Abstract
Purpose
To explore the value of a new simple lyophilized kit for labeling PRGD2 peptide (18F-ALF-NOTA-PRGD2, denoted as 18F-alfatide) in the determination of metabolic tumor volume (MTV) with micro-PET in lewis lung carcinoma (LLC) tumor-bearing C57BL/6 mice verified by pathologic examination and compared with those using 18F-fluorodeoxyglucose (FDG) PET.
Methods
All LLC tumor-bearing C57BL/6 mice underwent two attenuation-corrected whole-body micro-PET scans with the radiotracers 18F-alfatide and 18F-FDG within two days. 18F-alfatide metabolic tumor volume (VRGD) and 18F-FDG metabolic tumor volume (VFDG) were manually delineated slice by slice on PET images. Pathologic tumor volume (VPath) was measured in vitro after the xenografts were removed.
Results
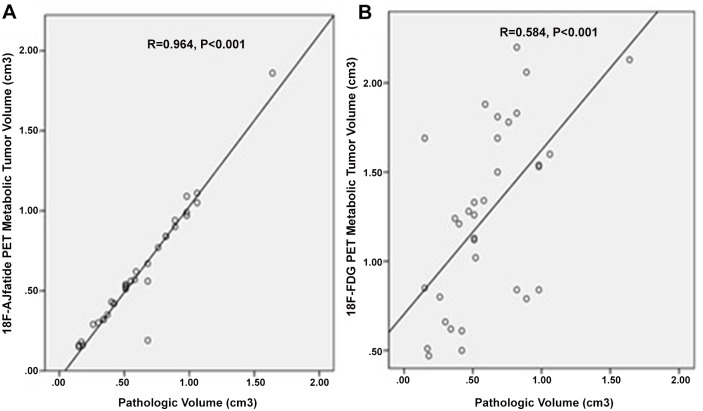
A total of 37 mice with NSCLC xenografts were enrolled and 33 of them underwent 18F-alfatide PET, and 35 of them underwent 18F-FDG PET and all underwent pathological examination. The mean ± standard deviation of VPath, VRGD, and VFDG were 0.59±0.32 cm3 (range,0.13~1.64 cm3), 0.61±0.37 cm3 (range,0.15~1.86 cm3), and 1.24±0.53 cm3 (range,0.17~2.20 cm3), respectively. VPath vs. VRGD, VPath vs. VFDG, and VRGD vs. VFDG comparisons were t = -0.145, P = 0.885, t = -6.239, P<0.001, and t = -5.661, P<0.001, respectively. No significant difference was found between VPath and VRGD. VFDG was much larger than VRGD and VPath. VRGD seemed more approximate to the pathologic gross tumor volume. Furthermore, VPath was more strongly correlated with VRGD (R = 0.964,P<0.001) than with VFDG (R = 0.584,P<0.001).
Conclusions
18F-alfatide PET provided a better estimation of gross tumor volume than 18F-FDG PET in LLC tumor-bearing C57BL/6 mice.
Introduction
Improving the accuracy of target volume estimation will help avoid unnecessary radiation of normal tissues and help avoid geographic tumor misses in patients with non-small cell lung cancer (NSCLC). Accurate metabolic tumor volume (MTV) assessment is a promising method for defining volumes in radiotherapy because of the development of functional imaging tools and image-guided radiotherapy.
PET with 18F-FDG has been widely used to define target volumes in radiotherapy with increased metabolism. Studies have shown that FDG PET/CT can reduce inter- and intra-observer variability of target volume delineation to improve radiotherapy treatment planning [1, 2]. In recent years, the possibly substantial impact of 18F-FDG PET on the size and form of target volumes in lung cancer was demonstrated [3]. Most methods currently used in clinical practice are based on the use of some form of binary threshold, either fixed [4] or adaptive, that use tumor-to-background ratios [5]. Unfortunately, sometimes 18F-FGD PET fail to provide satisfactory delineation of tumors characterized by heterogeneous activity distributions and fail to provide reproducible results for small tumors with low contrast because of intense cardiac uptake and high lung background [6]. Realistically, it is preferable that the radiotherapist uses the 18F-FDG PET images as a reference only [7]. The radiation oncologist are expecting more accurate tool to contour target volume precisely.
