Abstract
Anthracnose caused by Colletotrichum gloeosporioides has been destructive during pepper fruit production in outdoor fields in Korea. In vitro antifungal activities of 15 different plant essential oils or its components were evaluated during conidial germination and mycelial growth of C. gloeosporioides. In vitro conidial germination was most drastically inhibited by vapour treatments with carvacrol, cinnamon oil, trans-cinnamaldehyde, citral, p-cymene and linalool. Inhibition of the mycelial growth by indirect vapour treatment with essential oils was also demonstrated compared with untreated control. Carvacrol, cinnamon oil, trans-cinnamaldehyde, citral and eugenol were among the most inhibitory plant essential oils by the indirect antifungal efficacies. Plant protection efficacies of the plant essential oils were demonstrated by reduced lesion diameter on the C. gloeosporioides-inoculated immature green pepper fruits compared to the inoculated control fruits without any plant essential oil treatment. In planta test showed that all plant essential oils tested in this study demonstrated plant protection efficacies against pepper fruit anthracnose with similar levels. Thus, application of different plant essential oils can be used for eco-friendly disease management of anthracnose during pepper fruit production.
Keywords: Colletotrichum gloeosporioides, disease control, pepper anthracnose, plant essential oil, volatile
Anthracnose, one of the most destructive diseases during pepper fruit production, is caused by several different Colletotrichum spp. including C. acutatum and C. gloeosporioides in Korea (Park and Kim, 1992). Progressive lesion development on the C. gloeosporioides-infected pepper fruits was highly dependent on elevated temperature, higher relative humidity and prolonged free water period (Park and Kim, 1994). Longer wetness duration was also one of the factors to increase disease severity of pepper seedlings infected by Colletotrichum coccodes (Hong and Hwang, 1998). It was reported that disease incidence of pepper fruit anthracnose drastically increased in open pepper fields in Kyungbuk province of Korea from late August to mid September, and it is well matched with hot, rainy and windy seasons of the region (Kwon and Lee, 1999). Mechanical wounding accelerated anthracnose occurrence on pepper fruits, and it seems to be done by removed cuticular wax layers on the wounded fruits (Kim et al., 1999). Wounding and conidal dissemination on pepper fruits under heavy rain splash and windy environments have been supposed as critical reasons for drastic increase of pepper anthracnose in open pepper fields of Korea.
Rain-proofed fields for planting were suggested to protect pepper fruits against anthracnose occurring rainy period-dependently (Jee et al., 2010; Kwon and Lee, 1999). Recently, genetic regulations of disease resistance for resistant cultivar breeding have been investigated in alfalfa, common bean and strawberry to anthracnose caused by C. trifolii, C. linthemuthianum and C. gloeosporioides, respectively (Ferreira et al., 2012; Osorio et al., 2014; Yang et al., 2007). However, limited genetic resources are available for anthracnose resistance of pepper plants (Kim et al., 2007, 2010; Mahasuk et al., 2009). This is the reason why it is not easy to find commercialized anthracnose-resistant cultivars of different crops worldwide yet.
A variety of chemical pesticides have been suggested to control anthracnose in many crops such as banana, citrus, mango and strawberry (Freeman et al., 1997; Khan et al., 2001; Ojeda et al., 2012; Peres et al., 2004). Although in vitro antifungal activities of many fungicides were found against Colletotrichum spp. isolated from infected pepper fruits, efficacies of the fungicides for reducing anthracnose of the chili pepper fruits have been rarely investigated (Kim et al., 2003, 2004, 2005). Seventeen fungicides were registered commercially to apply to pepper anthracnose control for farmers in South Korea. Six fungicides containing effective ingredients of azoxystrobin, copper hydroxide, difenoconazole, famoxadone + cymoxanil, fludioxonil + cyprodinil and mancozeb, increased marketable yields of bell pepper fruits (Harp et al., 2014). Disease-forecasting system was designed based on leaf wetness duration and hourly temperature during wet cropping season to establish appropriate fungicide spraying schedule for pepper anthracnose (Ahn et al., 2008). However, occurrence of fungicide resistance in several fungal species also has been concerned in Colletotrichum spp. as well, and these urged us to find more eco-friendly and sustainable alternatives (Avila-Adame et al., 2003; Chung et al., 2006; Peres et al., 2004). Coating with edible composites was suggested to reduce banana anthracnose incidence during postharvest transportation and storage (Maqbool et al., 2010b). Application of chitosan-loaded nano-emulsions delayed onset of anthracnose on banana, papaya and dragon fruits during cold storage (Zahid et al., 2012). A variety of eco-friendly methods including agricultural organic materials, nano-particles and antagonistic bacteria have been applied to mitigate pepper anthracnose (Kim and Yun, 2011; Lamsal et al., 2011; Park et al., 2012; Sang et al., 2013).
Plant disease management with plant essential oils has been applied as one of the eco-friendly controls (Isman, 2000; Koul et al., 2008). A huge number of plant essential oils showed different levels of antimicrobial efficacies to various ranges of plant fungal and bacterial pathogens, and they efficiently reduced the major diseases in crops. Colletotrichum spp. infecting diverse economically important crops have been managed by plant essential oils and their individual components. In vitro mycelial growth of C. musae responsible for anthracnose peel blemishes of banana fruits was inhibited by cinnamon oil and clove oil (Ranasinghe et al., 2002). In vitro conidial germination of C. coccodes and lesion areas on infected tomato fruits were arrested by origanum oil treatment (Tzortzakis, 2010). C. gloeosporioides was mainly destructive to subtropical fruit vegetables such as avocado, mango and yellow passion fruit, and application of plant essential oils could decrease the anthracnose incidence on these fruits (Abd-Alla and Haggag, 2013; Anaruma et al., 2010; Sellamuthu et al., 2013a, 2013b). Various chemical and biological controls for pepper anthracnose reduction have been tried so far in Korea. However, efficacies of plant essential oils in the light of eco-friendly controls of pepper anthracnose control were not investigated.
In this study, in vitro antifungal activities of 15 plant essential oils or its components were evaluated during the conidal germination and mycelial growth of C. gloeosporioides. Anthracnose lesion reductions by application of the plant essential oils were also investigated using detached immature green pepper fruits.
