Abstract
Acute ablation of T cell antigen receptors (TCRs) in regulatory T cells (Treg cells) impairs the suppressive activity of these cells, even though they retain expression of Foxp3 and CD25. TCR signaling imparts a critical role in the suppressive function of Treg cells.
The development of Foxp3+ regulatory T cells (Treg cells) in the thymus is dependent on signaling via the T cell antigen receptor (TCR)1, but the TCR’s role in the function of Treg cells in the periphery remains elusive. As acute ablation of Treg cells from the normal adult mouse results in the rapid development of a lethal autoimmune syndrome2, it is likely that the suppressor function of Treg cells is constantly operative in vivo, yet evidence for TCR-mediated signaling in this process has been lacking. Levine et al.3 now provide, for the first time, compelling data indicating that continuous expression of the TCR is required for Treg cell suppressor function in vivo.
The major tool used in this study is deletion of the TCR from peripheral Treg cells by administration of tamoxifen to adult mice carrying Foxp3eGFP-Cre-ERT2 (a fusion expressing enhanced green fluorescent protein, Cre recombinase and the estrogen receptor ligand-binding domain protein) that have been crossed to TracFL/WT or TracFL/FL mice, in which one or both alleles of Tcra, specifically the gene segment (Trac) encoding the constant region of the TCR α-chain, were flanked by two loxP sites. A minor population (~20%) of TCR-deficient Treg cells was generated in the heterozygous cross, whereas in mice descended from the homozygous cross the majority (~70%) of Treg cells lacked the TCR. In addition, the authors bred TracFL/FL mice to Foxp3YFP-Cre mice to induce TCR deletion in newly generated Treg cells. In all of these models, most of the Treg cells that lacked the TCR expressed normal amounts of Foxp3 as well as other Treg signature molecules such as CD25, GITR, CD39 and CD73. However, CTLA-4 expression is induced by TCR signaling, as Treg cells with TCR ablation induced by Foxp3YFP-Cre failed to upregulate CTLA-4. Both Ctla4 mRNA and CTLA-4 protein were also reduced when the Tcra gene was deleted by Foxp3eGFP-Cre-ERT2 after tamoxifen treatment, indicating that CTLA-4 expression in Treg cells requires continuous TCR signaling. No evidence that loss of the TCR resulted in loss of Foxp3 expression was seen in fate-mapping studies.
The authors also report that just as in the conventional CD4+ T cell compartment, Treg cells consist of CD44loCD62Lhi ‘naive-like’ and CD44hiCD62Llo ‘effector-like’ populations. The most striking finding in this study was that the total numbers of TCR-negative Treg cells in the two inducible deletion models (Foxp3eGFP-Cre-ERT2 TracFL/WT and Foxp3eGFP-Cre-ERT2 TracFL/FL, of which only the latter resulted in autoimmunity) were essentially normal, but ablation of the TCR resulted in a marked loss of the Treg ‘effector-like’ cell population. On the other hand, Foxp3YFP-Cre-mediated deletion of Tcra prevented the generation of CD44hiCD62Llo ‘effector-like’ Treg cells. This finding was also correlated with decreased proliferation and severe reduction in cell numbers of the TCR-negative Treg cell population. These results are most consistent with the requirement of a TCR signal for the differentiation as well as maintenance of Treg ‘effector-like’ cells (Fig. 1). While reduction of CD44hi Treg cells resulted in generalized activation of conventional CD4+ or CD8+ T cells in Foxp3eGFP-Cre-ERT2 TracFL/FL mice, Foxp3YFP-Cre TracFL/FL mice that completely lacked the CD44hi Treg cell population developed severe autoimmunity and were moribund by day 13 of life. Thus, the suppressive functions of Treg cells are completely dependent on TCR signaling in vivo. Although interleukin 2 (IL-2) is required for the ‘fitness’ of Treg cells4, IL-2 signaling was normal in TCR-deficient Treg cells as measured ex vivo through STAT5 phosphorylation. Thus, IL-2 signaling in Treg cells does not require TCR activation. Furthermore, administration of IL-2 immune complexes did not reduce the activation of conventional CD4+ T cells in the Foxp3eGFP-Cre-ERT2 TracFL/FL mice.
