Abstract
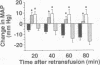
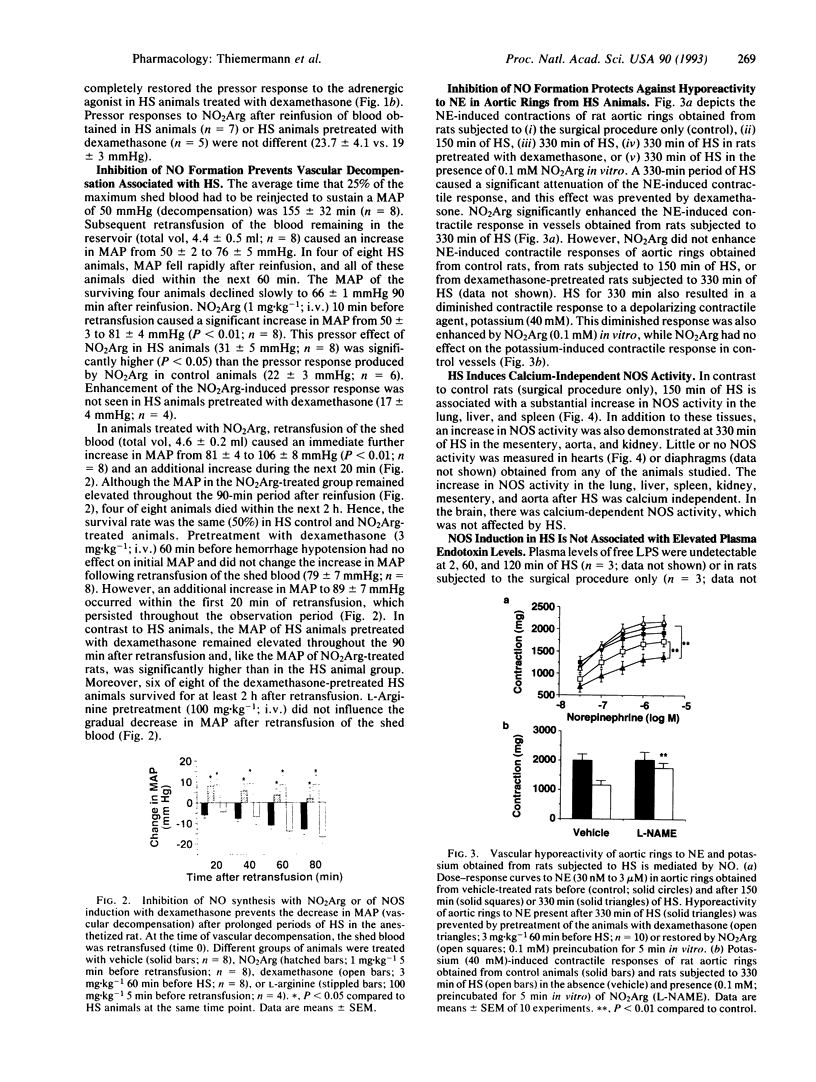
This study investigates the role of nitric oxide (NO) and the induction of a calcium-independent NO synthase (NOS) in development of vascular hyporeactivity to norepinephrine (NE) and vascular decompensation associated with hemorrhagic shock (HS) in the anesthetized rat. HS for 120 min caused a time-dependent reduction of the pressor responses to NE. This hyporeactivity is mediated by an enhanced release of NO by the constitutive NOS, for it was reversed by NG-nitro-L-arginine methyl ester (NO2Arg), an inhibitor of both constitutive and inducible NOS, but it was not prevented by dexamethasone, an inhibitor of NOS induction. Vascular decompensation following prolonged periods of HS was characterized by a failure of control animals to maintain arterial blood pressures despite reinfusion of blood. This progressive decrease in blood pressure is mediated by enhanced formation of NO by the inducible NOS, for it was prevented by NO2Arg or dexamethasone. A strong increase in calcium-independent (inducible) NOS activity was observed in several organs after 150 and 330 min of HS, being most pronounced in lung, liver, and spleen. HS for 330, but not 150, min also caused hyporeactivity of rat aortic rings to vasoconstrictors, which was associated with induction of calcium-independent NOS activity in this tissue. Aortic hyporeactivity was prevented by dexamethasone pretreatment in vivo and reversed by NO2Arg in vitro. HS was not associated with an increase in plasma endotoxin levels, showing that endotoxin does not account for induction of NOS in this model. Thus, excessive NO formation induces vascular hyporeactivity and decompensation in HS, indicating that NOS inhibitors, particularly of the inducible NOS, may improve the therapeutic outcome of patients suffering from HS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayala A., Perrin M. M., Meldrum D. R., Ertel W., Chaudry I. H. Hemorrhage induces an increase in serum TNF which is not associated with elevated levels of endotoxin. Cytokine. 1990 May;2(3):170–174. doi: 10.1016/1043-4666(90)90012-i. [DOI] [PubMed] [Google Scholar]
- Ayala A., Wang P., Ba Z. F., Perrin M. M., Ertel W., Chaudry I. H. Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol. 1991 Jan;260(1 Pt 2):R167–R171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond R. F., Johnson G., 3rd Vascular adrenergic interactions during hemorrhagic shock. Fed Proc. 1985 Feb;44(2):281–289. [PubMed] [Google Scholar]
- Bond R. F., Manley E. S., Jr, Green H. D. Cutaneous and skeletal muscle vascular responses to hemorrhage and irreversible shock. Am J Physiol. 1967 Feb;212(2):488–497. doi: 10.1152/ajplegacy.1967.212.2.488. [DOI] [PubMed] [Google Scholar]