Materials and Methods
Fungal culture
Colletotrichum gloeosporioides isolate 07-067 originated from infected pepper fruit was provided by Dr. Kyung-Sook Han (National Institute of Horticultural & Herbal Science, Rural Development Administration, Korea). The fungus was maintained on 1/2 potato dextrose agar (PDA) media at 25°C. Conidial suspension of the C. gloeosporioides was obtained from 10 day-old culture at 25°C by adding the sterile distilled water.
Preparation of paper discs containing plant essential oils
Fifteen plant essential oils and individual components used in this study were carvacrol, cinnamon oil, 1,8-cineole, trans-cinnamaldehyde, citral, clove oil, p-cymene, eugenol, geraniol, (±)-limonene, linalool, menthone, (1R)-(+)-α-pinene, (1S)-(−)-α-pinene and β-pinene. Cinnamon oil and clove oil were purchased from Sigma-Aldrich Co., LLC. Other 13 plant essential oils or individual components were purchased from Tokyo Chemical Industry Co., LTD. Each plant essential oil concentrate was diluted to 20% (v/v) solution in 95% ethanol to easily handle. Each 10 μl, 25 μl and 40 μl of the diluted plant essential oil solution was dropped onto sterile paper disc (8 mm in diameter) wetted with 30 μl, 15 μl and 0 μl of 95% ethanol to make each paper disc contain 2 μl, 5 μl and 8 μl of concentrates of plant essential oils, respectively. Forty μl of 95% ethanol was dropped onto paper disc as an untreated control. The paper discs were air-dried for 20 min at room temperature under sterile conditions to evaporate ethanol until used.
In vitro inhibition of conidial germination by volatiles of plant essential oils
Conidial suspension was adjusted to 2×105 conidia/ml using haemacytometer. Four drops of the conidial suspension (20 μl for each drop) were placed on glass slides without cover glass on the three-layered sterile distilled water-saturated gauze in plastic boxes (13 cm × 9 cm × 4.5 cm). Each paper disc was put in aluminum foil dish (2 cm in diameter) not to be wet by the water, and the aluminum foil dishes carrying paper disc was transferred into the plastic box. The plastic box was tightly covered and incubated at 25°C for 9 h under dark condition. After incubation, the conidia were stained with 1μl of lactophenol-trypan blue solution (0.05% trypan blue in phenol 20 ml, lactic acid 20 ml, glycerol 40 ml and distilled water 20 ml) and observed with light microscope to count number of germinated conidia.
In vitro fungal mycelial growth inhibition by volatile treatment with plant essential oils
For volatile effect of plant essential oils, mycelial agar plug (5 mm in diameter) was cut from the growing edge of the fungal culture, and inoculated at the center of the PDA medium. Paper disc containing plant essential oil was attached to the center inside plate cover. After covering, the plate was incubated upside down at 25°C, and the fungal colony diameter was measured at 10 d after inoculation.
Anthracnose suppression efficacies of indirect volatile treatments with plant essential oils on pepper fruits
Immature green pepper fruits (Capsicum annuum L. cv. Nockwang) were harvested from pepper plants grown in plastic house at Jinju, Gyeongnam province, Korea in April. Four pepper fruits were placed on the three-layered sterile water-saturated gauze in the plastic boxes same-sized as described above, and four drops of the conidial suspension (2×105 conidia/ml, 15 μl for each drop) was inoculated on the pin-wounded (1 mm in depth) pepper fruit surface. Paper disc in the aluminum foil dish was also placed into the plastic box. After covering, the inoculated pepper fruits were incubated at 25°C for 12 d for disease development, and then lesion diameter was measured. Four drops of the conidial suspension per pepper fruit were inoculated and four pepper fruits for each plant essential oil were used.
Statistical analyses
All experiments were conducted in a completely randomized block design with four replications. Four discs were prepared for repeated experiments per each biological replication. Four individual experiments were performed for in vitro and in planta assays. Data were subjected to analysis of variance using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) and means were separated by least significant difference (LSD) test at P<0.05.
Results
Volatile inhibitory effect of plant essential oils on in vitro conidial germination
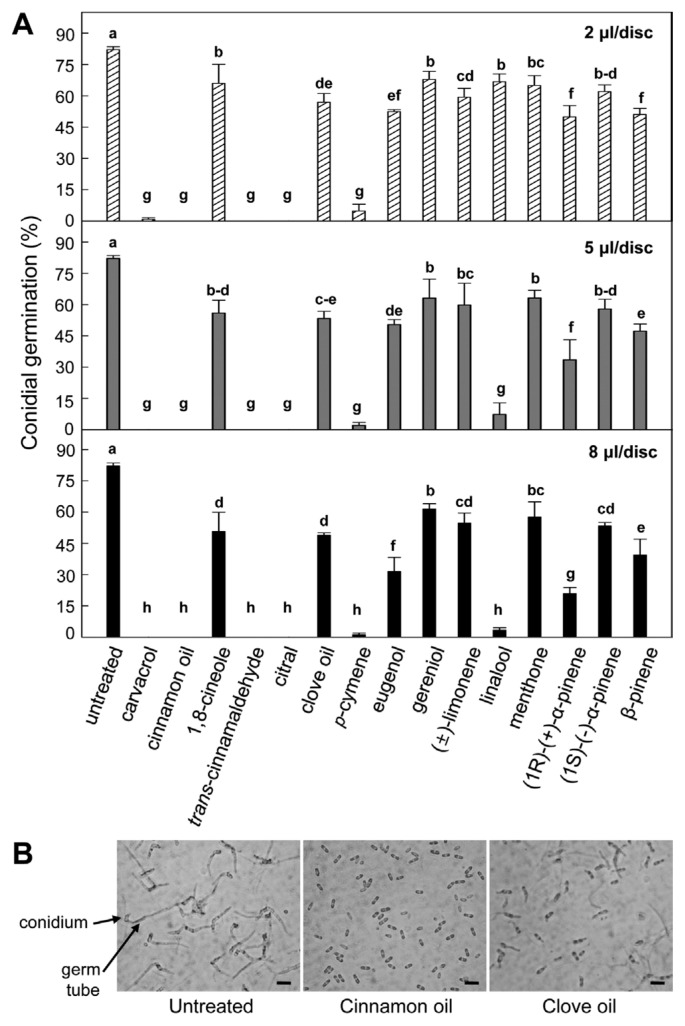
ca. 82.1% of the conidia were germinated on the glass slides in untreated control, and all 15 plant essential oils evaluated exerted volatile antifungal activities with different levels during the conidial germination (Fig. 1). No germination was found in the conidia treated with 2 μl/disc of five essential oils, carvacrol, cinnamon oil, trans-cinnamaldehyde, citral and p-cymene indicating that these are most effective to suppress conidial germination. Treatment with 2 μl/disc of linalool slightly decreased to ca. 66.7%, but increased doses of 5 and 8 μl/disc dramatically suppressed the conidial germination to ca. 7.3% and 3.4%, respectively (Table 1). (1R)-(+)-α-Pinene, β-pinene and eugenol showed moderate anti-germination activities. Increasing doses from 2 to 8 μl/disc of the (1R)-(+)-α-pinene, eugenol and β-pinene gradually lowered the germination, indicating ca. 49.9, 33.5 and 20.8% for (1R)-(+)-α-pinene, ca. 51.0, 47.3 and 39.5% for β-pinene and ca. 52.4, 50.4 and 31.5% for eugenol. 1,8-Cineole, clove oil, geraniol, (±)-limonene, menthone and (1S)-(−)-α-pinene have relatively lower antifungal activities showing that more than ca. 50% of the conidia were germinated by highest dose of 8 μl/disc.
