Abstract
HOXD10, a key regulator of cell-differentiated phenotype maintainence, has been demonstrated to be involved in the tumorigenesis of many human malignacies. However, the status of HOXD10 expression and its biological function in cholangiocellular carcinoma (CCC) remain to be clarified. In the present study, we investigated the clinical significance and biological functions of HOXD10 in CCC and found that the expression of HOXD10 and its downstream effector RHOC was significantly different in well-differentiated CCC tissues compared with poorly-differentiated lesions. We also observed a significant correlation between low HOXD10 and high RHOC expression levels and worse prognosis. The stable overexpression of HOXD10 by lentivirus vector significantly inhibited cell invasion partly by downregulating the expression of MMP2 and MMP9, and significantly increased early apoptosis in CCC cell lines and induced G1 phase cell cycle arrest, contributing to the inhibition of cell proliferation in vitro. Additionally, we demonstrated that the inactivation of the RHOC/AKT/MAPK pathway was involved in the tumor-suppressive functions of HOXD10 in CCC. These results suggested that HOXD10 may be a putative suppressor gene and can act as a prognostic marker and potentially a novel therapeutic target for CCC.
Keywords: RHOC/AKT/MAPK pathway, human cholangiocellular carcinoma, cell proliferation, apoptosis, prognosis, invasion, HOXD10, RHOC
Introduction
Cholangiocellular carcinoma (CCC) is an epithelial cell malignancy arising from various locations within the biliary tree, which is classified as intrahepatic, perihilar and distal cholangiocarcinoma based on anatomical location (1). CCC accounts for 3% of gastrointestinal tumors worldwide and is the second most common primary liver cancer (2). Even with greatly improved diagnosis and treatment technologies in the past 30 years, the 5-year survival rate including patients with radical surgical resection, the only means for cure, remain at <5% (3). High recurrence risks and poor prognoses in CCC patients have been a major challenge to medical workers (4). Therefore, it is necessary to identify a tumor marker for predicting therapeutic response, recurrence and the survival of CCC, and developing targeted therapy to improve patient prognosis.
HOX genes are an evolutionarily conserved family of genes that encode a class of important transcription factors which function in various developmental processes (5). In addition to their role in embryogenesis, HOX genes are expressed in adult cells where they regulate the expression of genes involved in cell proliferation as well as cell-cell and cell-extracellular matrix interactions (6). As one of such homeobox genes, the overexpression of HOXD10 has been demonstrated to maintain a quiescent, differentiated phenotype in endothelial cells by suppressing the expression of genes involved in remodeling the extracellular matrix and cell migration (7). In breast cancer cell lines, HOXD10 induces cell phenotypic reversion from highly invasive tumor cells to a non-malignant state in a three-dimensional culture model (8).
Overexpression or loss of HOXD10 is involved in tumorigenesis characterized as an oncogene or tumor-suppressor because of its importance in cancer cell survival, proliferation, invasion and migration in several malignancies, including breast, gastric, hepatocellular, ovarian, malignant glioma, head and neck, bladder, and colorectal cancer (9–16). However, the role of HOXD10 in CCC malignant progression remains elusive. Therefore, we investigated whether HOXD10 plays a role in the development and progression of CCC.
Materials and methods
Patients and clinicopathological data
Concurrent clinical and pathological data were retrospectively collected from 87 patients who underwent surgical resection of pathologically confirmed CCC between March 3, 2008 and December 20, 2013 at the First Affiliated Hospital of Xi'an Jiaotong University and the Tangdu Hospital (Shaanxi, China). Demographic data and pathological results were collected for each patient. The study was approved by the ethics committees of the above hospitals in March 2008. The patients provided signed informed consent. The follow up of patients was conducted through phone calls until death or the study deadline date of December 20, 2013. Additionally, 30 cases of para-carcinoma tissue were included as controls. Histopathological diagnosis was performed by the Department of Pathology of each Institution. Clinicopathological staging was determined by the TNM classification of the 7th edition American Joint Committee on Cancer (AJCC) for intrahepatic, perihilar and distal cholangiocarcinoma, respectively (17). Tissue samples were fixed in 4% formalin immediately after removal for immunohistochemical (IHC) staining.
Immunohistochemical analysis
Fixed tumor samples were processed conventionally for paraffin-embedded tumor slides. The slides were subjected to antigen retrieval and the endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. The sections were incubated with 1% bovine serum albumin to decrease non-specific staining and reduce endogenous peroxidase activity. For IHC staining, the sections were incubated with antibodies against HOXD10 (1:800; Abgent Corporation, San Diego, CA, USA) and RHOC (1:200; Abcam, Cambridge, MA, USA). PBS was used to replace the primary antibody as a negative control. After being washed with PBS, the slides were incubated with biotinylated secondary antibody followed by conjugated horseradish peroxidase (HRP)-labeled streptavidin (Dako, Glostrup, Denmark) and then washed with PBS. The slides were then incubated with diaminobenzidine (DAB; Sigma, St. Louis, MO, USA) as the chromogen, followed by counterstaining with diluted Harris's hematoxylin (Sigma). After staining, five high-power fields (×400) were randomly selected in each slide, and the average proportion of positive cells in each field was counted. The immunoreactivity score of each section was based on the proportion of immunopositive cells (0, <10%; 1, ≥10 to <25%; 2, ≥25 to <50%; 3, ≥50 to <75% and 4, ≥75%) x their intensity of staining (0, no positivity; 1, weak; 2, moderate and 3, strong) and was divided into 4 levels, 0 (negative), 1–4 (weak), 5–8 (moderate) and 9–12 (strong). The final immunoreaction score was defined as the sum of the two parameters (extension and intensity), and the samples were grouped as negative (0), weak (1–2), moderate (3) and strong (4–6) staining. For statistical purposes, only the final immunoreaction scores of moderate and strong were considered positive, and the other final scores negative.
Cell culture and transfection with lentiviral-mediated overexpression of HOXD10 vectors
The RBE cell line was purchased from the Shanghai Institute for Biological Sciences (Shanghai, China). The HCCC-9810 cell line was obtained from the Wuhan Boster Biological Engineering Corporation (Wuhan, China). The cells were maintained in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and were routinely cultivated in a humidified incubator at 37°C with 5% CO2.
