Abstract
Many birds and mammals produce distress calls when captured. Bats often approach speakers playing conspecific distress calls, which has led to the hypothesis that bat distress calls promote cooperative mobbing. An alternative explanation is that approaching bats are selfishly assessing predation risk. Previous playback studies on bat distress calls involved species with highly maneuverable flight, capable of making close passes and tight circles around speakers, which can look like mobbing. We broadcast distress calls recorded from the velvety free-tailed bat, Molossus molossus, a fast-flying aerial-hawker with relatively poor maneuverability. Based on their flight behavior, we predicted that, in response to distress call playbacks, M. molossus would make individual passing inspection flights but would not approach in groups or approach within a meter of the distress call source. By recording responses via ultrasonic recording and infrared video, we found that M. molossus, and to a lesser extent Saccopteryx bilineata, made more flight passes during distress call playbacks compared to noise. However, only the more maneuverable S. bilineata made close approaches to the speaker, and we found no evidence of mobbing in groups. Instead, our findings are consistent with the hypothesis that single bats approached distress calls simply to investigate the situation. These results suggest that approaches by bats to distress calls should not suffice as clear evidence for mobbing.
Introduction
“Distress calls” are produced by captured or trapped animals of many species, including frogs [1], lizards [2], crocodilians [3], birds [4–9], bats [10–17], and other mammals [18–22]. In primates, distress calls are produced by all age categories, sexes, and species in which vocal communication has been studied [17, 22]. Because primate distress calls will often solicit help from groupmates, distress call playbacks have been used to test social knowledge and social relationships [e.g. 20–21]. In contrast, it is still unclear whether distress calls even serve a social function in most other species. Authors have proposed a variety of non-mutually exclusive hypotheses for why distress calls might be adaptive [23]. For example, they might altruistically warn kin of danger or they might aid in escape by startling the predator, by attracting conspecifics, or by luring secondary predators to scare away the attacking predator [23]. Alternatively, distress calls might simply be a non-adaptive but non-costly byproduct of an ancestral trait.
Conspecifics and heterospecifics often approach the source of distress calls reliably enough to make them suitable as acoustic lures [5,10–16]. Several authors have suggested that the strong attraction of other bats to distress calls indicates that the function of these vocalizations is to evoke cooperative mobbing [11–15, 18], an antipredator behavior whereby groups of animals attack, harass, or approach potential predators to deter them.
There are however several reasons to be skeptical of the notion that distress calls function to provoke mobbing in bats. First, although cooperative mobbing is found in many social birds and some mammals, and sometimes triggered by alarm or mobbing calls [19, 24–31], these calls are typically distinct in structure and function from distress calls. Second, prey animals might merely approach potential predators to gather information [32–34]. Third, direct observational evidence for mobbing in bats has only been reported twice; greater-spear nosed bats Phyllostomus hastatus and naked-bellied tomb bats Taphozous nudiventris have each been observed harassing an owl [30–31]. This paucity of observations might simply result from the nocturnal flight behavior of bats being difficult to observe, but it might also accurately represent the rarity of cooperative mobbing by bats in nature.
A simpler explanation for why bats approach distress calls is that they are individually and selfishly investigating the situation. All bat species known to be attracted to distress call playbacks are highly maneuverable fliers capable of making tight swooping flights [10–16]. In these species, inspection flights can easily look like mobbing behavior because scanning with nocturnal vision or echolocation requires a closer approach than vision in daylight. We tested response to distress calls in a fast-flying, aerial-hawking bat with relatively poor maneuverability: the velvety free-tailed bat Molossus molossus. This species is a group-living and possibly group-hunting [35] insectivore that is highly adapted for hunting in open spaces, as reflected by its wing morphology [36, 37] and an echolocation call design that allows for long-range detection [38–40]. Given their relatively poor maneuverability, we therefore asked: Would playbacks of M. molossus distress calls attract groups of conspecifics to within one meter of the speaker, close enough to harass a predator? Or would M. molossus respond with passing investigative flights that maintain a safe distance? We also examined whether M. molossus would produce social calls more often during distress call playback than during controls.
On Barro Colorado Island, Panama where we conducted our study, several groups of M. molossus roost in the laboratory buildings and exit their roosts quickly using distinct flight paths. When returning from the foraging sites, bats often fly in the vicinity of the roost for some time before entering. Several groups of greater sac-winged bats (Saccopteryx bilineata) also live on the outside of the same buildings. This species departs earlier and forages nearby, is highly maneuverable and able to hover [41], and is also attracted by the distress calls of conspecifics (unpublished data). We therefore also tested if S. bilineata would be attracted by the heterospecific distress calls of M. molossus, and if so, whether their approaches would be closer to the speaker as predicted by their flight abilities.