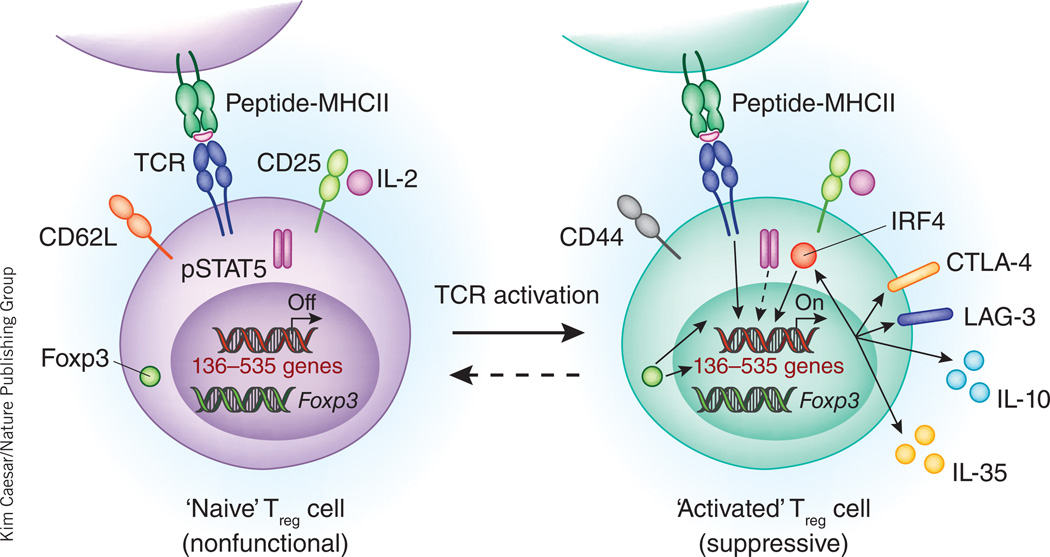
Figure 1.
Treg cells require continuous TCR stimulation to maintain their activated phenotype and suppressive activities in vivo. Upon TCR activation presumably by self-peptide, CD44loCD62Lhi ‘naive’ Treg cells may become CD44hiCD62Llo ‘activated’ Treg cells. Up to 535 genes are induced during this process. Among them, at least 136 genes require continuous TCR stimulation for their expression. The 136 genes encode many important molecules, including LAG-3 and IL-10, which are involved in exerting the suppressive functions of Treg cells. Consequently, inducible ablation of the TCR in Treg cells results in loss of suppressor function and autoimmunity. Induction of these 136 genes by TCR activation requires Foxp3 and is partially reinforced by IRF4-mediated positive feedforward regulation. Although continuous TCR signaling in Treg cells is essential for their suppressive function, it is not required for Foxp3 expression, IL-2 signaling or the expression of many Treg ‘signature’ molecules such as CD25 and GITR. Whether activated Treg cells will return to the naive state upon cessation of TCR signaling is unknown.
Levine et al. also compared gene expression by TCR-sufficient and TCR-deficient Treg cells isolated from Foxp3eGFP-Cre-ERT2 TracFL/FL mice3. Within the ‘effector-like’ Treg cell subset, 155 genes were significantly downregulated in the absence of the TCR, including those encoding transcription factors (NFATc1, c-Rel, IRF4 and Bcl-6) and inhibitory molecules (CD83, CD200, LAG-3, IL-10 and EBI3). Levine et al. raise the possibility that IRF4 has a critical role in Treg ‘effector-like’ cell differentiation downstream from TCR signals3. The transcription factor IRF4 is also highly upregulated by TCR stimulation in effector T cells. In fact, IRF4 plays a critical role in the differentiation of TH2, TH17 and follicular helper T cells (TFH cells) through its interaction with members of the BATF protein family5. In Treg cells, IRF4 interacts with Foxp3 and regulates ~20% of ‘Treg cell–specific’ genes, and specific deletion of Irf4 in Treg cells by Foxp3-Cre results in severe autoimmune lymphoproliferative diseases with uncontrolled TH2 responses6. IRF4 affects the expression of IL-10, ICOS and CCR8, but not CD25, GITR and CLTA-4. However, in the current study inducible deletion of Irf4 in mature Treg cells produced only a very modest effect. Although this small effect size may have resulted from inefficient ablation of IRF4, it is also possible that IRF4 may not be very critical for maintaining the activation status of Treg cells. To fully determine the contribution of an IRF4-mediated positive feedforward regulatory loop in the activation of Treg cells by the TCR, it is necessary to compare the genes regulated by IRF4 with those regulated by TCR activation. Interestingly, the genes that depend on continuous TCR signaling for their expression are largely regulated by Foxp3. Although Foxp3 by itself is not sufficient to induce and/or maintain the expression of these TCR-inducible genes, Foxp3 is needed to collaborate with TCR signaling to promote gene expression in ‘effector-like’ Treg cells.
