Abstract
Cytotoxic T-lymphocyte-associated antigen (CTLA-4) is a naturally occurring inhibitor of T-cell costimulation. Monoclonal antibody inhibition of CTLA-4 with ipilimumab blocks this negative regulator of costimulation, promoting T-cell activation and survival, and leads to melanoma regression. Findings of the Vogt-Koyanagi-Harada syndrome, an uveomeningitic syndrome that features neurologic, auditory, ophthalmologic, and cutaneous involvement due to autoimmune targeting of melanocytic antigen, have rarely been described in association with melanoma immunotherapy. We describe a case of Vogt-Koyanagi-Harada (VKH)-like syndrome in a 45-year-old HLA-A02-positive patient with metastatic melanoma treated with ipilimumab. Disruption of immune tolerance by ipilimumab led to melanoma remission while also inciting systemic and ophthalmic autoimmunity towards melanocytic antigen. These observations provide insight into the pathophysiology of the VKH syndrome, as well as the balance between tumor-associated tolerance and autoimmunity.
Keywords: Melanoma, immunotherapy, uveitis, autoimmunity, CTLA-4 antibody, ipilimumab
Introduction
The prognosis for stage IV metastatic melanoma is poor with 5-year survival rates reported between 6-8%.1-2 Chemotherapy, radiation, and surgical therapy, often employed in combination, may result in melanoma regression but is rarely curative. Immunotherapies evaluated include vaccine-based approaches, adoptive transfer of tumor-infiltrative lymphocytes (TIL), and biologic response modifiers.3-4 Ipilimumab (Yervoy, Bristol-Myers Squibb, Princeton, NJ) is a fully human monoclonal antibody biological response modifier directed against cytotoxic Tlymphocyte-associated antigen (CTLA-4), which was approved in 2011 for the treatment of unresectable or metastatic melanoma.5 CTLA-4 is naturally occurring competitive inhibitor of the CD28-B7.1-B7.2 costimulatory signal. Inhibition of CTLA-4 leads to unchecked costimulation and subsequent T-cell activation and survival with activity against tumor antigen.5
A Phase III clinical trial of ipilimumab with or without gp100 peptide vaccination versus gp100 vaccination alone showed improved median survival of 10.0 months with ipilimumab (with or without gp100) vs. 6.4 months with gp100 peptide alone (hazard ratio for death, 0.68; P<0.001).6 Grade 3 or 4 immune-related adverse events occurring from 10-15% include immunemediated enterocolitis, hepatitis, and endocrinopathies. Uveitis, episcleritis and scleritis were rare, occurring in less than 1% of patients. We describe a patient with retinal and choroidal pigment abnormalities, poliosis, diffuse cutaneous vitiligo, headaches, and auditory changes, which were consistent with a Vogt-Koyanagi-Harada (VKH)-like syndrome, which followed successful ipilimumab treatment for stage IV metastatic melanoma. These findings provide insight into the balance between the benefits of disruption of tumor immunotolerance and systemic and ocular autoimmunity.
Case Presentation
A 54 year-old HLA-A02+ woman presented with a subcutaneous multinodular abdominal mass in October 2010. Excisional biopsy of the abdominal mass revealed metastatic malignant melanoma and PET/CT and MRI showed widespread disease with brain, liver, lung, small bowel, adrenal and peritoneal involvement with an unknown primary. The patient reported a remote history of excision of two skin lesions, both of which were thought to be benign. Over the ensuing 3 months the patient underwent 3 cycles of temozolomide and stereotactic radiosurgery to the brain lesions, but progression of the subcutaneous masses, lung, peritoneum, adrenals, liver and brain was observed. She was started on ipilimumab in March 2011 and received ipilimumab (3 mg/kg) every 3 weeks for a total of 4 doses.
A partial response was observed during the first six-months of follow-up and by 1 year following the last ipilimumab infusion, PET/CT scan showed an excellent response to treatment with complete resolution of activity in multiple subcutaneous locations, lung, small bowel, peritoneal right adrenal and liver lesions (Figure 1). Repeat MRI scan showed complete resolution of the brain lesions. However, at 14 months following completion of ipilimumab therapy, a new lesion within the small bowel was observed, prompting surgical resection with no evidence of disease recurrence at 26 months following completion of ipilimumab (34 months from her initial diagnosis of metastatic melanoma). The ileal tumor was BRAF-V600E negative, and Melan-A and HMB-45 (i.e. gp100) positive.