Fig. 1.
Effect of different plant essential oils on in vitro conidial germination of Collectotrichum gloeosporioides. (A) Relative conidial germination of C. gloeosporioides treated with different plant essential oils on glass slide. Values presented are means and error bars represent the standard errors of the means of four independent experimental replications. Means followed by the same letter are not significantly different at 5% level by least significant difference test. Different essential oil doses were applied 8μl/disc demonstrated in upper (2 μl/disc), middle (5 μl/disc) and bottom (8 μl/disc) graphs. (B) Conidial germination of C. gloeosporioides treated with 8 μl/disc of cinnamon oil and clove oil. Conidial germination was observed under light microscope. Photos were taken at 9 h after treatment. Scale bar represents 20 μm.
Table 1.
Dose-dependent activities of different plant essential oils and their components against conidial germination and mycelial growth of Colletotrichum gloeosporioides, and anthracnose lesion development on inoculated pepper fruits
| Plant essential oils | Dose (μl/disc) | Conidial germination (%) | Colony diameter (mm)z | Lesion diameter (mm) |
|---|---|---|---|---|
| Carvacrol | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 0.6 | 0.0 | 10.9 | |
| 5 | 0.0 | 0.0 | 10.7 | |
| 8 | 0.0 | 0.0 | 9.1 | |
|
| ||||
| LSD0.05 y | 1.3 | 2.0 | 1.6 | |
|
| ||||
| Cinnamon oil | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 0.0 | 33.4 | 10.5 | |
| 5 | 0.0 | 10.8 | 8.7 | |
| 8 | 0.0 | 1.6 | 9.6 | |
|
| ||||
| LSD0.05 | 1.1 | 6.1 | 2.5 | |
|
| ||||
| 1,8-Cineole | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 66.0 | 52.0 | 10.5 | |
| 5 | 56.0 | 48.6 | 10.4 | |
| 8 | 50.7 | 46.2 | 9.6 | |
|
| ||||
| LSD0.05 | 10.3 | 6.9 | 1.5 | |
|
| ||||
| trans-Cinnamldehyde | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 0.0 | 37.0 | 10.3 | |
| 5 | 0.0 | 23.0 | 8.9 | |
| 8 | 0.0 | 9.3 | 7.5 | |
|
| ||||
| LSD0.05 | 1.1 | 6.7 | 3.3 | |
|
| ||||
| Citral | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 0.0 | 41.1 | 10.3 | |
| 5 | 0.0 | 19.5 | 10.5 | |
| 8 | 0.0 | 4.0 | 10.1 | |
|
| ||||
| LSD0.05 | 1.1 | 3.4 | 1.5 | |
|
| ||||
| Clove oil | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 56.9 | 26.3 | 9.9 | |
| 5 | 53.4 | 15.1 | 9.1 | |
| 8 | 48.9 | 8.5 | 8.2 | |
|
| ||||
| LSD0.05 | 4.1 | 7.5 | 1.9 | |
|
| ||||
| p-Cymene | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 4.8 | 49.8 | 9.1 | |
| 5 | 2.0 | 49.5 | 8.6 | |
| 8 | 1.2 | 44.4 | 8.4 | |
|
| ||||
| LSD0.05 | 3.4 | 5.2 | 2.5 | |
|
| ||||
| Eugenol | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 52.4 | 24.5 | 10.1 | |
| 5 | 50.4 | 8.0 | 8.6 | |
| 8 | 31.5 | 5.1 | 8.0 | |
|
| ||||
| LSD0.05 | 6.0 | 5.5 | 1.7 | |
|
| ||||
| Geraniol | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 67.9 | 43.1 | 8.9 | |
| 5 | 63.1 | 32.4 | 8.7 | |
| 8 | 61.5 | 24.6 | 7.7 | |
|
| ||||
| LSD0.05 | 8.7 | 4.0 | 1.7 | |
|
| ||||
| Limonene | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 59.4 | 48.8 | 8.9 | |
| 5 | 59.9 | 45.8 | 9.5 | |
| 8 | 54.8 | 45.4 | 8.6 | |
|
| ||||