Several questions raised by this work merit further investigation. Although it is clear from this study that TCR activation is required for the generation of ‘effector-like’ Treg cells with suppressor function and that CD44hi Treg cells with TCR ablation have changed their gene expression pattern, the requirements for TCR signaling for the maintenance of the CD44hiCD62Llo Treg cell population in vivo remain to be further elucidated. Approximately 50% of this subpopulation cycles every 2–3 days in vivo, as measured by Ki67 staining. Yet it is unknown whether TCR-derived signals play any role in this process, which must also include the death of a major proportion of the cycling cells, as absolute numbers of Treg cells remain constant for the life of the animal. In this regard, the cycling of Treg cells in vivo closely resembles the cycling of conventional memory phenotype CD44hiCD4+ T cells7, and the factors that regulate the proliferation of CD44hiCD4+ T cells also remain to be fully characterized. Normal adult thymus does contain a population of CD44hi Treg cells that cycle at a frequency slightly less than that of CD44hi Treg cells in the periphery (M. Holt and E.M.S., unpublished observations). It is thus possible that there are two waves of TCR-induced activation of Treg cells, with the first wave, in the thymus, resulting in partial T cell activation and the second wave, in the periphery, inducing suppressive activity. It will be important to determine whether Treg cells activated in the thymus already express suppression-related genes or whether these genes need to be induced in the periphery by TCR stimulation.
Studies performed in vitro have suggested that the suppressor function of Treg cells requires TCR activation but that, once activated, suppressor function is nonspecific8. Does a similar situation exist in vivo? It also remains unclear whether upon TCR activation, CD44loCD62Lhi ‘naive-like’ Treg cells can turn into CD44hiCD62Llo ‘effector-like’ Treg cells. In the future, it will be important to understand the dynamics between these two states. What is the rate of such transition at steady state? Are the TCR repertoires of these two populations the same, or is the CD44hi CD62Llo ‘effector-like’ population more self-reactive? Can CD44hiCD62Llow ‘effector-like’ Treg cells revert to CD44loCD62Lhi ‘naive-like’ Treg cells or are they terminally differentiated?
An additional issue raised by these studies is how the results might influence the design of therapies to maximize the abundance of Treg cells or, more importantly, their suppressive function in vivo. For example, would blocking the TCR specifically on Treg cells (if possible) impair their function and represent an adjunct to tumor therapy? At present, low-dose IL-2 therapy has been advocated as a potentially safe, effective approach to augmenting Treg cell function in a variety of experimental studies and human clinical trials9. Yet the target of low-dose IL-2 would be the nonsuppressive CD44loCD62Lhi population that expresses large amounts of CD25 (ref. 10). Do the beneficial effects of this therapy depend on simultaneous TCR activation and differentiation of the CD44loCD62Lhi Treg cells to ‘suppressor effector-like’ CD44hiCD62Llo Treg cells? The failure of IL-2 immune complexes to reverse the activation of conventional CD4+ T cells in the present study suggests that this is unlikely to be the mechanism. Conversely, how can we directly augment the numbers and function of the CD44hiCD62Llo ‘effector-like’ Treg cells? Smigiel et al. suggest that their maintenance depends of ICOS-ICOSL signaling10, but this pathway does not appear to mediate their proliferation, as inhibition of ICOS signaling did not reduce the cycling of the CD44hiCD62Llo Treg cells. Lastly, one potential alternative approach for the expansion of ‘effector-like’ Treg cells may be the delivery of costimulation in the absence of an overt TCR signal—for example, the delivery of CD28-mediated costimulation with a CD28 superagonist monoclonal antibody11. It remains to be determined whether this type of stimulation would selectively expand CD44hiCD62Llo Treg cells.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Jinfang Zhu, Email: jfzhu@niaid.nih.gov.
Ethan M Shevach, Email: eshevach@niaid.nih.gov.
References
- 1.Hsieh CS, Lee HM, Lio CW. Nat. Rev. Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Levine AG, Arvey A, Jin W, Rudensky AY. Nat. Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 5.Murphy TL, Tussiwand R, Murphy KM. Nat. Rev. Immunol. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, et al. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes SA, et al. PLoS Biol. 2011;9:e1001171. doi: 10.1371/journal.pbio.1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton AM, Shevach EM. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 9.Churlaud G, et al. Clin. Immunol. 2014;151:114–126. doi: 10.1016/j.clim.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Smigiel KS, et al. J. Exp. Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabares P, et al. Eur. J. Immunol. 2014;44:1225–1236. doi: 10.1002/eji.201343967. [DOI] [PubMed] [Google Scholar]