studies, which may provide a more comprehensive understanding of the regulatory roles of mechanical stretch in osteoblastic energy metabolism.
In conclusion, the present study demonstrated that cyclic stretch promoted energy metabolism in osteoblast-like MG-63 cells by regulating glucose consumption, lactate levels, ATP levels and energy metabolism-related enzymes partially through the Akt/mTOR/p70s6k signaling pathway. These findings not only enrich our basic knowledge of the molecular mechanisms involved in osteoblastic energy metabolism in response to cyclic stretch, but also provide new insight into the biological functions of osteoblasts from the perspective of energy metabolism.
Acknowledgments
The present study was supported by grants from the National Natural Science Foundation of China (nos. 31070836 and 81100750).
References
- 1.Ruff C, Holt B, Trinkaus E. Who's afraid of the big bad Wolff?: 'Wolff's law' and bone functional adaptation. Am J Phys Anthropol. 2006;129:484–498. doi: 10.1002/ajpa.20371. [DOI] [PubMed] [Google Scholar]
- 2.Jing D, Baik AD, Lu XL, Zhou B, Lai X, Wang L, Luo E, Guo XE. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014;28:1582–1592. doi: 10.1096/fj.13-237578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolam KA, van Uffelen JG, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men: a systematic review. Osteoporos Int. 2013;24:2749–2762. doi: 10.1007/s00198-013-2346-1. [DOI] [PubMed] [Google Scholar]
- 4.Schulte FA, Ruffoni D, Lambers FM, Christen D, Webster DJ, Kuhn G, Müller R. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS One. 2013;8:e62172. doi: 10.1371/journal.pone.0062172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd SA, Bandstra ER, Willey JS, Riffle SE, Tirado-Lee L, Nelson GA, Pecaut MJ, Bateman TA. Effect of proton irradiation followed by hindlimb unloading on bone in mature mice: a model of long-duration spaceflight. Bone. 2012;51:756–764. doi: 10.1016/j.bone.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaber EA, Dvorochkin N, Lee C, Alwood JS, Yousuf R, Pianetta P, Globus RK, Burns BP, Almeida EA. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS One. 2013;8:e61372. doi: 10.1371/journal.pone.0061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Jing D, Lu XL, Luo E, Sajda P, Leong PL, Guo XE. Spatiotemporal properties of intracellular calcium signaling in osteocytic and osteoblastic cell networks under fluid flow. Bone. 2013;53:531–540. doi: 10.1016/j.bone.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mai Z, Peng Z, Wu S, Zhang J, Chen L, Liang H, Bai D, Yan G, Ai H. Single bout short duration fluid shear stress induces osteogenic differentiation of MC3T3-E1 cells via integrin β1 and BMP2 signaling cross-talk. PLoS One. 2013;8:e61600. doi: 10.1371/journal.pone.0061600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young SR, Hum JM, Rodenberg E, Turner CH, Pavalko FM. Non-overlapping functions for Pyk2 and FAK in osteoblasts during fluid shear stress-induced mechanotransduction. PLoS One. 2011;6:e16026. doi: 10.1371/journal.pone.0016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans CE, Mylchreest S, Andrew JG. Age of donor alters the effect of cyclic hydrostatic pressure on production by human macrophages and osteoblasts of sRANKL, OPG and RANK. BMC Musculoskelet Disord. 2006;7(21) doi: 10.1186/1471-2474-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walboomers XF, Elder SE, Bumgardner JD, Jansen JA. Hydrodynamic compression of young and adult rat osteoblast-like cells on titanium fiber mesh. J Biomed Mater Res A. 2006;76:16–24. doi: 10.1002/jbm.a.30304. [DOI] [PubMed] [Google Scholar]
- 14.Matsui H, Fukuno N, Kanda Y, Kantoh Y, Chida T, Nagaura Y, Suzuki O, Nishitoh H, Takeda K, Ichijo H, et al. The expression of Fn14 via mechanical stress-activated JNK contributes to apoptosis induction in osteoblasts. J Biol Chem. 2014;289:6438–6450. doi: 10.1074/jbc.M113.536300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneuji T, Ariyoshi W, Okinaga T, Toshinaga A, Takahashi T, Nishihara T. Mechanisms involved in regulation of osteo-clastic differentiation by mechanical stress-loaded osteoblasts. Biochem Biophys Res Commun. 2011;408:103–109. doi: 10.1016/j.bbrc.2011.03.128. [DOI] [PubMed] [Google Scholar]
- 16.Kaspar D, Seidl W, Neidlinger-Wilke C, Beck A, Claes L, Ignatius A. Proliferation of human-derived osteoblast-like cells depends on the cycle number and frequency of uniaxial strain. J Biomech. 2002;35:873–880. doi: 10.1016/S0021-9290(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 17.Rosen CJ. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab. 2008;7:7–10. doi: 10.1016/j.cmet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Senin LL, Al-Massadi O, Folgueira C, Castelao C, Pardo M, Barja-Fernandez S, Roca-Rivada A, Amil M, Crujeiras AB, Garcia-Caballero T, et al. The gastric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS One. 2013;8:e80339. doi: 10.1371/journal.pone.0080339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijer AJ, Dubbelhuis PF. Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun. 2004;313:397–403. doi: 10.1016/j.bbrc.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubbelhuis PF, Meijer AJ. Hepatic amino acid-dependent signaling is under the control of AMP-dependent protein kinase. FEBS Lett. 2002;521:39–42. doi: 10.1016/S0014-5793(02)02815-6. [DOI] [PubMed] [Google Scholar]
- 25.Clover J, Gowen M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone. 1994;15:585–591. doi: 10.1016/8756-3282(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 26.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Wittig I, Schägger H. Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochim Biophys Acta. 2009;1787:672–680. doi: 10.1016/j.bbabio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Wang SB, Murray CI, Chung HS, Van Eyk JE. Redox regulation of mitochondrial ATP synthase. Trends Cardiovasc Med. 2013;23:14–18. doi: 10.1016/j.tcm.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adeva M, González-Lucán M, Seco M, Donapetry C. Enzymes involved in L-lactate metabolism in humans. Mitochondrion. 2013;13:615–629. doi: 10.1016/j.mito.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Díaz-Ramos A, Roig-Borrellas A, García-Melero A, López-Alemany R. α-Enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cillo JE, Jr, Gassner R, Koepsel RR, Buckley MJ. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: implications for distraction osteo-genesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:147–154. doi: 10.1067/moe.2000.107531. [DOI] [PubMed] [Google Scholar]
- 32.Gnudi L, Thomas SM, Viberti G. Mechanical forces in diabetic kidney disease: a trigger for impaired glucose metabolism. J Am Soc Nephrol. 2007;18:2226–2232. doi: 10.1681/ASN.2006121362. [DOI] [PubMed] [Google Scholar]
- 33.Lin G, Gai R, Chen Z, Wang Y, Liao S, Dong R, Zhu H, Gu Y, He Q, Yang B. The dual PI3K/mTOR inhibitor NVP-BEZ235 prevents epithelial-mesenchymal transition induced by hypoxia and TGF-β1. Eur J Pharmacol. 2014;729:45–53. doi: 10.1016/j.ejphar.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Case N, Thomas J, Sen B, Styner M, Xie Z, Galior K, Rubin J. Mechanical regulation of glycogen synthase kinase 3β (GSK3β) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein. J Biol Chem. 2011;286:39450–39456. doi: 10.1074/jbc.M111.265330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. 2011;43:1267–1276. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA. 2006;103:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol. 2011;589:1831–1846. doi: 10.1113/jphysiol.2011.205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804. doi: 10.1042/bj20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]