A lentivirus encoding the complete human HOXD10/GFP open reading frame (lentivirus-GFP-HOXD10) and a control negative lentivirus encoding a green fluorescent protein (GFP) open reading frame (lentivirus-GFP) were constructed by Genechem Biotechnology Inc., Ltd. (Shanghai, China). The HOXD10 coding sequence was amplified from human genomic DNA by PCR using the primers: sense, 5′-GAGGATCCCCGGGTACCGGTCGCCACCATGTCCTTTCCCAACAG CTC-3′ and antisense, 5′-TCCTTGTAGTCCATACCAGAA AACGTGAGGTTGGC-3′. The amplified HOXD10 gene was cloned into GV358 mammalian expressing vector, and transferred into the lentiviral vector, Ubi-MCS-3FLAG-SV40-EGFP-IRES-puromycin, using in vitro recombination, to generate the recombinant lentiviral expression vector, LV-HOXD10. The empty lentiviral vector LV-GFP was also packaged in the same manner and used as a negative control. The expression of GFP was examined under a fluorescence microscope, and the intensity of green fluorescence indicated the transduction efficiency. The two CCC cells were transfected with high titer (2×108 TU/ml) lentivirus-HOXD10 and negative control lentivirus expression vector particles (all with a MOI of 30 transfection concentration) according to the manufacturer's instructions. Stable HOXD10 overexpression and negative lentiviral transfectants were obtained by continuous treatment with 2 µg/ml puromycin in transfected RBE and HCCC-9810 cell lines.
Reverse transcription-PCR (RT-PCR)
Total RNA was extracted from the cultured cells using the TRIzol reagent (Invitrogen-Life Technologies). Oligo (dT)-primed RNA (1 µg) was reverse-transcribed with reverse transcriptase (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's instructions. The primers (Sangon Biotech, Shanghai, China) used to detect the expression of HOXD10, MMP2, MMP9, MMP14 and GAPDH (internal control) were: HOXD10 sense, 5′-GACATGGGGACCTATGGAATGC-3′ and antisense, 5′-TGGTGGTTCACTTCTCTTTTGG-3′; MMP2 sense, 5′-CTTCTTCCCTCGCAAGCC-3′ and anti-sense, 5′-ATGGATTCGAGAAAACCG-3′; MMP9 sense, 5′-ACGCAGACATCGTCATCC-3′ and antisense, 5′-AACCGAGTTGAACCACG-3′; MMP14 sense, 5′-TCCATCAACACTGCCTACGA-3′ and antisense, 5′-TGAATGACCCTCTGGGAGAC-3′; and GAPDH sense, 5′-AGGTCCACCACTGACACGTT-3′ and antisense, 5′-GCCTCAAGATCATCAGCAAT-3′.
Western blot analysis
Equal amounts of protein sample from the RBE and HCCC-9810 cells were separated by SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked and then probed with a primary antibody against HOXD10 (1:300; Abgent Corporation), RHOC, MMP2 and MMP9 (all at 1:1,000) and MMP14 (1:2,000), (all from Abcam), AKT, ERK1/2, p-AKT, p-ERK1/2 (all at 1:500; Wanleibio Co., Ltd., China) and β-actin (1:5,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After being washed, the membranes were incubated with HRP-conjugated goat anti-mouse/rabbit IgG (Santa Cruz Biotechnology, Inc.) and detected using the enhanced chemiluminescence (ECL) detection kit from Pierce (Rockford, IL, USA).
Cell viability assay
Cell viability was assessed using a MTT assay according to the manufacturer's instructions. The absorbance at 490 nm was detected using a microplate reader (Bio-Rad, Hercules, CA, USA). A growth curve was prepared according to the absorbance values at 490 nm. The results reflect the average of three replicates under the same conditions.
Colony formation assay
Non-transfected and transfected RBE and HCCC-9810 cells (200 cells/dish) were seeded in 60-mm culture dishes. After 14 days of culture, the adherent cells were washed twice with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. The colonies were stained with Giemsa solution for 15 min, washed with water and air-dried. The number of colonies was counted using a light microscope. The experiments were performed in triplicate.
Analysis of the cell cycle distribution and apoptosis
The effect of HOXD10 overexpression on cell cycle progression was determined using flow cytometry following propidium iodide (PI) staining. Briefly, the cells were harvested and fixed in 70% ethanol at 4°C overnight. For the analysis, 1 ml of freshly prepared PBS staining solution (50 µg/ml PI and 100 µg/ml RNase A) was added to the cells. Following incubation for 1 h in the dark at room temperature, the cells were analyzed using flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA). The proportion of DNA in different phases was analyzed using dedicated software (BD Biosciences). Quantification of apoptosis was determined by analyzing the percentages of apoptotic cells. The cells were stained using an Annexin V-APC/7-AAD apoptosis detection kit (KGA1023–1026; Nanjing KeyGen Biotech. Co., Ltd.) according to the manufacturer's instructions and were analyzed by flow cytometry using a Beckman Coulter flow cytometer.
Cell invasion assay
Cell invasion was assessed using 6.5-mm Transwell chambers with a pore size of 8 µm (Corning, NY, USA). The Transwell upper chambers were pre-coated with 50 µg/chamber of a solubilized basement membrane in the form of Matrigel (BD Biosciences). RPMI-1640/10% FBS was added to the bottom chamber. CCC cells (3×104/chamber) were seeded in serum-containing media in the upper well of the Transwell chambers. The cells were incubated for 48 h, and those that did not invade through the pores were removed with a cotton swab. The invasive cells in the lower chamber (below the filter surface) were fixed in 70% ethanol, stained with 0.1 mg/ml crystal violet solution, and counted under a microscope (×20 magnification). Individual experiments had triplicate inserts, and five randomly selected fields were counted per insert.
Statistical analysis
Data were analysed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). The data are presented as the mean ± SD. The data were analyzed using the χ2 test and the Student's t-test. The Kaplan-Meier test was used for univariate survival analysis. The Cox proportional hazard model was used for multivariate analysis and for determining the 95% confidence intervals. Differences with P<0.05 were considered to indicate a statistically significant result.
Results
Low HOXD10 and high RHOC expression in poorly-differentiated CCC tissues and the association with a poor outcome in CCC patients
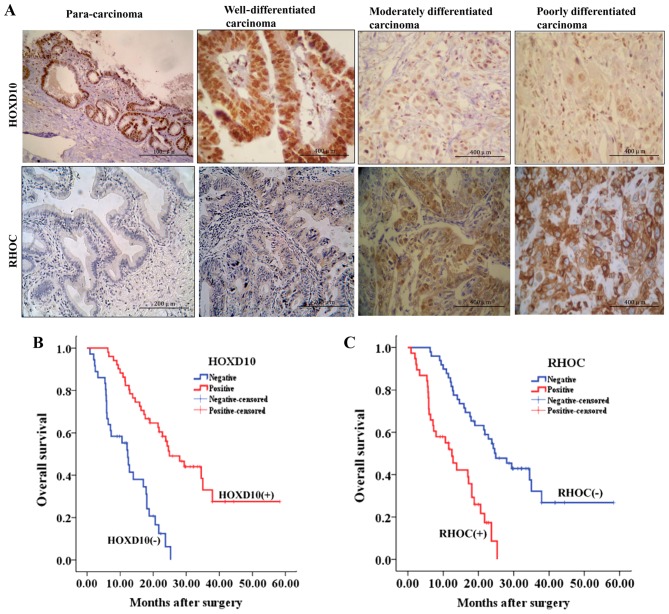
To determine the role of HOXD10 in CCC, we evaluated HOXD10 expression in tumor or para-carcinoma tissue from patients with CCC by using IHC. The positive expression rate of HOXD10 in well-differentiated cancerous tissues (25/28, 89.3%) was not significantly different compared to that of the adjacent para-carcinoma tissues (27/30, 90%) (P>0.05). However, the HOXD10-positive expression rate in well-differentiated cancerous tissues (25/28, 89.3%) was significantly higher than that of the poorly-differentiated cancerous tissues (4/21, 19%) (P<0.001). Furthermore, HOXD10 expression was progressively reduced with increasing degrees of malignancy (Fig. 1A and Table I, P<0.001).