| LSD0.05 | 7.4 | 2.9 | 1.6 | |
|
| ||||
| Linalool | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 66.7 | 46.6 | 9.6 | |
| 5 | 7.3 | 42.4 | 8.6 | |
| 8 | 3.4 | 35.6 | 8.3 | |
|
| ||||
| LSD0.05 | 5.3 | 2.4 | 1.7 | |
|
| ||||
| Menthone | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 65.0 | 47.7 | 9.3 | |
| 5 | 63.2 | 45.9 | 8.9 | |
| 8 | 57.7 | 44.8 | 9.0 | |
|
| ||||
| LSD0.05 | 7.9 | 4.0 | 1.5 | |
|
| ||||
| (1R)-(+)-α-Pinene | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 49.9 | 48.8 | 9.8 | |
| 5 | 33.5 | 47.6 | 8.9 | |
| 8 | 20.8 | 43.3 | 8.5 | |
|
| ||||
| LSD0.05 | 9.9 | 6.2 | 1.7 | |
|
| ||||
| (1S)-(−)-α-Pinene | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 62.0 | 48.8 | 10.1 | |
| 5 | 58.0 | 48.2 | 9.2 | |
| 8 | 53.3 | 47.4 | 8.2 | |
|
| ||||
| LSD0.05 | 5.0 | 2.2 | 1.7 | |
|
| ||||
| (1S)-(−)-β-Pinene | 0 | 82.1 | 51.8 | 13.0 |
| 2 | 51.0 | 47.4 | 9.2 | |
| 5 | 47.3 | 48.1 | 8.6 | |
| 8 | 39.5 | 47.2 | 8.1 | |
|
| ||||
| LSD0.05 | 6.8 | 2.3 | 1.5 | |
diameter of fungal colony formed on PDA media treated with volatile activity of plant essential oils
means are not significantly different using LSD at 5%
nd: not determined
ns: not significant
Volatile effect of plant essential oils on in vitro mycelial growth
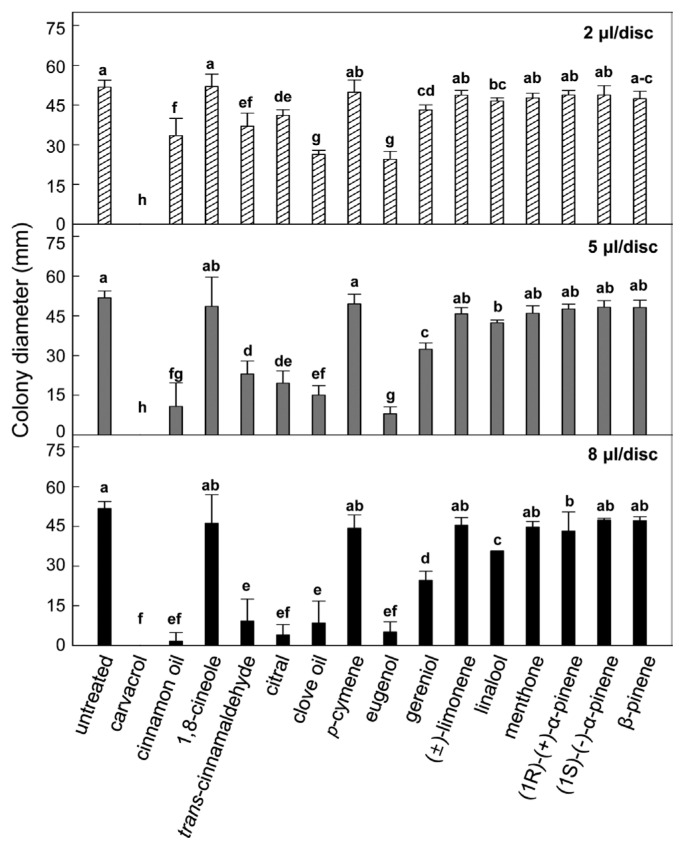
Various plant essential oils reduced colony diameter of the fungus by different volatile antifungal activities (Fig. 2). Without plant essential oil treatment, the fungal colony was grown to ca. 51.8 mm in diameter 10 days after inoculation except for diameter of fungal mycelial plugs. Three doses (2, 5 and 8 μl/disc) of carvacrol completely inhibited the fungal mycelial growth. Antifungal activities of cinnamon oil, trans-cinnamaldehyde, citral, clove oil, eugenol and geraniol appeared in dose-dependent manners, and relatively higher doses manifested stronger antifungal activities (Table 1). Suppressive efficacies of other essential oils such as 1,8-cineole, p-cymene, (±)-limonene, linalool, menthone, (1R)-(+)-α-pinene, (1S)-(−)-α-pinene and β-pinene demonstrated as colony size were marginal.
Fig. 2.
Effect of different plant essential oils on in vitro mycelial growth of Collectotrichum gloeosporioides by an indirect volatile activity. In vitro inhibitory activities of various plant essential oils against C. gloeosporioides mycelia were shown by colony diameter (mm). Values presented are means and error bars represent the standard deviation of the means of four independent experimental replications. Means followed by the same letter are not significantly different at 5% level by least significant difference test. nd, not determined. Different essential oil doses were applied 8 μl/disc demonstrated in upper (2 μl/disc), middle (5 μl/disc) and bottom (8 μl/disc) graphs.
Anthracnose reduction on pepper fruits by plant essential oils volatility
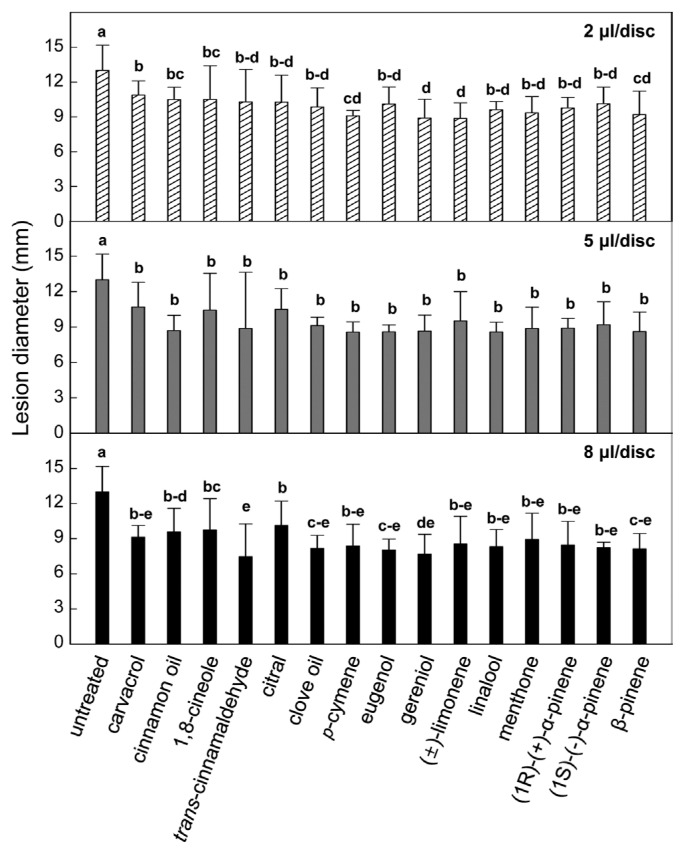
To investigate efficacies of different plant essential oils on control of pepper fruit anthracnose, lesion diameters on the inoculated fruits in the absence or presence of essential oil treatments were measured (Fig. 3).