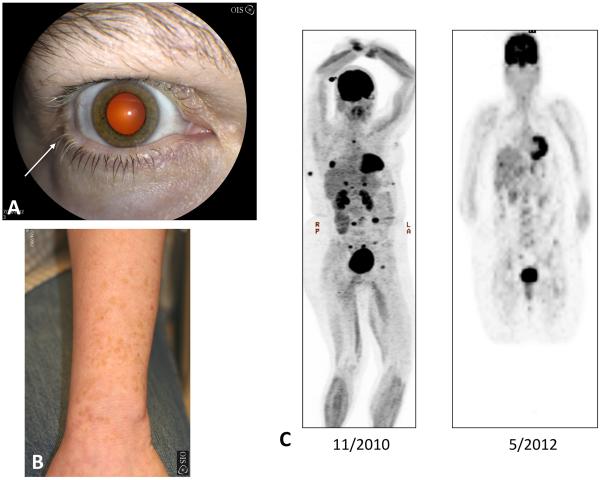
Figure 1. Forearm, external photographs, and maximum intensity projection (MIP) from PET scan.
An external photograph demonstrates vitiligo on the forearm. The darker areas are remnants of the patient's natural skin tone (Figure 1A). External photograph of the right eye shows whitening of the eyelashes (poliosis), which is more pronounced near the lateral canthus (arrow). Note the hypopigmentation of the periorbital skin (Figure 1B). MIP image from PET scan shows multiple foci of metastatic disease (C, left panel) with resolution of metastatic disease activity 18-months later (C, right panel).
Approximately one year following therapy with ipilimumab, the patient presented to an ophthalmologist for complaints of headache, blurry vision, and auditory changes. Specifically, she reported intermittent diffuse headaches, whitening of her eyelashes and difficulty with accommodation and right-sided tinnitus starting in August 2011, 2 months following her last ipilimumab infusion. She reported gradual resolution of her headaches over the ensuing six months and was asymptomatic at the time of her ophthalmologic examination with the exception of mild tinnitus in July 2012, 13 months following ipilimumab therapy. Dermatologic examination revealed vitiligo on the palmar surfaces of her hands and on her forearms. Amongst these areas of cutaneous vitiligo, there were rare, spotty areas of normal skin pigmentation. External examination showed poliosis of her upper and lower eyelashes in both eyes (Figure 1). Visual acuities were 20/20 in both eyes (OU) and intraocular pressures were physiologic at 11 mmHg OU. Slit lamp examination of the anterior segment was otherwise unremarkable without episcleritis, scleritis or anterior chamber cell. Dilated funduscopic examination demonstrated diffuse choroidal hypopigmentation and retinal pigmentary epithelial changes in circular configurations in the macula of both eyes. Funduscopic findings were consistent with the “sunset glow” fundus classically seen in VKH syndrome (Figures 2). Fluoroscein angiography demonstrated bilateral early hypofluorescence and late hyperfluorescence in the areas of retinal pigment epithelial changes, which were consistent with multifocal exudative retinal detachments of the VKH syndrome. Fundus autofluorescence demonstrated areas of hyperautofluorescence and hypoautofluorescence suggestive of multifocal areas of regressed exudative retinal detachment (Figure 3). Full-field electoretinography demonstrated normal rod and cone function in both eyes. Kinetic perimetry showed full visual fields both eyes. The patient was diagnosed with ipilimumab-associated uveitis, which met the diagnostic criteria for VKH syndrome.7 Because there was no active disease and vision was normal, routine surveillance for uveitis was recommended and corticosteroid therapy was deferred.
Figure 2. Fundus photograph montage both eyes.
Color fundus photograph montage of the right demonstrating the “sunset glow” fundus as seen in convalescent or chronic VKH syndrome. There is diffuse choroidal depigmentation with patches of remaining pigmentation seen within the inferior retina. The temporal and superotemporal aspects of the fundus are devoid of choroidal pigmentation (Figure 2A). Color fundus photograph montage of the left eye demonstrating “sunset-glow fundus” with macular retinal pigment epithelial mottling. There area patchy areas of residual choroidal pigmentation, which bear resemblance to the patches of pigmentation remaining on the patient's skin, which demonstrates diffuse cutaneous vitiligo (Figure 2B).
Figure 3. Fundus photography, autofluorescence and fluorescein angiography of left eye.