Figure 1.
Decreased HOXD10 and increased RHOC expression in CCC and the negative association of the dysregulated expressions with prognosis. (A) Immunohistochemical stainings for HOXD10 and RHOC expression. The positive HOXD10 staining was primarily localized in the nucleus, while the positive RHOC staining was localized in the cytoplasm. The HOXD10 stainings are strong, strong, moderate and weak in para-carcinoma (×100), well- (×400), moderately- (×400) and poorly-differentiated carcinoma (×400), respectively, while the RHOC stainings are weak, weak, moderate and strong in para-carcinoma (×200), well- (×200), moderately- (×400) and poorly-differentiated carcinoma (×400), respectively. (B) Kaplan-Meier plots of the overall survival rate of CCC patients based on nuclear HOXD10-positive or -negative expression. (C) Kaplan-Meier plots of the overall survival rate of CCC patients based on RHOC-positive or -negative expression. CCC, cholangiocellular carcinoma.
Table I.
Correlations between HOXD10 expression and the clinicopathological characteristics of CCC patients.
| Parameters/categories | No. of cases (n) | HOXD10
|
||
|---|---|---|---|---|
| No. of positive cases (%) | χ2 | P-value | ||
| Age | ||||
| <70 | 66 | 38 (57.6) | 0.123 | 0.726 |
| ≥70 | 21 | 13 (61.9) | ||
| Gender | ||||
| Male | 46 | 26 (56.5) | 0.177 | 0.674 |
| Female | 41 | 25 (61.0) | ||
| Histological grade | ||||
| G1 | 28 | 25 (89.3) | 24.420 | <0.001a |
| G2 | 38 | 22 (57.9) | ||
| G3 | 21 | 4 (19.0) | ||
| TNM stage | ||||
| I-II | 77 | 46 (59.7) | 0.346 | 0.556 |
| III-IV | 10 | 5 (50.0) | ||
| Lymph node metastasis | ||||
| Negative | 60 | 39 (65.0) | 3.244 | 0.072 |
| Positive | 27 | 12 (44.4) | ||
| CA-199 | ||||
| <35 | 39 | 27 (69.2) | 3.281 | 0.070 |
| ≥35 | 48 | 24 (50.0) | ||
| Venous/lymphatic invasion | ||||
| Negative | 44 | 24 (54.5) | 0.610 | 0.435 |
| Positive | 43 | 27 (62.8) | ||
| Perineural invasion | ||||
| Negative | 45 | 29 (64.4) | 1.303 | 0.254 |
| Positive | 42 | 22 (52.4) | ||
| Tumor location | ||||
| Intrahepatic | 8 | 3 (37.5) | 1.749 | 0.417 |
| Perihilar | 21 | 12 (57.1) | ||
| Distal | 58 | 36 (62.1) | ||
Statistically significant. G1, well-differentiated; G2, moderately-differentiated; G3, poorly-differentiated; CCC, cholangiocellular carcinoma.
We assessed the correlation between the expression of HOXD10 and the histopathological parameters in CCC patients. As shown in Table I, the positive expression of HOXD10 was significantly associated with histologic grade (P<0.001). However, HOXD10 expression was not correlated with gender, age, TNM stage, CA19–9 level, venous/lymphatic invasion, tumor location or lymph node metastasis (P>0.05). The results of the Kaplan-Meier analysis for overall survival based on HOXD10 expression are shown in Table II (P<0.001). The prognosis for patients with a positive HOXD10 expression was significantly better than that for patients with a negative HOXD10 expression (Fig. 1B).
Table II.
Univariate analyses showing the overall survival rate of CCC patients.
| Parameters/categories | No. of cases (n) | Mean survival time in months (95% CI) | P-value |
|---|---|---|---|
| Age | |||
| <70 | 66 | 18.1 (13.0–23.2) | 0.352 |
| ≥70 | 21 | 23.7 (13.7–33.7) | |
| Gender | |||
| Male | 46 | 19.0 (13.8–24.1) | 0.393 |
| Female | 41 | 17.2 (8.3–26.1) | |
| Histological grade | |||
| G1 | 28 | 35.0 (27.9–42.1) | <0.001a |
| G2 | 38 | 13.8 (11.6–16.0) | |
| G3 | 21 | 10.6 (0.1–21.1) | |
| TNM stage | |||
| I-II | 77 | 20.6 (15.5–25.7) | 0.007a |
| III-IV | 10 | 12.1 (9.1–15.1) | |
| Lymph node metastasis | |||
| Negative | 60 | 23.8 (17.7–29.9) | 0.005a |
| Positive | 27 | 14.6 (7.5–21.7) | |
| CA-199 | |||
| <35 | 39 | 22.8 (16.5–29.1) | 0.100 |
| ≥35 | 48 | 16.3 (11.9–20.7) | |
| Venous/lymphatic invasion | |||
| Negative | 44 | 19.0 (12.5–25.5) | 0.822 |
| Positive | 43 | 18.8 (12.2–25.4) | |
| Perineural invasion | |||
| Negative | 45 | 18.1 (10.8–25.4) | 0.586 |
| Positive | 42 | 18.8 (13.5–24.1) | |
| HOXD10 | |||
| Negative | 36 | 12.5 (8.3–16.7) | <0.001a |
| Positive | 51 | 24.2 (19.8–28.6) | |
| RHOC | |||
| Negative | 49 | 23.8 (17.8–29.8) | <0.001a |
| Positive | 38 | 13.8 (5.2–22.4) |
Statistically significant. G1, well-differentiated; G2, moderately-differentiated; G3, poorly-differentiated; CCC, cholangiocellular carcinoma.