Arginine-glycine-aspartic acid peptide (Arg-Gly-Asp, RGD) can specifically bind with integrin αvβ3, which is highly expressed in angiogenic tumors, to detect angiogenesis in non-invasive PET imaging. Angiogenesis plays an important role in the regulation of tumor growth, local invasiveness, and metastatic potential [8]. Chen et al invented a new simple lyophilized kit for labeling PRGD2 peptide (18F-ALF-NOTA-PRGD2, denoted as 18F-alfatide) [9]. PET scanning with RGD allows specific imaging of integrin αvβ3 expression with minimal nonspecific activity accumulation in normal lung and heart tissue. Therefore, RGD PET may render high-quality orthotopic lung cancer images, enabling clear demarcation of both the primary tumor at the upper lobe of the left lung, as well as metastases in the mediastinum, contralateral lung, and diaphragm.
This study is designed to explore the value of 18F-alfatide in the determination of MTV with micro-PET in lewis lung carcinoma (LLC) tumor-bearing C57BL/6 mice verified by pathologic examination and compared with those using 18F-FDG PET.
Materials and Methods
Cell Culture and Animal Tumor Model Preparation
Murine LLC cells, recently used in a number of high-profile preclinical studies [10,11], was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Murine LLC cells were grown in RPMI 1640, supplemented with 10% fetal bovine serum and 1% penicillin streptomycin antibiotic mixture in a humidified incubator (Heraeus, Hanau, Germany) at 37°C with 5% CO2 atmosphere. LLC was injected (2.5×105 cells/100 μl/mouse) into the right hind leg muscle of C57BL/6 mice.
Thirty-seven C57BL/6 inbred male mice were housed in a limited access animal facility. Animal room temperature and relative humidity were set at 22±2°C and 55±10%, respectively. Artificial lighting provided a 24 h cycle of 12 h light/12 h dark (7 a.m. to 7 p.m.). All animal procedures were in accordance with the Shandong Cancer Hospital & Institute Ethical Committee Guide for the care and use of Laboratory Animals. The Shandong Cancer Hospital & Institute Ethical Committee specifically approved this study.
18F-alfatide PET and 18F-FDG PET image acquisition
All mice underwent PET scans (Siemens Medical Solutions) using 18F-alfatide and 18F-FDG PET respectively within 2 days when tumor diameter reached approximately 10 mm. With the assistance of the Inveon system’s positioning laser, the LLC tumor-bearing mouse was placed with its tumor located at the center of the field of view (FOV), where the highest imaging sensitivity can be achieved. 18F-alfatide PET scans were performed 60 minutes after tail-vein injection of 2.4–3.5 MBp of 18F-Alfatide under isoflurane anesthesia. During the acquisition period, a thermostat-controlled thermal heater maintained the body temperature of the mice. 18F-FDG PET scans were performed for alll mice after 4 hours fasting and 60 minutes after injection of 18F-FDG at a dosage of 2.6–3.6 MBp. The mice rested quietly in a warm box under isoflurane anesthesia for approximately 1 hour. Subsequently, the mice underwent the scanning, using the same parameters that had been employed for the 18F-Alfatide PET scan.
The images were reconstructed and analyzed using Inveon Acquisition Workplace v1.4.3 SP1 software. In the bio-distribution analysis of static images, regions of interest (ROIs) were placed over the tumor. The attenuation-corrected PET images were reconstructed and reviewed in axial, coronal, and sagittal planes; the same procedure was performed with a cine display of maximum-intensity projections of the PET data.
The 18F-alfatide metabolic tumor volume (VRGD) and 18F-FDG metabolic tumor volume (VFDG) were manually delineated slice by slice on the 18F-alfatide and 18F-FDG PET images (Fig 1). As a first step, an experienced physician (Z.F) used the ROI standard evaluation tool provided by the manufacture of the micro-PET system and a global logarithmic scaling to generate a “visual” PET GTV, comprising the tissue considered visually as part of the malignant primary tumor. ROI was positioned around the tumors slice by slice and obtained a set of data such as max (ROImax), mean and so on. The results were expressed as the standardized uptake value (SUVmax), which was calculated according to the following formula: ROImax×9500(CF value)×body weight [g]/injected activity [Bq]). The MTV was delineated on the PET images in transaction slice by slice with the 40% of the SUVmax, a threshold that has been used for the delineation tumor volume in previously study [12].
Fig 1. PET imaging.