Fig. 3.
Effect of different plant essential oils on lesion development of anthracnose on immature green pepper fruits. Disease protection was evaluated by reduced lesion diameter formed on the detached pepper fruits inoculated by dropping conidial suspension (2×105 conidia/ml) of Colletotrichum gloeosporioides with different essential oil vapors. Lesion diameter was measured 12 days after inoculation. Values presented are means and error bars represent the standard errors of the means of four independent experimental replications. Means followed by the same letter are not significantly different at 5% level by least significant difference test. Different essential oil doses were applied 8 μl/disc demonstrated in upper (2 μl/disc), middle (5 μl/disc) and bottom (8 μl/disc) graphs.
Without plant essential oil treatment, anthracnose lesions of ca. 13.0 mm in diameter were formed on the inoculated pepper fruits at 12 days post-inoculation. Interestingly, all 15 plant essential oils showed disease reduction efficacies with similar activities. By 2 μl/disc treatments with carvacrol, cinnamon oil, 1,8-cineole, trans-cinnamaldehyde, citral, clove oil, eugenol, (1R)-(+)-α-pinene and (1S)-(−)-α-pinene, slight reduced lesion size (ca. 10.9-9.8 mm in diameter) on the fruit surfaces was found. p-Cymene, geraniol, (±)-limonene, linalool, menthone and β-pinene treatments with 2 μl/disc showed a bit more reduced lesion diameters ca. 9.6-8.9 mm. Increasing cinnamon oil, trans-cinnamaldehyde and eugenol to 5 μl/disc led to significantly more decreased the lesion sizes compared to those on the 2 μl/disc-treated pepper fruits. Other 12 plant essential oils did not demonstrate increased efficacies against pepper fruit anthracnose by 5 μl/disc. Only treatment with 8 μl/disc of carvacrol and trans-cinnamaldehyde increased protection effect compared to 5 μl/disc of each essential oil.
Discussion
A large number of essential oils have been originated from different plant species, and evaluated their antimicrobial activities against broad range of plant pathogens causing oomycete, bacterial and fungal diseases (Bajpai et al., 2011; Isman, 2000; Sivakumar and Bautista-Baños, 2014). Fungal and bacterial diseases in many economically important crops could be efficiently reduced by treatments with different plant essential oils. Spraying plant essential oils was widely applied to suppress foliar fungal diseases such as flax powdery mildew, grape grey mould and peanut late leaf spot in the fields (Aly et al., 2013; Kishore et al., 2007; Walter et al., 2001). Treatment with plant essential oils has been evident as useful eco-friendly control of postharvest decays which are caused by some fungal species during fruit storage (Sivakumar and Bautista-Baños, 2014). Appropriate biological and chemical managements had not been recommended for many bacterial plant diseases so far. Plant essential oils were also suggested as promising agents for controlling the bacterial plant diseases. Bacterial wilt caused by Ralstonia solanacearum could be suppressed by applying clove oil, palmarosa oil and thymol into tomato-growing soils (Ji et al., 2005; Lee et al., 2012). In vitro and in vivo antibacterial efficacies of various plant essential oils were found against different Xanthomonas species (Bajpai et al., 2011). Interestingly, recent reports demonstrated that application of plant essential oils suppressed Tomato yellow leaf curl virus disease in tomato plants and Tobacco mosaic virus disease in Nicotiana glutinosa with protective and curative effects, although mode-of-action for reducing the diseases were not fully understood (Min et al., 2013; Wang and Fan, 2014). Combined application of plant essential oils with other plant essential oils and disease controlling agents such as antagonistic bacteria and chitosan may enhance the protective effects of plant essential oils against pepper anthracnose (Arrebola et al., 2010; Shao et al., 2015; Xing et al., 2011). These demonstrated that plant essential oils can be suggested as eco-friendly alternatives for a variety of plant diseases caused by taxonomically different pathogens hard to be controlled.
Plant essential oils and individual compounds used in this study were evaluated for their inhibitory efficacies on in vitro conidial germination and mycelial growth of C. gloeosporioides. Carvacrol was most effective to suppress conidial germination and mycelial growth of C. gloeosporioides. Although anthracnose lesion sizes on the inoculated pepper fruits were also decreased by carvacrol, in planta inhibitory activity of carvacrol was not highest level. Carvacrol is an individual component of a monoterpenoid phenol, and identified as major component of 79.8% of oregano oil and 37.9% of thyme oil (Soylu et al., 2006). In vitro antioomycete and antifungal activity of carvacrol has been demonstrated against several phytopathogenic oomycete and fungi including Phytophthora citrophthora, Rhizopus stolonifer, Botrytis cinerea and Penicillium expansum (Camele et al., 2012). Carvacrol reduced Dutch elm disease caused by Ophiostoma novo-ulmi fungus in planta (Martín et al., 2012). Oregano and thyme oils containing carvacrol as an ingredient have been shown their in vitro antifungal activities against C. gloeosporioides (Lee et al., 2007). Significantly higher antifungal activities of cinnamon oil and its main constituent trans-cinnamaldehyde against C. gloeosporioides were seen in this study. Cinnamon oil has been known as effective agent for inhibiting in vitro growth of B. cinerea and Fusarium proliferatum (Wilson et al., 1997). Periwinkle Phytophthora blight, peanut crown rot and banana anthracnose diseases were efficiently reduced by cinnamon oil treatment (Bowers et al., 2004; Kishore et al., 2007; Maqbool et al., 2010a; Ranasinghe et al., 2002). In vitro conidial germination and mycelial growth of Monilinia laxa were inhibited by trans-cinnamaldehyde (Neri et al., 2007). However, these carvacrol and trans-cinnamaldehyde were not suggested to control anthracnose occurred in many crops including pepper fruits. Many plant essential oils without antifungal activities on in vitro conidial germination and mycelial growth demonstrated in planta disease suppression effects on the pepper anthracnose in the present study. Activated plant defences or enhanced antioxidant capacity of pepper fruit tissues by treatment with the plant essential oils could not be excluded, which remains elucidated (Jin et al., 2012).