RHOC, a member of the Rho family GTPases, is involved at multiple stages of tumorigenesis and progression, and is HOXD10-dependent in breast and gastric cancer cell lines (18–22). To analyze the role of HOXD10 in CCC, we investigated the RHOC protein expression using the same set of CCC samples. The IHC analysis revealed tumors with lower HOXD10 levels also exhibited a higher RHOC expression (Fig. 1A), showing a direct negative association between HOXD10 and RHOC expression (Table III, P<0.001). Moreover, the results of the Kaplan-Meier analysis for overall survival based on RHOC expression are shown in Table II (P<0.001). The patients with a negative RHOC expression had a longer survival than those with a positive RHOC expression (Fig. 1C).
Table III.
Correlation between HOXD10 and RHOC expression in CCC patients.
| HOXD10 expression | RHOC expression
|
χ2 | P-value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 6 | 45 | 51.026 | <0.001a |
| Negative | 32 | 4 | ||
Statistically significant. P-values were calculated using the χ2 test. CCC, cholangiocellular carcinoma.
To obtain a more precise estimate regarding patient prognosis, the Cox proportional hazard regression model was applied. The results confirmed that a positive HOXD10 expression was an independent prognostic factor for CCC patients, and a positive HOXD10 expression may contribute to the reduced mortality rate following surgery by 65.9% (HR=0.341, Table IV).
Table IV.
Multivariate analysis of the overall survival rate of CCC patients.
| Parameters/categories | HR (95% CI) | P-value |
|---|---|---|
| HOXD10 expression | ||
| Negative | 0.341 (0.139–0.838) | 0.019a |
| Positive | ||
| RHOC expression | ||
| Negative | 1.744 (0.691–4.402) | 0.239 |
| Positive | ||
| TNM stage | ||
| I-II | 2.923 (1.210–7.064) | 0.017a |
| III-IV | ||
| Lymph node metastasis | ||
| Negative | 1.311 (0.713–2.411) | 0.383 |
| Positive | ||
| Histological grade | ||
| G1–G2 | 1.215 (0.646–2.284) | 0.545 |
| G3 |
Statistically significant. HR, hazard ratio; G1, well-differentiated; G2, moderately-differentiated; G3, poorly-differentiated; CCC, cholangiocellular carcinoma.
HOXD10 mRNA and protein expression is effectively induced by lentiviral-mediated overexpression of HOXD10 in RBE and HCCC-9810 cell lines
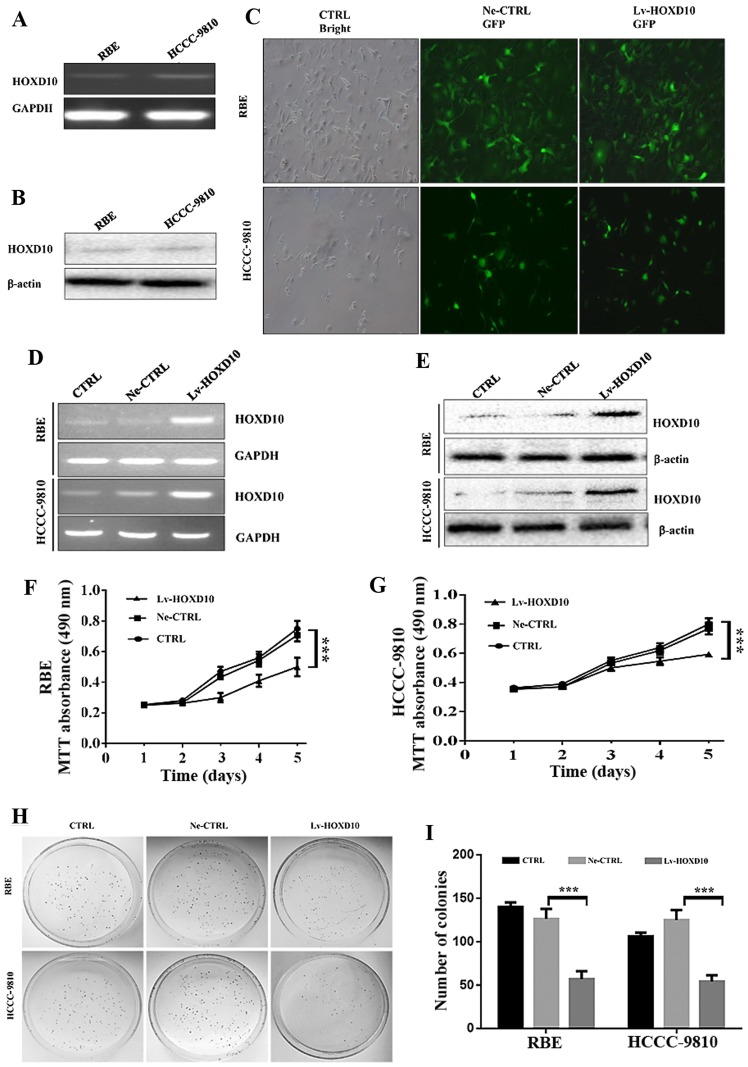
Since HOXD10 protein expression was downregulated in CCC tissues and associated with poor outcome of CCC patients, the function of HOXD10 was determined using a lentivirus-mediated HOXD10 overexpression vector in the RBE and HCCC-9810CCC cell lines that had low endogenous HOXD10 mRNA and protein expression levels (Fig. 2A and B). To observe the lentiviral transfection efficiency in RBE and HCCC-9810 cells, GFP expression was examined using fluorescent microscopy 4 days after infection (Fig. 2C). The efficiency of lentiviral transfection in RBE and HCCC-9810 cells was ≥95% on the 4th day or later at the MOI of 30.
Figure 2.
Effect of HOXD10 overexpression on RBE and HCCC-9810 cell proliferation. (A and B) Low endogenous HOXD10 mRNA and protein expression levels in RBE and HCCC-9810 cell lines. (C) Lentiviral transduction efficiency. Compared with the control cell under a light microscope (×100), a fluorescence microscopy (×100) shows >95% RBE and HCCC-9810 cells are effectively transfected with negative HOXD10 lentivirus and lentivirus-HOXD10 4 days after transfection at a MOI of 30. (D and E) Relative HOXD10 mRNA and proteins expression 5 days after lentivirus transfection. (F and G) Cell viability of untransfected and stably transfected RBE and HCCC-9810 cells by an MTT assay, ***P<0.001. (H) Colony formation assay of RBE and HCCC-9810 cells. (I) A statistical plot of the average number of RBE and HCCC-9810 cells forming colonies in each group. The graph shows the mean ± SD; ***P<0.001. CTRL, blank control; Ne-CTRL, cells transduced with negative HOXD10 lentivirus; Lv-HOXD10, cells transduced with positive HOXD10 lentivirus; CCC, cholangiocellular carcinoma.