Lastly, we investigated if the responsiveness of M. molossus to distress calls would increase over time. At our study site, M. molossus depart from their roosts at sunset, forage intensely for only ~50 min, then return to their roosts [42]. If bats respond to distress calls in order to mob predators, then we predicted that M. molossus might be less responsive to distress calls before foraging, and would be increasingly responsive to distress calls as the night progressed. This prediction is based on the assumption that the the expected fitness costs to an individual mobbing bat would be greater at emergence when their energy reserves are lower and avian predators would have more light. On the other hand, if the approaches do not pose a significant cost or risk, then we do not expect a difference in responsiveness over time.
Methods
Ethics statement
This study was approved by the Institutional Animal Care and Use Committee of the University of Maryland College Park (IACUC Ref#: R-14-07) and of the Smithsonian Tropical Research Institute (IACUC Ref# 2013-1015-2016 and 2014-0815-2017).
Recording and measuring distress calls
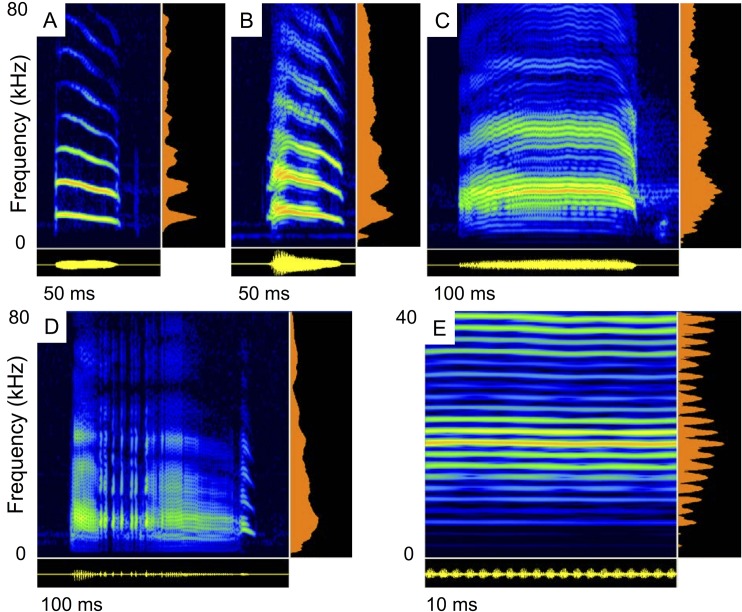
We located eight roosts of Molossus molossus in the laboratory buildings at the Smithsonian Tropical Research Station at Barro Colorado Island, Panama (9°9´17´´ N, 79°51´53´´ W). We recorded distress calls (Fig 1) from 9 adult male M. molossus that were entangled in mist-nets (Ultrathin Mist Nets M-14; Ecotone, Gdynia, Poland) at ~1 m distance with a handheld USG 116Hm (Avisoft Bioacoustics, Berlin, Germany, Avisoft.com; gain set to 0 dB, sample rate 500 kHz, frequency response: 5–30 kHz ± 4dB, 30–100 kHz ± 2dB). We analyzed the M. molossus distress calls used in our playback sequences via color spectrograms (FFT 512, Blackman, dynamic range 90 dB) and waveforms displayed with the software Selena (Animal Physiology, University of Tuebingen). Call duration and call interval (time between the onsets of two consecutive calls) were measured from waveforms. Upper and lower frequency limits were set 25 dB below maximum amplitude. Sideband modulations [43] were measured (FFT 2048) in five calls per bat.
Fig 1. Variation of distress calls of Molossus molossus.
Spectrograms (FFT 1024, Blackman, auto padding, dynamic range of 90 dB) of distress calls playbacks with averaged power spectrum (aside) and waveform (below). Distress calls structures included multiharmonic shallowly modulated calls (A), calls with sideband modulations (B, C), and some calls with nonlinear phenomena (D). Sideband modulations of the distress call shown in C are depicted in E (FFT 2048).
Constructing playback sequences
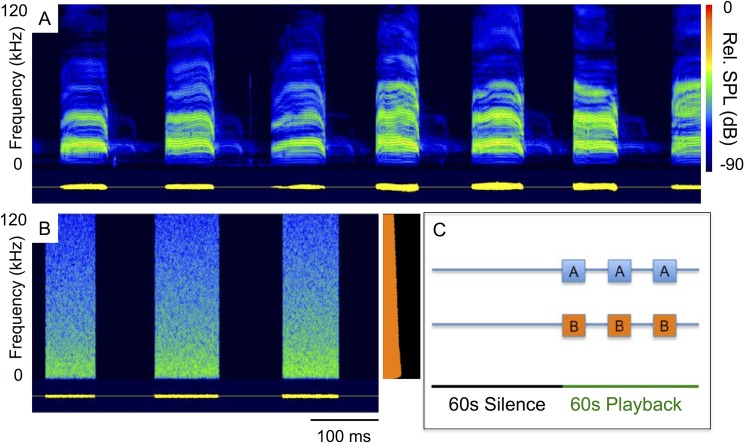
Using BatSound Pro (Pettersson Elektronik, Uppsala, Sweden), we constructed 12 unique 10 s clips of distress calls that preserved the spacing in the original distress call recordings (Fig 2A). Different calls of one male were used for two clips, different calls of a second male were used for three clips and the remaining seven males contributed to one clip each. Each clip included 35–60 calls.