Volatility was one of the beneficial effects of plant essential oils for plant disease management. Foliar spraying and soil-drenching chemical pesticides or antagonistic microbes occasionally did not efficiently suppress disease occurrence in the crop fields, although those abiotic and biotic agents significantly arrested in vitro growth of plant pathogens. It may be due to insufficient direct contacts between plant pathogens and active compounds of the pesticides or the microorganisms under the field conditions. Various plant essential oils manifested their volatile antifungal activities without direct contacts to inhibit in vitro conidial germination and mycelial growth of C. gloeosporioides. Moreover, arrested lesions on the inoculated fruits in the sealed plastic boxes were also achieved by volatile effects of plant essential oils. No visible phytotoxic effects were observed on the pepper fruits treated with plant essential oils in this study. Increasing doses of the plant essential oils should be evaluated to improve the plant protection efficacies against the anthracnose.
Taken together, fungal development of C. gloeosporioides including conidial germination and mycelial growth can be controlled by plant essential oil treatments via indirect volatile antifungal activities. In addition, anthracnose control efficacies of plant essential oils on the detached green fruits were achieved. Feasible disease protection by treatment with plant essential oils need to be proved under rain-splashed and rain-proof field conditions during pepper growing season for eco-friendly disease management in the further studies.
Acknowledgments
This research was supported by Gyeongnam National University of Science and Technology (GNTech) Grant 2014 to Jeum Kyu Hong.
References
- Abd-Alla MA, Haggag WM. Use of some plant essential oils as post-harvest botanical fungicides in the management of anthracnose disease of mango fruits (Mangifera indica L.) caused by Colletotrichum gloeosporioides (Penz) Int J Agric Forest. 2013;3:1–6. [Google Scholar]
- Ahn M-I, Kang WS, Park EW, Yun S-C. Validation of an anthracnose forecaster to schedule fungicide spraying for pepper. Plant Pathol J. 2008;24:46–51. doi: 10.5423/PPJ.2008.24.1.046. [DOI] [Google Scholar]
- Aly AA, Mohamed HI, Mansour MTM, Omar MR. Suppression of powdery mildew on flax by foliar application of essential oils. J Phytopathol. 2013;161:376–381. doi: 10.1111/jph.12080. [DOI] [Google Scholar]
- Anaruma ND, Schmidt FL, Duarte MCT, Figueira GM, Delarmelina C, Benato EA, Sartoratto A. Control of Colletotrichum gloeosporioides (Penz.) Sacc. in yellow passion fruit using Cymbopogon citratus essential oil. Braz J Microbiol. 2010;41:66–73. doi: 10.1590/S1517-83822010000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrebola E, Sivakumar D, Korsten L. Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol Cont. 2010;53:122–128. doi: 10.1016/j.biocontrol.2009.11.010. [DOI] [Google Scholar]
- Avila-Adame C, Olaya G, Köller W. Characterization of Colletotrichum graminicola isolates resistant to strobilurin-related QoI fungicides. Plant Dis. 2003;87:1426–1432. doi: 10.1094/PDIS.2003.87.12.1426. [DOI] [PubMed] [Google Scholar]
- Bajpai VK, Kang S, Xu H, Lee S-G, Beak KH, Kang SC. Potential roles of essential oils on controlling plant pathogenic bacteria Xanthomonas species: a review. Plant Pathol J. 2011;27:207–224. doi: 10.5423/PPJ.2011.27.3.207. [DOI] [Google Scholar]
- Bowers JH, Locke JC. Effect of formulated plant extracts and oils on population density of Phytophthora nicotianae in soil and control of Phytophthora blight in the greenhouse. Plant Dis. 2004;88:11–16. doi: 10.1094/PDIS.2004.88.1.11. [DOI] [PubMed] [Google Scholar]
- Camele I, Altieri L, De Martino L, De Feo V, Mancini E, Rana GL. In vitro control of post-harvest fruit rot fungi by some plant essential oil components. Int J Mol Sci. 2012;13:2290–2300. doi: 10.3390/ijms13022290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W-H, Ishii H, Nishimura K, Fukaya M, Yano K, Kajitani Y. Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Dis. 2006;90:506–512. doi: 10.1094/PD-90-0506. [DOI] [PubMed] [Google Scholar]
- Ferreira JJ, Campa A, Pérez-Vega E, Rodríguez-Suárez C, Giraldez R. Introgression and pyramiding into common bean market class fabada of genes conferring resistance to anthracnose and potyvirus. Theor Appl Genet. 2012;124:777–788. doi: 10.1007/s00122-011-1746-x. [DOI] [PubMed] [Google Scholar]
- Freeman S, Nizani Y, Dotan S, Even S, Sando T. Control of Colletotrichum acutatum in strawberry under laboratory, greenhouse, and field conditions. Plant Dis. 1997;81:749–752. doi: 10.1094/PDIS.1997.81.7.749. [DOI] [PubMed] [Google Scholar]
- Harp T, Kuhn P, Roberts PD, Pernezny KL. Management and cross-infectivity potential of Colletotrichum acutatum causing anthracnose on bell pepper in Florida. Phytoparasitica. 2014;42:31–39. doi: 10.1007/s12600-013-0334-9. [DOI] [Google Scholar]
- Hong JK, Hwang BK. Influence of inoculum density, wetness duration, plant age, inoculation method, and cultivar resistance on infection of pepper plants by Colletotrichum coccodes. Plant Dis. 1998;82:1079–1083. doi: 10.1094/PDIS.1998.82.10.1079. [DOI] [PubMed] [Google Scholar]