Five days after transfection, the HOXD10 mRNA and protein expressions in RBE and HCCC-9810 cells were analyzed using RT-PCR and western blotting, respectively. The levels of HOXD10 mRNA and protein in RBE and HCCC-9810 cells transfected with recombinant lentivirus HOXD10 were significantly increased, compared with cells transfected with negative lentivirus HOXD10 and the blank control cells (Fig. 2D and E). These results indicated that a lentivirus-mediated overexpression vector effectively and specifically induced HOXD10 overexpression in RBE and HCCC-9810 cells.
Establishment of RBE and HCCC-9810 cells stably overexpressing HOXD10
In preliminary studies, adequate selection pressure was maintained with 2 µg/ml of puromycin. The expression of GFP and HOXD10 was observed. After the cells were frozen in liquid nitrogen for 6 months and revived monthly, GFP expression was observed in >95% of the lentivirus-transfected RBE and HCCC-9810 cells treated with 2 µg/ml of puromycin. PCR and western blotting showed the stable overexpresion of HOXD10 mRNA and protein, respectively, in the same cells. These results showed that the stably transfected RBE and HCCC-9810 cell lines were successfully established with lentivirus and that the overexpression of HOXD10 lasted ≥6 months. The stably transfected RBE and HCCC-9810 cell lines were defined as lentiviral-HOXD10 (Lv-HOXD10) cells and negative lentiviral-HOXD10 (Ne-CTRL) control cells, respectively, and used in subsequent experiments.
Effect of HOXD10 upregulation on RBE and HCCC-9810 cell growth and proliferation
The viability of the transfected cell lines was detected using an MTT assay (Fig. 2F and G). The sustainable HOXD10 overexpression markedly inhibited the viability of RBE and HCCC-9810 cells in a time-dependent manner. The percentage of viable cells in the RBE and HCCC-9810 cells with Lv-HOXD10 significantly decreased compared with the Ne-CTRL and the blank control (CTRL) cells (P<0.001).
Furthermore, the colony formation analysis revealed that stably enhancing HOXD10 significantly decreased the number of colonies of RBE and HCCC-9810 cells compared with the Ne-CTRL and CTRL groups (Fig. 2H and I, P=0.001). These results suggested that HOXD10 may play a vital-suppressive role in CCC cell growth and proliferation.
G1/S progression arrest and apoptosis promotion of RBE and HCCC-9810 cells induced by upregulation of HOXD10 expression
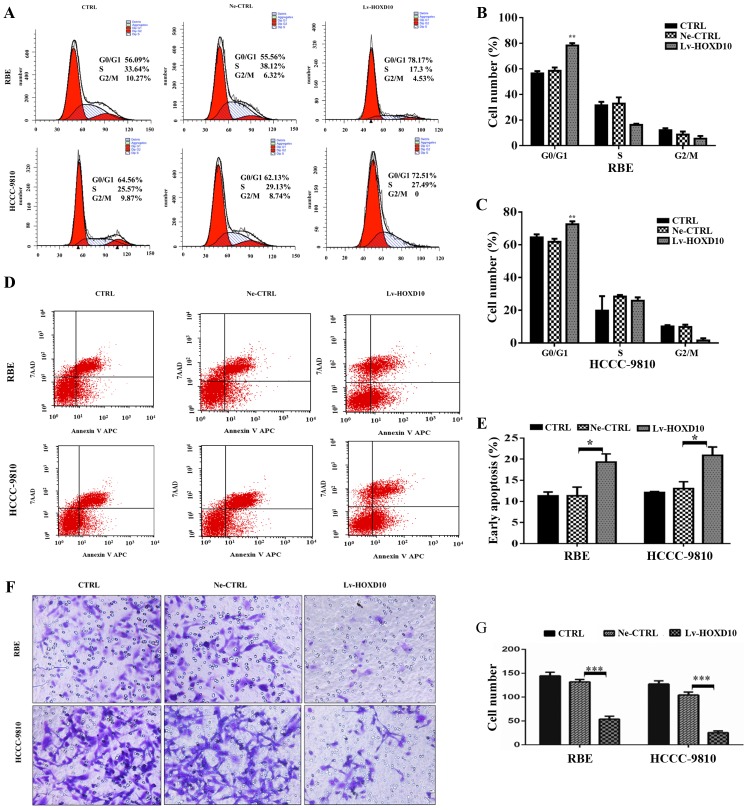
The effect of overexpression of HOXD10 on cell cycle progression was examined via PI staining followed by FACS analysis. As shown in Fig. 3A–C, cells overexpressing HOXD10 accumulated in the G1 phase compared with the control cells and showed a corresponding decrease in cell numbers in the S and G2/M phase. Additionally, the percentages of early cell apoptosis (right lower quadrant) were markedly increased in the two CCC cell lines after transfection with HOXD10 lentivirus (Fig. 3D and E, P<0.05). These results indicated that stable HOXD10 overexpression inhibited RBE and HCCC-9810 cell proliferation by inducing G1/S phase arrest and apoptosis.
Figure 3.
Effects of HOXD10 overexpression on cell cycle progression, apoptosis and cell invasion. (A) G1 phase cell cycle arrest induced by overexpression of HOXD10 in RBE and HCCC-9810 cells. (B and C) A statistical plot of cell cycle distributions of RBE and HCCC-9810 cells in each group, **P=0.003. (D) Population of early apoptosis in RBE and HCCC-9810 cells. (E) Statistical plot of percentages of early apoptosis in RBE and HCCC-9810 cells, *P<0.05. (F) Representative photos of crystal violet-stained RBE and HCCC-9810 cells that invaded through Matrigel of the invasion chamber. (G) Statistical plot of the average number of invaded RBE and HCCC-9810 cells in each group. The graph shows the mean ± SD, ***P<0.001. CTRL, blank control; Ne-CTRL, cells transduced with negative HOXD10 lentivirus; Lv-HOXD10, cells transfected with positive HOXD10 lentivirus; CCC, cholangiocellular carcinoma.
Effect of HOXD10 overexpression on invasive potential of RBE and HCCC-9810 cells
The clinical spread of CCC is characterized by expansion and invasive growth along the lymphangion or perineurium or direct invasion into the liver. HOXD10 has been shown to mediate the invasion of breast, gastric, malignant glioma, ovarian, bladder and hepatocellular cancer (12–15,18,19). Thus, we examined whether HOXD10 was involved in the invasion of RBE and HCCC-9810 cells. A Matrigel invasion Transwell assay was conducted to confirm the effect of HOXD10 on the invasive properties of cholangiocarcinoma cell lines. As expected, stable overexpression of HOXD10 significantly decreased the invasive potential of both the RBE and HCCC-9810 cell lines (Fig. 3F and G, P<0.001), suggesting that HOXD10 was associated with the metastatic capacity of CCC. These results elucidated the aforementioned correlation of lower HOXD10 expression with poor prognosis of CCC patients. Furthermore, the effects of upregulated HOXD10 on the expression of tumor invasion compared to the regulators were examined using RT-PCR and western blot analysis. As shown in Fig. 4A and B, the mRNA and protein expression of MMP2 and MMP9 was significantly inhibited in the Lv-HOXD10 group compared with the Ne-CTRL and CTRL group, while no significant change was observed in the expression of MMP14. These results suggested that overexpression of HOXD10 inhibited the invasion of CCC cells, possibly partly by disturbing the expression of MMP2 and MMP9.