Fig 2. Distress calls and pink noise stimuli.
Spectrograms (500 kHz sampling rate, 16 bit resolution, 1024 FFT, Blackman window) of a sequence of Molossus molossus distress calls (A) and pink noise controls (B) with averaged power spectrum of the third pink noise stimulus. Each 2-min playback sequence (C) begins with a 1-min silent period followed by a 1-min playback period with either distress calls (A boxes) or pink noise bursts (B boxes) repeated three times and spaced apart by 10 s of silence.
For each distress call clip, we created a paired pink noise control clip that roughly matched the variation in amplitude and temporal spacing of each distress call clip (Fig 2B). We then expanded each 10 s clip (12 distress and 12 paired noise clips) into a 120-s playback sequence (Fig 2C) consisting of 60 s of silence (“silent period”) followed by a 60 s period with three repeats of 10 s silence and the 10 s distress call or noise clip (“playback period”).
To eliminate background noise, we bandpass-filtered all the sequences at 2.5–200 kHz (Butterworth filter, filter order = 2). To equalize and maximize signal strength, we adjusted the amplitude of signals in Batsound Pro so that the highest amplitude signal was near 100% without being clipped (adjustments ranged from 1-3x the original).
We measured the SPL range of playbacks to be 77–94 dB SPL rms re to 20 μPa at 1m. We obtained these measures by recording the playback of three paired exemplar distress call and noise stimuli (lowest, highest, and 50% relative amplitude) using a 1/8 inch precision pressure microphone (40DP, G.R.A.S. Sound & Vibration, Denmark, gras.dk) linked to a 12AK Power module (G.R.A.S.) at a distance of 23 cm in a room covered in acoustic foam, and then measured peak-to-peak amplitude (mV) on a Tektronix TDS2014 digital oscilloscope (Tektronix, Inc., tek.com).
Experimental design
We recorded bat passes while broadcasting playbacks during 12 playback sessions on 8 nights at 8 different sites at the Barro Colorado Island field station. Each playback session included 12 unique distress call playback sequences and 12 unique noise playback sequences in alternating order (e.g. distress, noise, distress, noise, etc) spaced apart by at least 60 s of silence. Playback sequences in each session were looped so that the first playback sequence began again after the last. Equipment errors and weather led to some playbacks being interrupted, so playback sessions included 6–50 playback sequences (12 min–100 min).
In total, we broadcast 308 playback sequences (155 and 153 distress and noise playbacks respectively) using an Avisoft USG Player BL Pro speaker (frequency response: 5–80 kHz ± 4 dB). We simultaneously recorded continuously at constant gain with an Avisoft CM16 microphone connected to an UltrasoundGate 116Hn (500 kHz sampling rate, 16 bit resolution) that was pointed in the same direction as the speaker such that bat calls directed towards the speaker would be highest in amplitude. By visual inspection of the spectrograms, we could easily discriminate between our playbacks and real bat calls.
Measuring bat responses
We defined a “pass” as one or more echolocation calls where at least one call exceeded an amplitude threshold of 10% in BatSound Pro. A typical single pass consists of a series of echolocation pulses that begin below 10% amplitude, increase over 10%, then decreases again below 10%. This pattern indicates that a bat was flying nearby the speaker or turning towards it.
By examining the spectrograms, we categorized each pass as Molossus sp. (probably M. molossus), Saccopteryx bilineata or “other” (usually Myotis nigricans). We assumed that Molossus calls we recorded were M. molossus, because this species was by far the most abundant molossid bat in the area: more than 95% of molossid bats (n = 256) caught in the areas where we conducted our experiments were M. molossus, and others were M. sinaloae or M. bondae (unpublished data 2010–2014).
We measured two responses in M. molossus and S. bilineata. “Activity” is the number of bat passes occurring during the 2-min playback sequence. “Responsiveness” is the change in the number of passes after onset of playback (i.e., number of passes during the 1-min playback minus the number of passes during previous 1-min silence; Fig 2C). Additionally, we scored if a M. molossus pass included a social call.
To observe close approaches to the speaker, we illuminated and videotaped the speaker using an infrared (IR) spotlight (IRLamp6, Wildlife Engineering, irlight.com) and a Sony DCR-SR85 Nightshot camcorder. This allowed us to observe an area of more than a square meter in front of the speaker. We reviewed video footage in fast-forward during periods with no recordings detected, and carefully reviewed footage in real time or slower during periods with an increase of 3 passes during the 1-min playback (responsiveness > 3 passes).
Statistical analysis
We tested (1) if the number of bat passes increased from the 1-min silent period to the 1-min playback period (mean responsiveness > 0) and (2) if this increase in bat passes (responsiveness) was greater during distress call playback compared to noise playback. We inspected histograms and normal quantile plots to confirm normality of responsiveness values. To test the effect of playback treatment on responsiveness, we used restricted maximum likelihood to fit a linear mixed model in JMP 12 [44] with playback treatment (distress or noise) as a fixed factor and playback sequence (1–12) as a random factor nested within treatment.