- Isman MB. Plant essential oils for pest and disease management. Crop Protect. 2000;19:603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- Jee H-J, Shin S-S, Lee J-H, Kim W-I, Hong SJ, Kim Y-K. Conidial disperse of the pepper anthracnose fungus Colletotrichum acutatum and its density on infected fruits. Res Plant Dis. 2010;16:101–105. doi: 10.5423/RPD.2010.16.1.101. (in Korean) [DOI] [Google Scholar]
- Ji P, Momol MT, Olson SM, Pradhanang PM, Jones JB. Evaluation of thymol as biofumigant for control of bacterial wilt of tomato under field conditions. Plant Dis. 2005;89:497–500. doi: 10.1094/PD-89-0497. [DOI] [PubMed] [Google Scholar]
- Jin P, Wang SY, Gao H, Chen H, Zheng Y, Wang CY. Effect of cultural system and essential oil treatment on antioxidant capacity in raspberries. Food Chem. 2012;132:399–405. doi: 10.1016/j.foodchem.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Khan SH, Aked J, Magan N. Control of the anthracnose pathogen of banana (Colletotrichum musae) using antioxidants alone and in combination with thiabendazole or imazalil. Plant Pathol. 2001;50:601–608. doi: 10.1046/j.1365-3059.2001.00599.x. [DOI] [Google Scholar]
- Kim J-S, Jee H-J, Gwag J-G, Kim C-K, Shim C-K. Evaluation on red pepper germplasm lines (Capsicum spp.) for resistance to anthracnose caused by Colletotrichum acutatum. Plant Pathol J. 2010;26:273–279. doi: 10.5423/PPJ.2010.26.3.273. [DOI] [Google Scholar]
- Kim JJ, Kim JT, Park SW, Park ES, Kim HT. Development of assay method for activities of new compounds, and effect of several fungicides against spore germination, adhesion, and mycelial growth of Colletotrichum sp. causing red pepper anthracnose. Kor J Pest Sci. 2003;7:159–168. (in Korean) [Google Scholar]
- Kim JT, Lee KH, Min JY, Cho IJ, Kang BK, Park SW, Van Bach N, Kim Y-S, Hong ST, Rho CW, Kim HT. Fluctuation of the sensitivity of Colletotrichum spp. causing the red-pepper anthracnose to chlorothalonil. Kor J Pest Sci. 2004;8:231–237. (in Korean) [Google Scholar]
- Kim JT, Lee KH, Min JY, Kang BW, Rho CW, Hong ST, Kim HT. Response of Colletotrichum spp. causing red pepper anthracnose to protective and ergosterol biosynthesis-inhibiting fungicides. Res Plant Dis. 2005;11:185–192. doi: 10.5423/RPD.2005.11.2.185. (in Korean) [DOI] [Google Scholar]
- Kim KD, Oh BJ, Yang J. Differential interactions of a Colletotrichum gloeosporioides isolate with green and red pepper fruits. Phytoparasitica. 1999;27:97–106. doi: 10.1007/BF03015074. [DOI] [Google Scholar]
- Kim S-T, Yun S-C. Biocontrol with Myxococcus sp. KYC 1126 against anthracnose in hot pepper. Plant Pathol J. 2011;27:156–163. doi: 10.5423/PPJ.2011.27.2.156. [DOI] [Google Scholar]
- Kim SH, Yoon JB, Do JW, Park HG. Resistance to anthracnose caused by Colletotrichum acutatum in chili pepper (Capsicum annuum L.) J Crop Sci Biotechnol. 2007;10:277–280. [Google Scholar]
- Kishore GK, Pande S, Harish S. Evaluation of essential oils and their components for broad-spectrum antifungal activity and control of late leaf spot and crown rot diseases in peanut. Plant Dis. 2007;91:375–379. doi: 10.1094/PDIS-91-4-0375. [DOI] [PubMed] [Google Scholar]
- Koul O, Walia S, Dhaliwal GS. Essential oils as green pesticides: potential and constraints. Biopestic Int. 2008;4:63–84. [Google Scholar]
- Kwon C-S, Lee S-G. Occurrence and ecological characteristics of red pepper anthracnose. Res Plant Dis. 1999;8:120–123. doi: 10.5423/RPD.2002.8.2.120. (in Korean) [DOI] [Google Scholar]
- Lamsal K, Kim SW, Jung JH, Kim YS, Kim KS, Lee YS. Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology. 2011;39:194–199. doi: 10.5941/MYCO.2011.39.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Choi CW, Kim SH, Yun JG, Chang SW, Kim YS, Hong JK. Chemical pesticides and plant essential oils for disease control of tomato bacterial wilt. Plant Pathol J. 2012;28:32–39. doi: 10.5423/PPJ.OA.10.2011.0200. [DOI] [Google Scholar]
- Lee SO, Choi GJ, Jang KS, Lim HK, Cho KY, Kim J-C. Antifungal activity of five plant essential oils as fumigant against postharvest and soilborne plant pathogenic fungi. Plant Pathol J. 2007;23:97–102. doi: 10.5423/PPJ.2007.23.2.097. [DOI] [Google Scholar]
- Mahasuk P, Taylor PWJ, Mongkolporn O. Identification of two new genes conferring resistance to Colletotrichum acutatum in Capsicum baccatum. Phytopathology. 2009;99:1100–1104. doi: 10.1094/PHYTO-99-9-1100. [DOI] [PubMed] [Google Scholar]
- Maqbool M, Ali A, Alderson PG. Effect of cinnamon oil in incidence of anthracnose disease and postharvest quality of banana during storage. Int J Agric Biol. 2010a;12:516–520. [Google Scholar]
- Maqbool M, Ali A, Ramachadran S, Smith DR, Alderson PG. Control of postharvest anthracnose of banana using a new edible composite coating. Crop Protect. 2010b;29:1136–1141. doi: 10.1016/j.cropro.2010.06.005. [DOI] [Google Scholar]
- Martín JA, Solla A, Carcía-Vallejo MC, Gil L. Chemical changes in Ulmus minor xylem tissue after salicylic acid or carvacrol treatments are associated with enhanced resistance to Ophiostoma novo-ulmi. Phytochemistry. 2012;83:104–109. doi: 10.1016/j.phytochem.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Min L, Han Z, Xu Y, Yao L. In vitro and in vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. J Microbiol Biotechnol. 2013;23:771–778. doi: 10.4014/jmb.1210.10078. [DOI] [PubMed] [Google Scholar]