Figure 4.
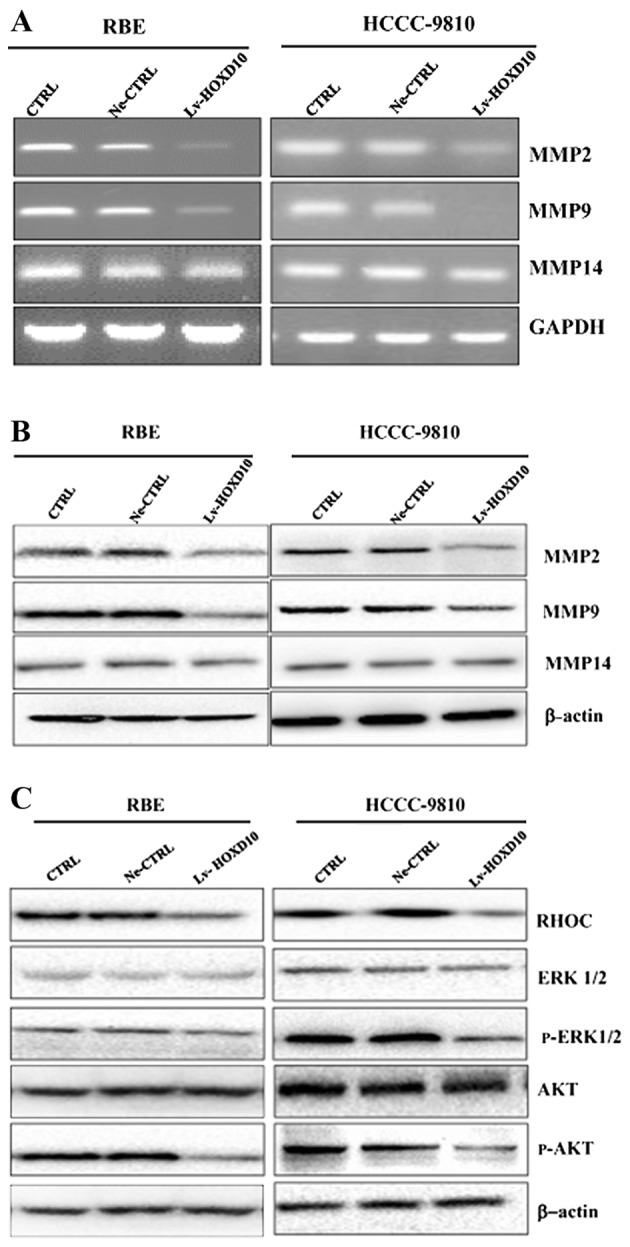
Expression of MMP2, MMP9 and MMP14 and RHOC/AKT/ERK activities in RBE and HCCC-9810 cells. (A and B) Decreased relative mRNA and protein expressions of MMP2, MMP9 and the unchanged expression of MMP14 in RBE and HCCC-9810 cells. (C) Suppressed protein expression levels of RHOC, phosphorylated AKT, and phosphorylated ERK (1/2), and unchanged expression of AKT and ERK (1/2) in RBE and HCCC-9810 cells compared with the control groups. CTRL, blank control; Ne-CTRL, cells transfected with negative HOXD10 lentivirus; Lv-HOXD10, cells transfected with positive HOXD10 lentivirus; CCC, cholangiocellular carcinoma.
Involvement of RHOC/AKT and MAPK pathways in tumor-suppresive effect of HOXD10
Upregulated expression of HOXD10 resulted in the decreased expression of tumor metastatic-associated gene, RHOC. Since we observed a significant correlation between HOXD10 and RHOC expression and CCC patient prognosis, it was suggested that HOXD10 may inhibit CCC cell growth and metastasis by reducing RHOC signaling. As shown in Fig. 4C, the RHOC protein levels were markedly reduced in the Lv-HOXD10 group. As RHOC promotes tumor metastasis in distinct carcinomas by stimulating the activity of a series of kinases including PI3K/AKT and mitogen-activated protein kinase (MAPK) in prostate, head and neck, as well as gastric and breast cancers, we investigated the AKT and ERK phosphorylation states in the two CCC cell lines (18,19,22–24). As expected, the upregulated expression of HOXD10 led to AKT and ERK dephosphorylation, showing that the PI3K/AKT and MAPK pathways were significantly inactivated (Fig. 4C). These results suggested that RHOC was a key negative mediator of HOXD10-dependent CCC development and progression and that the PI3K/AKT and MAPK pathways were involved in this process.
Discussion
CCC is a highly lethal cancer due to lack of early detection, resistance to chemoradiotherapy and high propensity to invade locally and distantly (25). Current therapeutic approaches for highly invasive cholangiocarcinoma are not very effective (26). The low overall survival rate of CCC has led investigators to investigate the mechanisms involved in the characteristic development and progression of CCC to identify effective therapeutic methods.
In the present study, IHC staining for HOXD10 was performed in a large cohort of CCC patients with complete clinicopathological and follow-up data. Our results clearly showed that the positive rate of HOXD10 protein decreased gradually with the increase of histological grade in CCC patients, which suggests HOXD10 is involved in the malignant progression of CCC. These results are consistent with those of previous studies in gastric, ovarian and hepatocellular carcinoma (11,13,27,28). In the univariate analysis of the present study, we found that the prognosis for patients with a positive HOXD10 protein expression was better than that of patients with a negative HOXD10 protein expression. Furthermore, a positive expression of HOXD10 was an independent predictor for longer survival in CCC patients as evidenced by the Kaplan-Meier curves and multivariable Cox proportional hazards regression analysis. Therefore, HOXD10 is a potential therapeutic target for CCC treatment and may also be used as a potential prognostic marker for CCC patients.