We excluded observations of zero bat passes during both the silent and playback period, because these cases indicated an absence of bats, and a zero-inflated dataset would inflate sample size, reduce effect size estimates, and create deviation from normality. To check the robustness of our results, we also repeated our analyses including these cases with zero bat passes, using a permuted linear model (lmPerm package in R) for inference. We only present the analysis excluding the sequences with no bat passes present, but our conclusions were the same using either approach.
To determine if M. molossus responsiveness to distress calls differed between when bats were either departing or returning to roosts, we first plotted M. molossus activity and responsiveness during distress call sequences over time. We analyzed recordings with bats present from four evenings (n = 54 playback sequences; 108 min) where the microphone and speaker were positioned within ~20 meters of a roost but facing in such a way that the bats would need to deviate from their normal flight paths to approach the speaker. We then tested if M. molossus activity or responsiveness increased from emergence (1840 h) until return (2000 h) by fitting a linear model in JMP 12.
Results
Distress call variation
Distress call structure from the nine male Molossus molossus varied both within and among individuals (Fig 1), from pure multiharmonic shallowly modulated signals to calls with nonlinear phenomena. Most distress calls had sideband modulations [43] (Fig 1E). The mean modulation frequency was 1.7 kHz. The mean overall bandwidth set by the upper and lower frequency limits was 45 kHz with a mean lower frequency limit of 10 kHz. Average call duration was 64 ms and call interval ranged from 0.06 to 1.3 s (Table 1). The peak frequency corresponded to the first harmonic in 90% of the calls (n = 506; mean peak frequency = 16.0 kHz ± 3.6 kHz S.E.), to the second harmonic in 9% of calls (n = 51; mean = 32.9± 7.1 kHz), and to the third harmonic in 1% of calls (n = 4; mean = 50.9 ± 9.6 kHz).
Table 1. Call parameters of playback distress calls (mean ± standard deviation).
| Bat | N | Call duration(ms) | Call interval (ms) | Peak freq. (1st harm.) (kHz) (N) | Max freq (kHz) | Min freq (kHz) | Bandwidth (kHz) | Freq. of sideband modulation (kHz) |
|---|---|---|---|---|---|---|---|---|
| 1 | 111 | 73.1 ± 10.9 | 179.0 ± 39.6 | 18.4 ± 1.7 (103) | 12.5 ± 1.7 | 61.3 ± 10.6 | 48.9 ± 10.9 | 2.0 ± 0.2 |
| 2 | 145 | 54.1 ± 16.6 | 207.7 ± 140.5 | 15.2 ± 3.2 (141) | 9.1 ± 1.6 | 58.0 ± 10.5 | 48.9 ± 10.8 | 2.0 ± 0.1 |
| 3 | 60 | 50.3 ± 15.0 | 164.1 ± 26.9 | 12.0 ± 1.1 (58) | 8.1 ± 1.5 | 39.2 ± 8.7 | 31.2 ± 9.1 | 1.4 ± 0.1 |
| 4 | 18 | 63.0 ± 18.1 | 263.1 ± 227.8 | 16.8 ± 6.5 (17) | 7.9 ± 3.4 | 52.7 ± 3.4 | 44.9 ± 8.6 | 1.6 ± 0.1 |
| 5 | 39 | 65.1 ± 14.7 | 248.7 ± 145.8 | 17.9 ± 4.6 (32) | 9.7 ± 3.0 | 49.8 ± 7.7 | 40.1 ± 7.8 | 1.9 ± 0.2 |
| 6 | 41 | 68.3 ± 27.9 | 241.3 ± 129.1 | 16.3 ± 4.3 (41) | 9.9 ± 2.3 | 56.1 ± 8.6 | 46.2 ± 10.0 | 1.8 ± 0.3 |
| 7 | 54 | 72.5 ± 15.8 | 183.2 ± 35.2 | 16.8 ± 4.2 (36) | 11.3 ± 2.4 | 58.9 ± 9.0 | 47.6 ± 9.2 | 1.9 ± 0.1 |
| 8 | 44 | 65.9 ± 25.5 | 225.1 ± 179.0 | 19.9 ± 1.5 (32) | 10.3 ± 3.8 | 50.7 ± 11.0 | 40.4 ± 11.1 | 1.6 ± 0.1 |
| 9 | 49 | 75.1 ± 14.9 | 206.1 ± 39.2 | 15.6 ± 2.1 (46) | 9.2 ± 2.8 | 56.5 ± 9.1 | 47.3 ± 9.7 | 1.6 ± 0.0 |
| 64.1 ± 19.3 | 203.1 ± 113.8 | 16.0 ± 3.6 | 10.0 ± 2.7 | 55.1 ± 11.5 | 45.1 ± 6.9 | 1.7 ± 0.2 |
Response to playback
Molossus molossus activity was twice as high as Saccopteryx bilineata at our recording sites. We recorded 1–16 M. molossus passes during 116 of the 308 two-min playback sequences (Table 2), and 1–9 S. bilineata passes during 59 playback sequences. Overall, we recorded 154 M. molossus passes during distress call playback periods and 90 passes during the paired silent periods. During noise playback and paired silent periods, we recorded 76 and 90 passes, respectively. For S. bilineata, there were 58 and 36 passes during distress call and paired silent periods, respectively. During the noise playback and paired silent periods, we recorded 28 and 54 S. bilineata passes, respectively.