- Bottoms G. D., Gimarc S., Pfeifer C. Plasma concentrations of endotoxin following jugular or portal injections of endotoxin and following gastrointestinal ischemia due to hemorrhage. Circ Shock. 1991 Jan;33(1):1–6. [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- CATCHPOLE B. N., HACKEL D. B., SIMEONE F. A. Coronary and peripheral blood flow in experimental hemorrhagic hypotension treated with 1-nor-epinephrine. Ann Surg. 1955 Sep;142(3):372–380. doi: 10.1097/00000658-195509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Julou-Schaeffer G., Parratt J. R., Stoclet J. C. Incubation with endotoxin activates the L-arginine pathway in vascular tissue. Biochem Biophys Res Commun. 1990 Sep 14;171(2):562–568. doi: 10.1016/0006-291x(90)91183-s. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Botting R. M., Vane J. R. Mediators produced by the endothelial cell. Hypertension. 1988 Dec;12(6):530–548. doi: 10.1161/01.hyp.12.6.530. [DOI] [PubMed] [Google Scholar]
- HARRISON D. C., CHIDSEY C. A., BRAUNWALD E. EFFECT OF HEMORRHAGIC SHOCK ON RELEASE OF NOREPINEPHRINE BY TYRAMINE. Am J Physiol. 1964 Jun;206:1262–1266. doi: 10.1152/ajplegacy.1964.206.6.1262. [DOI] [PubMed] [Google Scholar]
- Harris R. I., Stone P. C., Stuart J. An improved chromogenic substrate endotoxin assay for clinical use. J Clin Pathol. 1983 Oct;36(10):1145–1149. doi: 10.1136/jcp.36.10.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- KOVACH A. G. B., TAKACS L. Response of the vascular system to sympathetic stimuli during shock. Nature. 1953 Mar 7;171(4349):433–434. doi: 10.1038/171433a0. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- MANGER W. M., BOLLMAN J. L., MAHER F. T., BERKSON J. Plasma concentration of epinephrine and norepinephrine in hemorrhagic and anaphylactic shock. Am J Physiol. 1957 Aug;190(2):310–316. doi: 10.1152/ajplegacy.1957.190.2.310. [DOI] [PubMed] [Google Scholar]
- MELLANDER S., LEWIS D. H. EFFECT OF HEMORRHAGIC SHOCK ON THE REACTIVITY OF RESISTANCE AND CAPACITANCE VESSELS AND ON CAPILLARY FILTRATION TRANSFER IN CAT SKELETAL MUSCLE. Circ Res. 1963 Aug;13:105–118. doi: 10.1161/01.res.13.2.105. [DOI] [PubMed] [Google Scholar]
- Miller V. M., Vanhoutte P. M. Endothelium-dependent responses and their potential relevance to the distribution of blood flow during hemorrhagic shock. Resuscitation. 1989 Dec;18(2-3):165–171. doi: 10.1016/0300-9572(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbeck L., Hedqvist P. Blood pressure responses associated with hemorrhagic shock in anesthetized rats. Acta Chir Scand. 1982;148(1):3–8. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Mustafa M., Mester P. A., Mitchell J. A., Hecker M., Vane J. R. Inhibition of the release of endothelium-derived relaxing factor in vitro and in vivo by dipeptides containing NG-nitro-L-arginine. Br J Pharmacol. 1991 Sep;104(1):31–38. doi: 10.1111/j.1476-5381.1991.tb12380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]