To evaluate the biological significance of HOXD10 in CCC pathogenesis, we employed a lentiviral-mediated HOXD10 overexpression vector to effectively and sustainably increase HOXD10 expression in the RBE and HCCC-9810 cholangiocarcinoma cell lines. We found that ectopic overexpression of HOXD10 significantly inhibited RBE and HCCC-9810 cell proliferation, which can be partly attributed to the induction of a G1-phase cell cycle arrest and increased apoptosis in vitro, suggesting that HOXD10 plays an important suppressive role in CCC cells. Additionally, the overexpression of HOXD10 markedly inhibited the cell invasion potential of cholangiocarcinoma cells, at least partly, by downregulating the expression of invasive factors MMP2 and MMP9, but not that of MMP14. Thus, more studies are needed to determine the precise molecular mechanisms by which HOXD10 overexpression inhibits invasion in RBE and HCCC-9810 cell lines.
The results of the present study provide evidence for the tumor-suppressive properties of HOXD10 in CCC cell lines. However, the underlying mechanisms of HOXD10 in the malignant progression of cholangiocarcinoma, particularly the downstream signaling pathways, remain unclear. RHOC belongs to RHO family proteins, which regulate extracellular stimuli-mediated signaling pathways that control numerous intracellular processes, including actin cytoskeletal organization, gene expression, invasion and cell cycle progression (22,29–31). RHOC has been characterized as an oncogenic kinase that promotes cancer cell proliferation, invasion and migration (32–34). High levels of RHOC activity are associated with metastatic lesions and a poor clinical outcome (35,36). RHOC has been identified as a downstream major effector of HOXD10 in breast and gastric cancer cells (18,19). In the present study, the IHC analysis of CCC samples showed that a lower HOXD10 expression was directly associated with a higher expression of RHOC. Additionally, western blot analysis indicated that the overexpression of HOXD10 significantly downregulated RHOC expression in RBE and HCCC-9810 cell lines. Since AKT and MAPK (ERK) kinases are known to be involved in RHOC downstream signaling pathways, we determined AKT and ERK1/2 activities and found that HOXD10/RHOC expression affected AKT and ERK1/2 phosphorylation, suggesting that these signaling pathways are involved in the development of HOXD10-dependent cholangiocarcinoma (15,23,24).
PI3K/AKT/MAPK pathways have been found to play a vital role in cholangiocarcinoma (37–39). Okada et al reported that rapamycin, an inhibitor of the PI3K/AKT/mTOR pathway, effectively inhibited the growth of cholangiocarcinoma cell lines with a decreased AKT expression significantly in a dose-dependent manner (40). Sorafenib, a specific inhibitor of the Ras-Raf-Mek-Erk pathway, was administered in patients with advanced biliary cancers in a phase II clinical study. The median progression-free and overall survival with sorafenib were not inferior to those reported with cytotoxic chemotherapy regimens in biliary cancer patients (41). The aforementioned studies revealed the potential value of targeting the AKT/MAPK (ERK) pathway in CCC treatment. The results of this study provide a further molecular basis for this role.
Although the functions of tumor suppression of HOXD10 have been evaluated, the results of previous studies have also indicated that HOXD10 has complicated roles in certain cell type tumors. For example, the expression levels of HOXD10 were higher in oral squamous cell carcinoma samples compared to those in normal oral mucosa (16). Overexpression of HOXD10 decreased cell invasion but increased proliferation, adhesion and migration in HNSCC cell lines (16). In addition, it was reported that HOXD10 is not involved in the inhibition of the tumor growth of primary breast cancer but markedly suppresses the formation of lung metastases in a mouse mammary tumor model (42). Thus, whether HOXD10 is anti-tumorigenic or pro-tumorigenic depends on the cell context and the type of stimulus (7). In the present study, downregulation or loss of HOXD10 may contribute to CCC malignant progression. However, the precise mechanism of dysregulation of HOXD10 in CCC is not clear. In gastric carcinoma, the downregulation or loss of HOXD10 expression occurs due to promoter methylation (11). By contrast, HOXD10 was negatively regulated by miR-10b via a specific target site within the 3′UTR of HOXD10 at the post-transcriptional level in breast cancer (18). Other regulation mechanisms of HOXD10 include the possible role of long non-coding RNAs such as HOX transcript antisense RNA (HOTAIR) and histone modification (7,43). However, the underlying mechanism of HOXD10 downregulation in CCC remains to be investigated.
In conclusion, HOXD10 plays an inhibitory role in CCC development and progression possibly via the RHOC/AKT/MAPK (ERK) pathways, suggesting that HOXD10 is a potential target for treating CCC.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81172026).
References
- 1.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69:259–270. doi: 10.1016/j.critrevonc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardinale V, Semeraro R, Torrice A, Gatto M, Napoli C, Bragazzi MC, Gentile R, Alvaro D. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol. 2010;2:407–416. doi: 10.4251/wjgo.v2.i11.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cillo C. HOX genes in human cancers. Invasion Metastasis. 1994–1995;14:38–49. [PubMed] [Google Scholar]
- 6.Quinonez SC, Innis JW. Human HOX gene disorders. Mol Genet Metab. 2014;111:4–15. doi: 10.1016/j.ymgme.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 8.Carrio M, Arderiu G, Myers C, Boudreau NJ. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer Res. 2005;65:7177–7185. doi: 10.1158/0008-5472.CAN-04-1717. [DOI] [PubMed] [Google Scholar]
- 9.Wang YF, Li Z, Zhao XH, Zuo XM, Zhang Y, Xiao YH, Li J, Peng ZH. MicroRNA-10b is upregulated and has an invasive role in colorectal cancer through enhanced Rhoc expression. Oncol Rep. 2015;33:1275–1283. doi: 10.3892/or.2015.3737. [DOI] [PubMed] [Google Scholar]
- 10.Vardhini NV, Rao PJ, Murthy PB, Sudhakar G. HOXD10 expression in human breast cancer. Tumour Biol. 2014;35:10855–10860. doi: 10.1007/s13277-014-2324-z. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Chen S, Xue M, Zhong J, Wang X, Gan L, Lam EK, Liu X, Zhang J, Zhou T, et al. Homeobox D10 gene, a candidate tumor suppressor, is downregulated through promoter hypermethylation and associated with gastric carcinogenesis. Mol Med. 2012;18:389–400. doi: 10.2119/molmed.2011.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Ding C, Chen C, Zhang Z, Xiao H, Xie F, Lei L, Chen Y, Mao B, Jiang M, et al. miR-224 promotion of cell migration and invasion by targeting homeobox D 10 gene in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:835–842. doi: 10.1111/jgh.12429. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama I, Shibazaki M, Yashima-Abo A, Miura F, Sugiyama T, Masuda T, Maesawa C. Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int J Oncol. 2013;43:63–71. doi: 10.3892/ijo.2013.1935. [DOI] [PubMed] [Google Scholar]