Table 2. Number of playback sequence leading to more or fewer passes.
| Bat response | M. molossus distress call playbacks | Pink noise playbacks |
|---|---|---|
| M. molossus | ||
| Passes increase | 39 | 15 |
| No change | 8 | 12 |
| Passes decrease | 18 | 24 |
| S. bilineata | ||
| Passes increase | 18 | 5 |
| No change | 4 | 4 |
| Passes decrease | 11 | 17 |
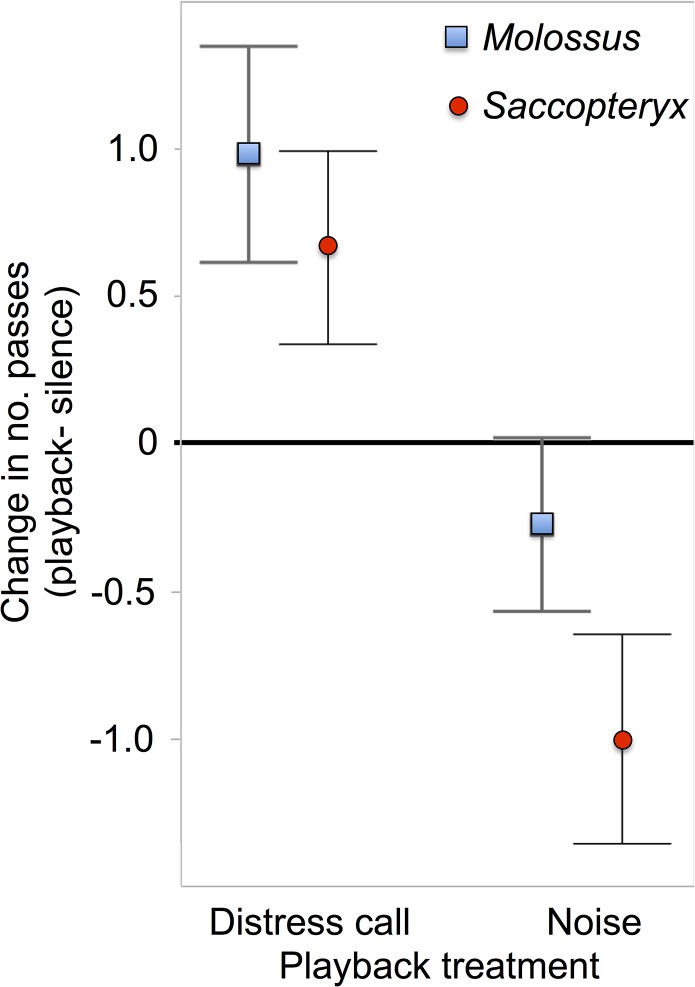
M. molossus activity increased more during distress call playbacks than during noise playbacks (F(1,20.12) = 4.47, p = 0.0298: Fig 3), and this difference was driven by attraction to distress calls (t = 2.68, df = 64, one-sided p = 0.0047) rather than avoidance of noise (t = 0.93, df = 50, one-sided p = 0.16; Fig 4). Mean S. bilineata activity also increased more during playbacks of M. molossus distress calls compared to noise (F(1,16.79) = 14.95, p = 0.0013; Fig 3), and this difference was driven by both an attraction to distress calls (t = 2.04, df = 32, one-sided p = 0.0251) and an avoidance of noise (t = 2.82, df = 25, one-sided p = 0.0047; Fig 5). We observed passes by other unidentified bat species in only 20 of 308 playback sequences, and found no effect of playback on this limited activity (F(1,22) = 0.39, p = 0.5).
Fig 3. Effect of playback treatment on bat activity.
The mean responsiveness (number of passes during playback period–silent period) is shown for M. molossus (blue squares) and S. bilineata (red circles) in response to either M. molossus distress calls or pink noise. Errors bars show standard error of the mean.