- Neri F, Mari M, Brigati S, Bertolini P. Fungicidal activity of plant volatile compounds for controlling Monilinia laxa in stone fruit. Plant Dis. 2007;91:30–35. doi: 10.1094/PD-91-0030. [DOI] [PubMed] [Google Scholar]
- Ojeda AM, Aguilera JAM, Monter AV, Diaz CN, Castro EH, Otero-Colina G, Morales JH. Temporal analysis and fungicide management strategies to control mango anthracnose epidemics in Guerrero, Mexico. Trop Plant Pathol. 2012;37:375–385. doi: 10.1590/S1982-56762012000600001. [DOI] [Google Scholar]
- Osorio LF, Pattison JA, Peres NA, Whitaker VM. Genetic variation and gains in resistance of strawberry to Colletotrichum gloeosporioides. Phytopathology. 2014;104:67–74. doi: 10.1094/PHYTO-02-13-0032-R. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim CH. Identification, distribution and etiological characteristics of anthracnose fungi of red pepper in Korea. Kor J Plant Pathol. 1992;8:61–69. [Google Scholar]
- Park KS, Kim CH. Effect of temperature, relative humidity, and free water period on lesion development and acervulus formation of Colletotrichum gloeosporioides on red pepper. Kor J Plant Pathol. 1994;10:34–38. [Google Scholar]
- Park S-J, Kim G-H, Kim AH, Lee H, Gwon H-W, Kim J, Lee KH, Kim HT. Controlling effect of agricultural organic materials on Phytophthora blight and anthracnose in red pepper. Res Plant Dis. 2012;18:1–9. doi: 10.5423/RPD.2012.18.1.001. (in Korean) [DOI] [Google Scholar]
- Peres NAR, Souza NL, Furtado EL, Timmer LW. Evaluation of systems for timing of fungicide sprays for control of postbloom fruit drop of citrus in Brazil. Plant Dis. 2004;88:731–735. doi: 10.1094/PDIS.2004.88.7.731. [DOI] [PubMed] [Google Scholar]
- Peres NAR, Souza NL, Peever TL, Timmer LW. Benomyl sensitivity of isolates of Colletotrichum acutatum and C. gloeosporioides from citrus. Plant Dis. 2004;88:125–130. doi: 10.1094/PDIS.2004.88.2.125. [DOI] [PubMed] [Google Scholar]
- Ranasinghe L, Jayawardena B, Abeywickrama K. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L.M. Perry against crown rot and anthracnose pathogens isolated from banana. Lett Appl Microbiol. 2002;35:208–211. doi: 10.1046/j.1472-765X.2002.01165.x. [DOI] [PubMed] [Google Scholar]
- Sang MK, Shrestha A, Kim D-Y, Park K, Pak CH, Kim KD. Biocontrol of Phytophthora blight and anthracnose in pepper by sequentially selected antagonistic rhizobacteria against Phytophthora capsici. Plant Pathol J. 2013;29:154–167. doi: 10.5423/PPJ.OA.07.2012.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellamuthu PS, Mafune M, Sivakumar D, Soundy P. Thyme oil vapour and modified atmosphere packaging reduce anthracnose incidence and maintain fruit quality in avocado. J Sci Food Agri. 2013a;93:3024–3031. doi: 10.1002/jsfa.6135. [DOI] [PubMed] [Google Scholar]
- Sellamuthu PS, Sivakumar D, Soundy P, Korsten L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol Technol. 2013b;81:66–72. doi: 10.1016/j.postharvbio.2013.02.007. [DOI] [Google Scholar]
- Shao X, Cao B, Xu F, Xie S, Yu D, Wang H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol Technol. 2015;99:37–43. doi: 10.1016/j.postharvbio.2014.07.014. [DOI] [Google Scholar]
- Sivakumar D, Bautista-Baños S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Protect. 2014;64:27–37. doi: 10.1016/j.cropro.2014.05.012. [DOI] [Google Scholar]
- Soylu EM, Soylu S, Kurt S. Antimicrobial activities of the essential oils of various plants against late blight disease agent Phytophthora infestans. Mycopathologia. 2006;161:119–128. doi: 10.1007/s11046-005-0206-z. [DOI] [PubMed] [Google Scholar]
- Tzortzakis NG. Ethanol, vinegar and Origanum vulgare oil vapour suppress the development of anthracnose rot in tomato fruit. Int J Food Microbiol. 2010;142:14–18. doi: 10.1016/j.ijfoodmicro.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Walter M, Jaspers MV, Eade K, Frampton CM, Stewart A. Control of Botrytis cinerea in grape using thyme oil. Austral Plant Pathol. 2001;30:21–25. doi: 10.1071/AP00059. [DOI] [Google Scholar]
- Wang C, Fan Y. Eugenol enhances the resistance of tomato against tomato yellow leaf curl virus. J Sci Food Agric. 2014;94:677–682. doi: 10.1002/jsfa.6304. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Solar JM, El Ghaouth A, Wisniewski ME. Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 1997;81:204–210. doi: 10.1094/PDIS.1997.81.2.204. [DOI] [PubMed] [Google Scholar]
- Xing Y, Li X, Xu Q, Yun J, Lu Y, Tang Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.) Food Chem. 2011;124:1443–1450. doi: 10.1016/j.foodchem.2010.07.105. [DOI] [Google Scholar]
- Yang S, Gao M, Deshpande S, Lin S, Roe BA, Zhu H. Genetic and physical localization of an anthracnose resistance gene in Medicago truncatula. Theor Appl Genet. 2007;116:45–52. doi: 10.1007/s00122-007-0645-7. [DOI] [PubMed] [Google Scholar]
- Zahid N, Ali A, Manickam S, Siddiqui Y, Maqbool M. Potential of chitosan-loaded nanoemulsions to control different Colletotrichum spp. and maintain quality of tropical fruits during cold storage. J Appl Microbiol. 2012;113:925–939. doi: 10.1111/j.1365-2672.2012.05398.x. [DOI] [PubMed] [Google Scholar]