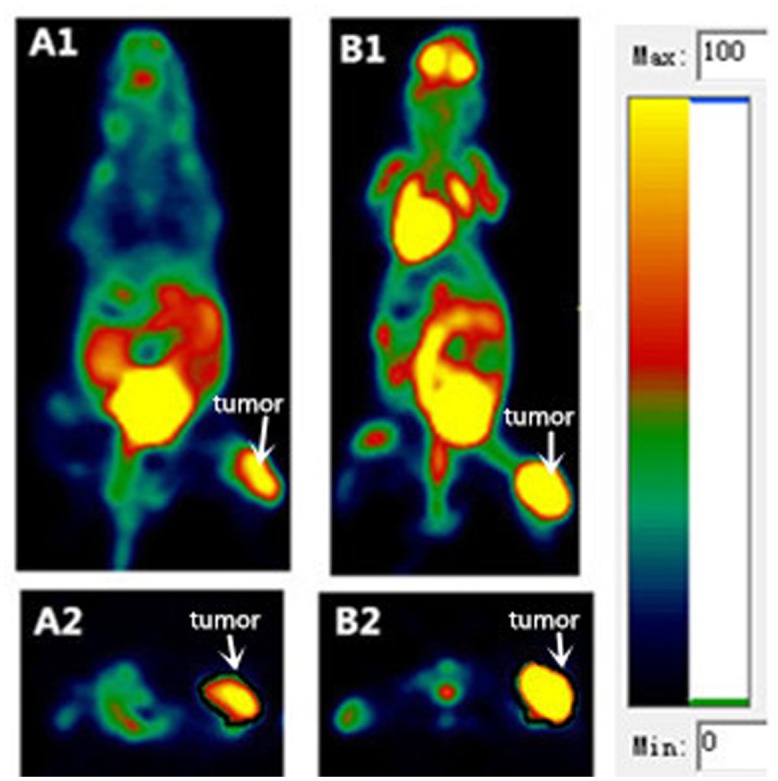
PET images showing localization of 18F-alfatide (A1, coronal; A2, transverse) and 18F-FDG (B1, coronal; B2 transverse) in the same mice with lung cancer xenografts. See the color bar for PET images.
Pathologic volume measurement
All mice underwent tumor resection after the end of imaging (Fig 2). Specimens that were submitted fresh from the operating laboratory had three dimensional gross measurements taken from the tumor before being placed into 10% formalin. The pathologic tumor volume (VPath) was estimated from the volume of an ellipsoid:
where Dlong and Dshort were the longest and shortest diameters on the transverse plane before formalin fixation. Dlong and Dshort were the two orthogonal diameters obtained from the resected tumor specimen.
Fig 2. Correlation between 18F-alfatide metabolic tumor volume (VRGD) and 18F-FDG PET metabolic tumor volume (VFDG) and pathologic volume(VPath).
A strong significant correlation was found between VRGD and VPath (A, R = 0.964, P<0.001). A moderately significant correlation was found between VFDG and VPath (B, R = 0.584, P<0.001).
Statistical analysis
Statistical analysis was performed using SPSS software (version 17.0; SPSS, Inc). Student’s unpaired t-test was used to detect differences between two sample means. The relationships between VPath and VRGD and between VPath and VFDG were tested by the Linear Regression Equation. All analyses were 2-sided, and a P value of less than 0.05 was considered statistically significant.
Results
A total of 37 mice with NSCLC xenografts underwent pathologic examination after completing the PET imaging. Each mouse underwent 18F-alfatide and 18F-FDG PET imaging. In the 18F-alfatide PET imaging process, 4 mice were not scanned because of intravenous administration failure. For the same reason, 2 mice did not undergo 18F-FDG PET scanning.
Tumor volume was measured by various methods (Table 1). The MTV was delineated in PET imaging performance of a novel αVβ3 integrin radiotracer, denoted as 18F-alfatide, and 18F-FDG. VRGD (n = 33) was significantly smaller than VFDG (n = 35), 0.61±0.37 vs. 1.24±0.53 cm3, t = -5.661, P<0.001. This finding is illustrated by Fig 1-A1 and 1-A2 and Fig 1-B1 and 1-B2, where length×width of the xenograft on 18F-FDG PET images were larger than 18F-alfatide PET images.