Fundus photograph of the left eye highlights retinal pigment epithelial changes indicative of prior underlying choroidal inflammation overlying a backdrop of choroidal depigmentation (Figure 3A). Fundus autofluorescence image, which highlights outer retinal and retinal pigment epithelial injury, shows multiple ring-shaped areas of hyperautofluoresence (white areas, yellow arrows) with adjacent hypoautoflouresence (dark areas). These areas of abnormal autofluorescence correspond to areas of injured retinal pigment epithelium and outer retina from regressed subretinal fluid. Fluid accumulates in the subretinal space and damages the retinal pigment epithelium and outer retina due to underlying choroidal inflammation, which is commonly observed in acute Vogt-Koyanagi-Harada syndrome and sympathetic ophthalmia (Figure 3B). A fluorescein angiogram of the left eye (arteriovenous phase) demonstrates reticular areas of hyperfluorescence with staining in areas of retinal pigment epithelial mottling (Figure 3C). Fluorescein angiography (late recirculation phase) shows hyperfluorescence in a ring-like configuration, which is indicative of prior exudative subretinal fluid, which has resolved (Figure 3D).
Discussion
Robinson et al first reported two patients who developed anterior uveitis, vitritis, and papillitis following anti-CTLA-4 monoclonal antibody (mAb) in conjunction with gp100 tumor vaccine for stage IV metastatic melanoma.8 Because gp100 vaccines had been previously administered with IL-2 therapy without any occurrence of uveitis, the role of CTLA-4 blockade was implicated in these initial reports of uveitis. Besides this report, Wong et al described bilateral vitritis, choroidits, and serous retinal detachments two weeks after the initiation of ipilimumab in the context of a phase I trial, prompting treatment with high-dose systemic corticosteroids. Vitiligo and poliosis ensued later and was suggestive of the active and chronic phases of VKH syndrome.9
In a Phase III clinical trial of 676 patients treated with ipilimumab, ophthalmic inflammatory conditions were rare with uveitis, episcleritis, and scleritis occurring in less than 1% of patients.6, Topical corticosteroids have been recommended for patients with uveitis.10 It is further notable that several reports have correlated autoimmune manifestations with clinical benefit in patients treated with ipilimumab and other melanoma immunotherapies.11-13 In an early dose escalation trial of 56 patients treated with anti-CTLA-4 monoclonal antibody, objective tumor regression was correlated with the development of grade 3/4 autoimune toxicities.11 Later evaluation of this correlation in Phase II studies demonstrated similar findings with a greater proportion of patients with grade 2 or greater immune-related adverse events demonstrating melanoma disease control.12-13
Our patient presented with diffuse choroidal hypopigmentation, poliosis, vitiligo and signs of prior exudative retinal detachment, which was diagnosed approximately 13 months following completion of induction ipilimumab. Interestingly, she did not demonstrate the exudative retinal detachments, optic disc edema, and severe vitritis, which are typical of the acute VKH syndrome and instead, showed progressive hypopigmentation consistent with the chronic VKH features. Her chronic features were remarkably similar to a prior patient who received immunotherapy with ex vivo expanded, autologous, tumor-reactive, tumor infiltrating lymphocyte (TIL) accompanied by interleukin-2.14 Dudley et al previously described 5 patients out of 35 treated with TIL plus IL-2 that developed mild anterior uveitis responsive to topical steroids.15
VKH is an idiopathic inflammatory disease syndrome characterized by chronic, diffuse granulomatous panuveitis often with associated neurological, integumentary, and auditory manifestations.7 Ophthalmic manifestations are divided into early VKH disease (i.e. choroidal thickening, serous retinal detachments, or characteristic findings on fluoroscein angiography or ultrasonography) and late VKH disease (i.e. retinal pigment changes or the sunset-glow fundus as seen in our patient). Neurologic or auditory symptoms include headache, meningimus and tinnitus. Skin and integumentary findings include alopecia, poliosis, and vitiligo.7 The diagnosis of VKH also requires the absence of prior ocular trauma or surgery and the exclusion of another ocular disease based on clinical or laboratory evidence.
The pathogenesis of VKH involves CD4+ and CD8+ cells targeting of melanocytic antigens, and an association exists between VKH and HLA-DR4. CD4+ T-cell clones harvested from HLA-DR4+ VKH patients produce interferon gamma in response to gp100 and tyrosinase antigens, while CD8+ T-cells from VKH patients can lyse melanoma cells.16 Interestingly, sympathetic ophthalmia, a closely related ocular inflammatory disease, also features diffuse, bilateral granulomatous panuveitis, which is thought to be mediated by ocular melanocytic antigen exposure following accidental penetrating trauma or ocular surgery. One prior study showed that T-cell clones harvested from patients with VKH and one patient with sympathetic ophthalmia were able to lyse melanocytes when pulsed with HLA-A2-binding MART-1 peptide, but not HLA-A2-binding pmel-17 (gp100) or tyrosinase peptide, in a HLA-A2-restricted manner.17 These findings suggest similar immunopathogenic mechanisms in VKH and sympathetic ophthalmia given the prior findings of autoimmunity triggered against melanocyte antigen. In the phase III trial of ipilimumab for metastatic melanoma, HLA-A0201 was a criterion for entry because two of the arms contained HLA-A0201-restricted gp100 vaccination. However, a recent study of pooled data from phase II and phase III showed that immune-related adverse events associated with ipilimumab occurred at similar frequencies regardless of HLA-A0201 status.18 While it is not clear whether the HLA-A2 positive status of our patient may have made her more prone to develop the VKH-like syndrome, prior reports have described the positive association of HLA-A2 and HLA-DR4 haplotypes in association with VKH.17,19
Although our patient had no antecedent history of trauma, her condition also demonstrated several clinical features of sympathetic ophthalmia. Several months after initiating ipilimumab, the patient expressed difficulty with accommodation, which is thought to be the first sign of sympathetic ophthalmia. The diffuse choroidal hypopigmentation or “sunset-glow” fundus, was observed at long-term follow-up and may be observed in both sympathetic ophthalmia and VKH.