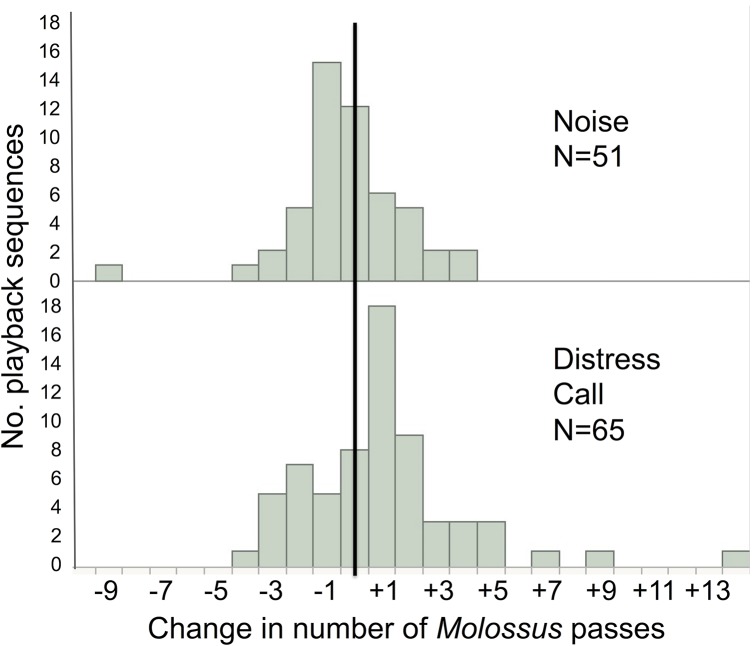
Fig 4. Distributions of Molossus molossus responsiveness.
Frequency histograms of responsiveness during playback sequences that had passes of M. molossus (n = 116). Increases in activity are to the right of the solid line.
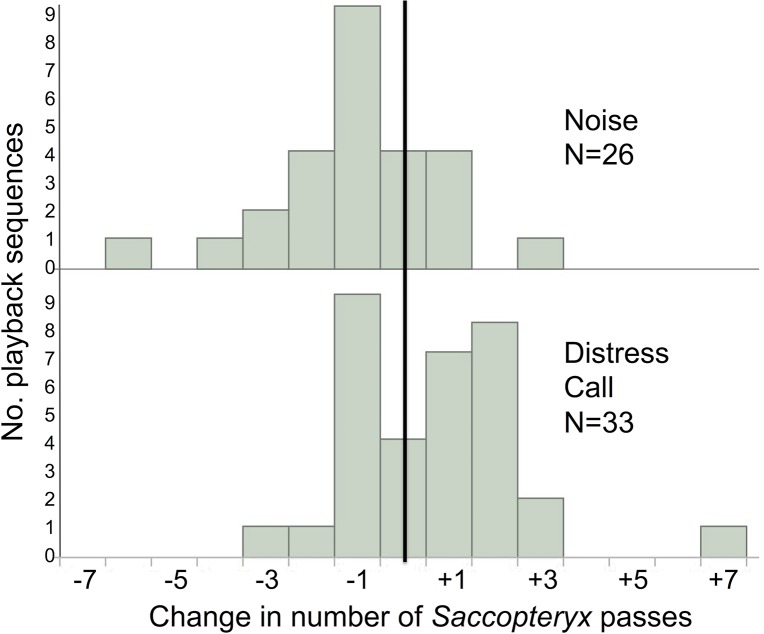
Fig 5. Distributions of Saccopteryx bilineata responsiveness.
Frequency histograms of responsiveness during playback sequences that had passes of S. bilineata (n = 59). Increases in activity are to the right of the solid line.
Social calls during passes by M. molossus occurred in only 17 of 308 playback sequences. During distress call sequences, passing M. molossus produced 3 social calls during silent periods and 9 social calls during distress call playback. During noise sequences, the 8 social calls were evenly divided between silent and noise periods. With this small sample size, we failed to detect an effect of playback treatment (t = 1.1, df = 15, p = 0.29) or a difference in social calling between distress call playback periods and silent periods (t = 1.96, df = 9, p = 0.08; 95% confidence interval of distress call playback effect on social call production = -0.09 to +1.29 social calls).
Behavior of passing bats during periods of high responsiveness
Bats approached the speaker during distress calls, but they did not approach closely, and if they ever approached in groups they did so either rarely or not at all. Across 308 playback sequences, there were only 11 sequences that led to responsiveness of >3 passes. For M. molossus, this occurred during two noise sequences and 9 distress call sequences. However, in the video recordings we saw no visible evidence of approaches to within 1 meter of the speaker, either as individuals or in groups. If bats were inspecting the location for predation risk, they did so primarily as individuals. Single M. molossus passes consisted of fast straight flights that did not approach the speaker. In S. bilineata, responsiveness exceeded 3 passes in only one case where an individual (probably a territorial male) circled very closely around the speakers, but we did not see multiple S. bilineata making close approaches.