Table 1. Tumor size, actual pathologic volume, 18F-alfatide metabolic volume, and 18F-FDG metabolic volume for each case.
| NO | Dlong(cm) | Dshort(cm) | Vpath(cc) | VRGD(cc) | VFDG(cc) |
|---|---|---|---|---|---|
| 1 | 1.00 | 0.70 | 0.26 | 0.29 | 0.80 |
| 2 | 1.20 | 0.90 | 0.51 | 0.51 | 1.13 |
| 3 | 1.30 | 1.20 | 0.98 | 0.97 | 0.84 |
| 4 | 1.60 | 1.40 | 1.64 | 1.86 | 2.13 |
| 5 | 1.40 | 1.20 | 1.06 | 1.11 | 1.60 |
| 6 | 1.40 | 1.20 | 1.06 | 1.05 | —— |
| 7 | 1.30 | 1.00 | 0.68 | 0.56 | 1.81 |
| 8 | 1.00 | 0.90 | 0.42 | 0.42 | 0.61 |
| 9 | 1.00 | 1.00 | 0.52 | —— | 1.02 |
| 10 | 1.20 | 0.90 | 0.51 | 0.53 | 1.26 |
| 11 | 1.30 | 1.00 | 0.68 | 0.19 | 1.69 |
| 12 | 1.30 | 1.10 | 0.82 | 0.84 | 0.84 |
| 13 | 1.10 | 1.00 | 0.58 | 0.57 | 1.34 |
| 14 | 1.30 | 0.90 | 0.55 | 0.56 | —— |
| 15 | 1.20 | 0.90 | 0.51 | 0.54 | 1.33 |
| 16 | 0.80 | 0.60 | 0.15 | 0.15 | 1.69 |
| 17 | 1.40 | 0.90 | 0.59 | 0.62 | 1.88 |
| 18 | 1.30 | 1.00 | 0.68 | 0.67 | 1.50 |
| 19 | 1.00 | 0.80 | 0.34 | 0.32 | 0.62 |
| 20 | 1.10 | 0.90 | 0.47 | —— | 1.28 |
| 21 | 1.40 | 1.10 | 0.89 | 0.94 | 2.06 |
| 22 | 1.10 | 0.80 | 0.37 | 0.35 | 1.24 |
| 23 | 0.90 | 0.60 | 0.17 | 0.18 | 0.51 |
| 24 | 1.20 | 0.80 | 0.40 | 0.43 | 1.21 |
| 25 | 1.20 | 1.10 | 0.76 | 0.77 | 1.78 |
| 26 | 1.30 | 1.10 | 0.82 | 0.84 | 2.20 |
| 27 | 1.30 | 1.20 | 0.98 | 1.09 | 1.53 |
| 28 | 1.30 | 1.20 | 0.98 | 0.99 | 1.54 |
| 29 | 1.30 | 1.10 | 0.82 | —— | 1.83 |
| 30 | 1.20 | 0.90 | 0.51 | 0.52 | 1.12 |
| 31 | 1.40 | 1.10 | 0.89 | 0.90 | 0.79 |
| 32 | 0.80 | 0.60 | 0.15 | 0.16 | 0.85 |
| 33 | 0.70 | 0.70 | 0.18 | 0.16 | 0.47 |
| 34 | 1.00 | 0.90 | 0.42 | 0.42 | 0.50 |
| 35 | 0.90 | 0.80 | 0.30 | 0.30 | 0.66 |
| 36 | 1.00 | 0.80 | 0.34 | 0.32 | 1.68 |
| 37 | 0.70 | 0.60 | 0.13 | —— | 0.17 |
Dlong = long diameter on transverse plane; Dshort = short diameter on transverse plane; Vpath = pathologic volume; VRGD = 18F-alfatide metabolic tumor volume; VFDG = 18F-FDG metabolic tumor volume.
Table 2 shows the comparison of VPath (n = 37) with VRGD and VFDG. VPath was considered the gold standard for MTV as it provides the closest estimation of real tumor volume. No difference was found between VPath and VRGD (0.59±0.32 vs. 0.61±0.37, t = -0.145, P = 0.885). VPath was significantly smaller than VFDG (0.59±0.32 vs. 1.24±0.53 cm3, t = -6.24, P<0.001).
Table 2. Pathologic Volume compared with 18F-alfatide and 18F-FDG PET MTV in LLC tumor-bearing C57BL/6 mice.
| Group | Median (cm3) | Mean±SD (cm3) | Mean difference | 95%CI difference | t | P |
|---|---|---|---|---|---|---|
| Vpath | 0.52 | 0.59±0.32 | —— | —— | —— | —— |
| VRGD | 0.54 | 0.61±0.37 | -0.01±0.10 | -0.21±0.19 | -0.145 | 0.885 |
| VFDG | 1.26 | 1.24±0.53 | -0.64±0.09 | -0.84±0.44 | -6.24 | <0.001 |
MTV = metabolic tumor volume; Vpath = pathologic volume; VRGD = 18F-alfatide metabolic tumor volume; VFDG = 18F-FDG metabolic tumor volume.