- 14.Sasayama T, Nishihara M, Kondoh T, Hosoda K, Kohmura E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int J Cancer. 2009;125:1407–1413. doi: 10.1002/ijc.24522. [DOI] [PubMed] [Google Scholar]
- 15.Xiao H, Li H, Yu G, Xiao W, Hu J, Tang K, Zeng J, He W, Zeng G, Ye Z, et al. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol Rep. 2014;31:1832–1838. doi: 10.3892/or.2014.3048. [DOI] [PubMed] [Google Scholar]
- 16.Hakami F, Darda L, Stafford P, Woll P, Lambert DW, Hunter KD. The roles of HOXD10 in the development and progression of head and neck squamous cell carcinoma (HNSCC) Br J Cancer. 2014;111:807–816. doi: 10.1038/bjc.2014.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SBBD; Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th edition. Springer; New York: 2010. pp. 60–62.pp. 66–71. [Google Scholar]
- 18.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Zhu J, Cao H, Ren H, Fang X. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int J Oncol. 2012;40:1553–1560. doi: 10.3892/ijo.2012.1342. [DOI] [PubMed] [Google Scholar]
- 20.Zhao ZH, Tian Y, Yang JP, Zhou J, Chen KS. RhoC, vascular endothelial growth factor and microvascular density in esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:905–912. doi: 10.3748/wjg.v21.i3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gou WF, Zhao Y, Lu H, Yang XF, Xiu YL, Zhao S, Liu JM, Zhu ZT, Sun HZ, Liu YP, et al. The role of RhoC in epithelial-to-mesenchymal transition of ovarian carcinoma cells. BMC Cancer. 2014;14:477. doi: 10.1186/1471-2407-14-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, et al. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68:7613–7620. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu Q, Tang HM, Tan J, Hu X, Wang DW. Expression of RhoC and ROCK-1 and their effects on MAPK and Akt proteins in prostate carcinoma. Zhonghua Zhong Liu Za Zhi. 2011;33:202–206. In Chinese. [PubMed] [Google Scholar]
- 24.Islam M, Datta J, Lang JC, Teknos TN. Downregulation of RhoC by microRNA-138 results in de-activation of FAK, Src and Erk1/2 signaling pathway in head and neck squamous cell carcinoma. Oral Oncol. 2014;50:448–456. doi: 10.1016/j.oraloncology.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathema VB, Na-Bangchang K. Current insights on cholangiocarcinoma research: A brief review. Asian Pac J Cancer Prev. 2015;16:1307–1313. doi: 10.7314/APJCP.2015.16.4.1307. [DOI] [PubMed] [Google Scholar]
- 26.Ulahannan SV, Rahma OE, Duffy AG, Makarova-Rusher OV, Kurtoglu M, Liewehr DJ, Steinberg SM, Greten TF. Identification of active chemotherapy regimens in advanced biliary tract carcinoma: A review of chemotherapy trials in the past two decades. Hepat Oncol. 2015;2:39–50. doi: 10.2217/hep.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao CG, Kong LM, Zhou P, Yang XL, Huang JG, Zhang HL, Lu N. miR-10b is overexpressed in hepatocellular carcinoma and promotes cell proliferation, migration and invasion through RhoC, uPAR and MMPs. J Transl Med. 2014;12:234. doi: 10.1186/s12967-014-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redline RW, Hudock P, MacFee M, Patterson P. Expression of AbdB-type homeobox genes in human tumors. Lab Invest. 1994;71:663–670. [PubMed] [Google Scholar]
- 29.Ruth MC, Xu Y, Maxwell IH, Ahn NG, Norris DA, Shellman YG. RhoC promotes human melanoma invasion in a PI3K/Akt-dependent pathway. J Invest Dermatol. 2006;126:862–868. doi: 10.1038/sj.jid.5700211. [DOI] [PubMed] [Google Scholar]
- 30.Ridley AJ. RhoA, RhoB and RhoC have different roles in cancer cell migration. J Microsc. 2013;251:242–249. doi: 10.1111/jmi.12025. [DOI] [PubMed] [Google Scholar]
- 31.Oxford G, Theodorescu D. Ras superfamily monomeric G proteins in carcinoma cell motility. Cancer Lett. 2003;189:117–128. doi: 10.1016/S0304-3835(02)00510-4. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Tumur Z, Katebzadeh S, Guerra C, Bhushan L, Alkam T, Henson BS. RhoC mediates epidermal growth factor-stimulated migration and invasion in head and neck squamous cell carcinoma. Neoplasia. 2015;17:141–151. doi: 10.1016/j.neo.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen R, Cheng Y, Zhang Y, Li Z, Geng L. RhoC mediates invasion and migration of CaSki cells through the Rho-associated serine-threonine protein kinase 1 signaling pathway. Int J Gynecol Cancer. 2014;24:184–191. doi: 10.1097/IGC.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 35.Bellovin DI, Simpson KJ, Danilov T, Maynard E, Rimm DL, Oettgen P, Mercurio AM. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene. 2006;25:6959–6967. doi: 10.1038/sj.onc.1209682. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Yang LY, Yang ZL, Peng JX, Yang JQ. Elevated expression of autocrine motility factor receptor correlates with overexpression of RhoC and indicates poor prognosis in hepato-cellular carcinoma. Dig Dis Sci. 2007;52:770–775. doi: 10.1007/s10620-006-9479-4. [DOI] [PubMed] [Google Scholar]
- 37.Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21:754–760. doi: 10.1002/jhbp.126. [DOI] [PubMed] [Google Scholar]
- 38.Dokduang H, Juntana S, Techasen A, Namwat N, Yongvanit P, Khuntikeo N, Riggins GJ, Loilome W. Survey of activated kinase proteins reveals potential targets for cholangiocarcinoma treatment. Tumour Biol. 2013;34:3519–3528. doi: 10.1007/s13277-013-0930-9. [DOI] [PubMed] [Google Scholar]
- 39.Leelawat K, Udomchaiprasertkul W, Narong S, Leelawat S. Induction of MKP-1 prevents the cytotoxic effects of PI3K inhibition in hilar cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2010;136:1537–1544. doi: 10.1007/s00432-010-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okada T, Sawada T, Kubota K. Rapamycin inhibits growth of cholangiocarcinoma cells. Hepatogastroenterology. 2009;56:6–10. [PubMed] [Google Scholar]
- 41.El-Khoueiry AB, Rankin CJ, Ben-Josef E, Lenz HJ, Gold PJ, Hamilton RD, Govindarajan R, Eng C, Blanke CD. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30:1646–1651. doi: 10.1007/s10637-011-9719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Cai K, Wang J, Wang X, Cheng K, Shi F, Jiang L, Zhang Y, Dou J. miR-7, inhibited indirectly by lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cells. 2014;32:2858–2868. doi: 10.1002/stem.1795. [DOI] [PubMed] [Google Scholar]