Molossus molossus activity and responsiveness over time
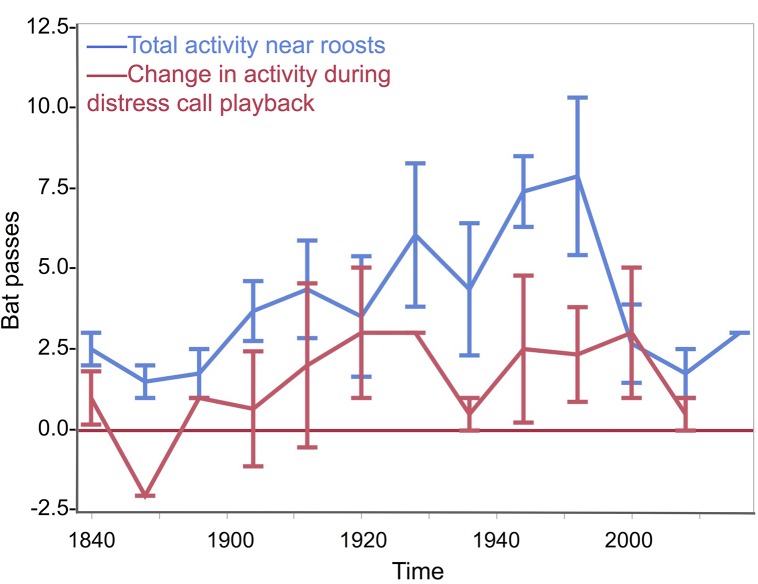
M. molossus passes near roosts increased steadily after emergence at sunset then declined rapidly after about 1.5 h (Fig 6). Starting at emergence at 1840 h, activity increased until 2000 h (R2 = 0.28, F(1,44) = 17.02, p = 0.0002); but we found no evidence that distress call responsiveness increased over this same time period (R2 = 0.07, F(1,23) = 1.64, p = 0.21; Fig 6).
Fig 6. Molossus molossus activity and responsiveness to distress call playback near roosts over time.
Mean number of passes (and standard errors) during the 2-min playback sequences recorded on four evenings from 1838 to 2020 h. Data are pooled in 8-min time bins (2–6 counts per bin). Counts do not include observations of zero passes. Blue line shows the total number of M. molossus passes during both distress call and noise playbacks. Red line shows the responsiveness (number of passes during the 1-min playback period minus 1-min silent period) during distress call playbacks.
Discussion
Response to playback
Both the free-tailed bats Molossus molossus and sac-winged bats Saccopteryx bilineata were more attracted to the M. molossus distress call playbacks than to playbacks of silence or pink noise. S. bilineata also actively avoided the noise playbacks. The attraction of both bat species to distress calls cannot therefore be explained as a general investigation of broadband sounds of similar duration. The increase in activity in response to the M. molossus distress calls was larger for conspecifics than for S. bilineata.
The effect of distress call playbacks on bat activity was more consistent with individual inspection flights than mobbing by individuals or groups. M. molossus most often responded to distress calls with one additional pass (+1 pass after silence; +1.26 passes compared to noise); multiple passes during the 1-min playback were rare (Fig 4). Video analyses suggested that multiple passes were more likely to represent a single bat circling than a group of bats.
Why did S. bilineata respond to M. molossus distress calls? Several species of bats are known to respond to heterospecific distress calls [12, 15], and tests on the response of European pipistrelles to experimentally synthesized or modified distress calls show that some bats will respond to a range of conspecific-like distress calls depending on the spectral and temporal call characteristics [16]. Therefore, heterospecific bats may be generally responsive to such distress calls and S. bilineata were simply the most commonly detected heterospecific bats at our study site. Alternatively, responses from this species might have only occurred because individuals of these two species roost nearby each other at this site and therefore face shared predation threats. In either case, the pattern of responses by S. bilineata was also more consistent with individual inspections than cooperative mobbing.
Our findings should be considered with two limitations in mind. First, our results do not prove that mobbing does not occur or would not occur in this species under different circumstances. For example, bats may have reacted differently if we presented a life-like predator model. Second, responses may have differed if we played the distress calls of females rather than males. It is unclear if adult bat distress calls convey the sex or individual identity of callers, but males are more likely to produce distress calls in at least some species (e.g. Sturnira lilium [17]) and social relationships in most bat species are female-biased [45]. Finally, the hypothesis that distress calls promote mobbing in other bat species is not altogether unlikely given previous evidence of mobbing [30–31] and other similar cooperative behaviors in at least some bats [45]. However, future studies should examine the mobbing hypothesis more critically.
Activity and responsiveness over time
In our study, M. molossus activity near roosts increased as the evening progressed from sunset until the bats returned to roosts after their first foraging bout. However, we failed to detect an increase over time in responsiveness (Fig 6). This may have been due to the large variance (range: -2 to +9 passes) and small sample (n = 25) of distress call responsiveness values, but it is also possible that bats are just as responsive to predation risk when departing from roosts as when returning to roosts. This finding is also more consistent with the predator inspection hypothesis than the mobbing hypothesis.
Distress call structure and function
Many authors have proposed non-mutually-exclusive functions of distress calls in vertebrates, but the fitness benefits of distress calls remain ambiguous [1–23]. Russ et al. [14–15] concluded that distress calls by pipistrelles likely function to attract conspecifics that perform mobbing behavior. Unlike pipistrelles, however, the poor maneuverability of M. molossus could severely limit their ability to perform flight patterns needed for effective mobbing, such as tight circling within a cluttered environment. We observed no mobbing behaviors or close approaches to the speaker during the times when we recorded a large increase in M. molossus passes. Instead, bats typically flew by quickly in straight paths. These observations combined with the relatively weak response of conspecifics compared to pipistrelles [14–15], and the observation that highly maneuverable S. bilineata approached and circles the speakers more closely than M. molossus, suggest that M. molossus distress calling does not promote conspecific mobbing behavior.