The relationship between MTV and VPath (Fig 2) was also analyzed. VPath was strongly correlated with VRGD (n = 33, R = 0.964, P<0.001). VPath also had a moderately positive correlation with VFDG (n = 35, R = 0.584, P<0.001). The change in MTV of various specimens measured by 18F-alfatide and 18F-FDG PET imaging was consistent with the actual tumor volume. However, 18F-alfatide PET more accurately reflected the actual tumor size than 18F-FDG PET.
Discussion
The present study showed that larger MTVs were obtained with 18F-FDG PET imaging than 18F-alfatide PET imaging, and VRGD was similar to VPath in a mice model with NSCLC.
A study showed similar results in which the average tumor volume shown by 18F-FDG PET imaging at 6 hours (0.37±0.08 cm3), 24 hours (0.29±0.07cm3), and 48 hours (0.18±0.03 cm3) was larger than that estimated by histology (0.16±0.05cm3), (0.17±0.06 cm3), (0.06±0.02 cm3) [13]. In 18F-FDG PET imaging, many normal tissues display high uptake of 18F-FDG [14], and this process is modulated by disease states such as diabetes [15] or physical exertion before and during scanning. These factors complicate the interpretation of 18F-FDG PET imaging and help motivate the development of novel imaging tracers, such as 18F-alfatide in this present study. In contrast to VFDG, we did not find any prior studies evaluating the measurement of MTV by 18F-alfatide PET. 18F-FDG uptake is significantly correlated with glucose metabolism, but it is phosphorylated and trapped within cells rather than metabolized and is rapidly cleared from the bloodstream [16]. Tumors are metabolically active but many normal tissues also display high uptake of 18F-FDG. Para-tumor inflammation and infection may lead to high 18F-FDG uptake, which potentially confounds cancer imaging. Kyoichi Kaira [17] et al showed that in tumor tissues, the amount of FDG uptake is not only associated with molecules relevant to glucose metabolism but also has a close relationship with hypoxia, angiogenesis and the mTOR signaling pathway. Research has reported that the amount of FDG uptake is determined by the expression of glucose transporter-1 (GLUT1), hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), and microvessel density (MVD) [18, 19]. These factors may explain why the MTV of 18F-FDG PET is larger than VPath. Integrin αVβ3 is a member of the integrins family, and it can bind to a variety of plasma and extracellular matrix proteins containing the conserved amino acid sequence RGD [20]. The integrin αVβ3 receptor was expressed preferentially on various tumor cells and endothelial cells but was low on mature endothelial and epithelial cells [21, 22]. Our findings indicate that RGD has high affinity for NSCLC in this study. However, the mechanism underlying why the MTV of 18F-alfatide PET is closer to actual tumor volume is still unclear. Additionally, 18F-alfatide PET is still not a perfect tool for tumor contouring due to the heterogeneity of the malignant lesions and partial volume effect. The criteria used to define MTV are subjective, and defining MTV depends on the interpretation of appropriate parameter settings in the evaluation of the image and resulting target volume contours [23]. The tumors had to be imaged with two tracers sequentially within two days as opposed to simultaneously, and therefore, variations in growth patterns could have partially influenced the results. Another limitation is that the LLC tumor model involves implantation of a tumor into a known location, and therefore, observers were aware of the tumor location. Furthermore, clinical situations are far more complicated than the animal models. Whether 18F-alfatide PET has additional value than 18F-FDG PET on tumor volume contouring is still unknown in patients with NSCLC and it deserves further study.
Conclusions
18F-alfatide PET provided a better estimation of gross tumor volume than 18F-FDG PET in LLC tumor-bearing C57BL/6 mice.