In summary, our patient demonstrated that ipilimumab may trigger a VKH-like uveitis syndrome whose phenotype may cause mild ocular symptoms despite the development of systemic findings. In our patient, these findings were correlated temporally with melanoma regression. The breakdown of immune tolerance via CTLA-4 inhibition is likely involved in the pathogenesis of VKH. Oncologists should be aware of this spectrum of ophthalmic and systemic findings and consider ophthalmologic consultation in patients presenting with disease features either in isolation or over time as the VKH syndrome develops.
Acknowledgments
Competing Interests and Funding Sources
This work was supported in part by an unrestricted departmental grant from Research to Prevent Blindness (New York, NY) to the Emory Eye Center and an NEI Core Grant for Vision Research (P30 EY 006360). DHL has served on medical advisory boards and conducted clinical trials with Bristol-Meyers-Squibb.
References
- 1.Neuman HB, Patel A, Hanlon C, et al. Stage-IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol. 2007;14:2847–53. doi: 10.1245/s10434-007-9448-y. [DOI] [PubMed] [Google Scholar]
- 2.Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193–201. [PubMed] [Google Scholar]
- 3.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–3. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Hodi SF, Weber JD, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N.Y. Acad. Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada Disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–652. doi: 10.1016/s0002-9394(01)00925-4. [DOI] [PubMed] [Google Scholar]
- 8.Robinson MR, Chi-Chao C, Yang CJ, et al. Cytotoxic T lympocyte-associated antigen 4 blockade in patients with metastatic melanoma. J Immunother. 2014;27:478–9. doi: 10.1097/00002371-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Wong RK, Lee JK, Huang JJ. Bilateral drug (ipilimumab)-induced vitritis, choroiditis, and serous retinal detachments suggestive of Vogt-Koyanagi-Harada syndrome. Retina Cases & Brief Reports. 2012;6(4):423–6. doi: 10.1097/ICB.0b013e31824f7130. Fall. [DOI] [PubMed] [Google Scholar]
- 10.Ipilimumab (Yervoy ®) package insert Bristol-Myers Squibb. 2011.
- 11.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005 Sep 1;23(25):6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarnaik AA, Yu B, Yu D, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011 Feb 15;17(4):896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutzky J, Wolchok J, Hamid O, et al. Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol. 2009;27(15S) Abstract 9034. [Google Scholar]
- 14.Yeh S, Karne NK, Kerkar SP, et al. Ocular and systemic autoimmunity following successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma Ophthalmology. 2009;116:981–9. doi: 10.1016/j.ophtha.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley ME, Wunderlich JR, Yang JC, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:136–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugita S, Takase H, Taguchi C, et al. Ocular infiltrating CD4+ T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Invest Ophthalmol Vis Sci. 2006;47(6):2547–54. doi: 10.1167/iovs.05-1547. [DOI] [PubMed] [Google Scholar]
- 17.Sugita S, Sagawa K, Mochizuki M, et al. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with Vogt-Koyanagi-Harada disease. Int Immunol. 1996;8(5):799–803. doi: 10.1093/intimm/8.5.799. [DOI] [PubMed] [Google Scholar]
- 18.Wolchok JD, Weber JS, Hamid O, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010 Oct 20;10:9. [PMC free article] [PubMed] [Google Scholar]
- 19.Davis JL, Mittal KK, Freidlin V, et al. HLA associations and ancestry in Vogt-Koyanagi-Harada disease and sympathetic ophthalmia. Ophthalmology. 1990;97(9):1137–42. doi: 10.1016/s0161-6420(90)32446-6. [DOI] [PubMed] [Google Scholar]