Supporting Information
(DOCX)
Acknowledgments
Ethical approval: Shandong Cancer Hospital & Institute Ethical Committee Guide for the Care and Use of Laboratory Animals was followed. This ethical committee specifically approved this study. This article does not contain any studies with human participants performed by any of the authors.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Buijsen J, van den Bogaard J, van der Weide H, Engelsman S, van Stiphout R, Janssen M, et al. FDG-PET-CT reduces the interobserver variability in rectal tumor delineation. Radiother Oncol. 2012; 102: 371–376. 10.1016/j.radonc.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 2. Steenbakkers RJ, Duppen JC, Fitton I, Deurloo KE, Zijp LJ, Comans EF, et al. Reduction of observer variation using matched CT-PET for lung cancer delineation: a three-dimensional analysis. Int J Radiat Oncol Biol Phys. 2006; 64: 435–448. [DOI] [PubMed] [Google Scholar]
- 3. Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 59: 78–86. [DOI] [PubMed] [Google Scholar]
- 4. Paulino AC, Johnstone PA FDG-PET in radiotherapy treatment planning: Pandora's box. Int J Radiat Oncol Biol Phys. 2004; 59: 4–5. [DOI] [PubMed] [Google Scholar]
- 5. Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rube C, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med. 2005; 46: 1342–1348. [PubMed] [Google Scholar]
- 6. Hatt M, Visvikis D, Albarghach NM, Tixier F, Pradier O, Cheze-le RC Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011; 38: 1191–1202. 10.1007/s00259-011-1755-7 [DOI] [PubMed] [Google Scholar]
- 7. MacManus M, Nestle U, Rosenzweig KE, Carrio I, Messa C, Belohlavek O, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother Oncol. 2009; 91: 85–94. 10.1016/j.radonc.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 8. Hood JD, Cheresh DA Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002; 2: 91–100. [DOI] [PubMed] [Google Scholar]
- 9. Wu C, Yue X, Lang L, Kiesewetter DO, Li F, Zhu Z, et al. Longitudinal PET imaging of muscular inflammation using 18F-DPA-714 and 18F-Alfatide II and differentiation with tumors. Theranostics. 2014; 4: 546–555. 10.7150/thno.8159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Day CP, Carter J, Bonomi C, Hollingshead M, Merlino G Preclinical therapeutic response of residual metastatic disease is distinct from its primary tumor of origin. Int J Cancer. 2012; 130: 190–199. 10.1002/ijc.25978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008; 319: 195–198. 10.1126/science.1150224 [DOI] [PubMed] [Google Scholar]
- 12. Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rube C, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med. 2005; 46: 1342–1348. [PubMed] [Google Scholar]
- 13. Wong AW, Ormsby E, Zhang H, Seo JW, Mahakian LM, Caskey CF, et al. A comparison of image contrast with (64)Cu-labeled long circulating liposomes and (18)F-FDG in a murine model of mammary carcinoma. Am J Nucl Med Mol Imaging. 2013; 3: 32–43. [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamoto Y, Tatsumi M, Hammoud D, Cohade C, Osman MM, Wahl RL Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology. 2005; 234: 879–885. 10.1148/radiol.2343030301 [DOI] [PubMed] [Google Scholar]
- 15. Diederichs CG, Staib L, Glatting G, Beger HG, Reske SN FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med. 1998; 39: 1030–1033. [PubMed] [Google Scholar]
- 16. Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, et al. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001; 42: 1551–1555. [PubMed] [Google Scholar]
- 17. Kaira K, Serizawa M, Koh Y, Takahashi T, Yamaguchi A, Hanaoka H, et al. Biological significance of 18F-FDG uptake on PET in patients with non-small-cell lung cancer. Lung Cancer. 2014; 83: 197–204. 10.1016/j.lungcan.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 18. van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007; 43: 1392–1398. [DOI] [PubMed] [Google Scholar]
- 19. Guo J, Higashi K, Ueda Y, Oguchi M, Takegami T, Toga H, et al. Microvessel density: correlation with 18F-FDG uptake and prognostic impact in lung adenocarcinomas. J Nucl Med. 2006; 47: 419–425. [PubMed] [Google Scholar]
- 20. Liu S Radiolabeled cyclic RGD peptides as integrin alpha(v)beta(3)-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem. 2009; 20: 2199–2213. 10.1021/bc900167c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danhier F, Le BA, Preat V RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012; 9: 2961–2973. 10.1021/mp3002733 [DOI] [PubMed] [Google Scholar]
- 22. Desgrosellier JS, Cheresh DA Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010; 10: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van de Steene J, Linthout N, de Mey J, Vinh-Hung V, Claassens C, Noppen M, et al. Definition of gross tumor volume in lung cancer: inter-observer variability. Radiother Oncol. 2002; 62: 37–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.