A simpler adaptive explanation is that captured bats produce distress calls to startle naive or inexperienced predators into releasing the caller [6–8, 17, 23]. This startle hypothesis is consistent with the presence of nonlinearities, such as sideband modulations [43], and a large variation in call structure both among and within callers, which can reduce habituation [46–50]. Russ et al. [14] argued that pipistrelle distress calls would not be effective at startling avian predators because the lower frequency limit of these calls is about 17 kHz, which is beyond the audible range of raptors and owls (up to 10–12.5 kHz). In contrast, M. molossus distress calls have a mean lower frequency limit of 10 kHz and could more easily serve as signals to both avian and mammalian predators. It would be interesting to see if M. molossus distress calls are structurally similar to calls given in agonistic contexts, because the startle hypothesis predicts that calls produced in either context should provide the same information regarding the ability of the caller to attack or defend itself. For example, avian distress calls contain information about health and thus ability to defend or escape [8].
Distress call structure varies across bat species, but common design features include longer durations, lower frequencies, and greater bandwidth relative to other social or echolocation calls [17]. These general acoustic characteristics do not implicate a single clear function because the same design might reflect multiple possible selective pressures, such as maximizing travel distance, conveying honest information about the caller’s ability to defend itself, or ensuring the signal is heard by a broadest range of predators. The potential fitness benefits of distress calls are also difficult to confirm because predation events are rare but have extreme fitness consequences. A single escape, however unlikely, could easily outweigh the fitness costs of being a frequent screamer when captured. That is, even if the success of mobbing, startling, or attracting other predators, is very unlikely—these rare positive outcomes may still provide an adequate benefit to maintain the trait of distress calling over evolutionary time.
Regardless of the adaptive function of distress calls, the most parsimonious explanation for the response of both conspecifics and heterospecifics is that approaching bystanders are merely investigating sources of distress calls to gather information about predation risk. Our results suggest that mobbing in response to male M. molossus distress calls is rare or nonexistent. M. molossus distress calls likely have other functions because this species frequently produces distress calls (even more so than many other bats), but does not appear to cooperatively mob in response to them (even less so than other bats). Based on our results, we suggest that the observation of bats approaching distress call playbacks does not itself provide clear evidence for mobbing. Future studies seeking to test the mobbing hypothesis would benefit from playing calls of both familiar and unfamiliar conspecifics of both sexes, and presenting visible life-like predator models, e.g. [51].
Acknowledgments
Two anonymous reviewers provided valuable feedback on the manuscript. We thank the other faculty of the animal communication field course: Hans-Ulrich Schnitzler (University of Tuebingen), Cindy Moss and Jerry Wilkinson (University Maryland, College Park). Ed Smith of the Center for Comparative and Evolutionary Biology of Hearing helped us measure dB SPL of our playbacks. This work was supported by the UMD-UT collaboration program of the University of Maryland and the University of Tuebingen. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tuebingen.
Data Availability
Playback response data can be found at Figshare: http://dx.doi.org/10.6084/m9.figshare.1454597.
Funding Statement
This project was funded by the UMD-UT collaboration program of the University of Maryland and the University of Tuebingen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. De Toledo LF, Fernando C, Haddad B. Defensive vocalizations of Neotropical anurans. S Am J Herpetol. 2009;4: 25–42. [Google Scholar]
- 2. Frankenberg E. Distress calls of gekkonid lizards from Israel and Sinai. Isr J Zool. 1975;24: 43–53. [Google Scholar]
- 3. Staton MA. “Distress Calls" of crocodilians–whom do they benefit? Am Nat. 1978;112: 327–332. [Google Scholar]
- 4. Perrone M. Factors affecting the incidence of distress calls in passerines. Wilson Bull. 1980;92: 404–408. [Google Scholar]
- 5. Hill G E The function of distress calls given by tufted titmice (Parus bicolor): an experimental approach. Anim Behav. 1986;34: 590–598. [Google Scholar]
- 6. Conover MR. Stimuli eliciting distress calls in adult passerines and response of predators and birds to their broadcast. Behav. 1994;131:19–37. [Google Scholar]
- 7. Wise KK, Conover MR, Knowlton FF. Response of coyotes to avian distress calls: testing the startle-predator and predator-attraction hypotheses. Behav. 1999;136: 935–950. [Google Scholar]
- 8. Laiolo P, Tella JL, Carrete M, Serrano D, López G. Distress calls may honestly signal bird quality to predators. Proc R Soc B. 2004; 271: S513–S515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Branch CL, Freeberg TM. Distress calls in tufted titmice (Baeolophus bicolor): are conspecifics or predators the target?. Behav Ecol 2012;23:854–862. [Google Scholar]
- 10. Fenton MB, Belwood JJ, Fullard JH, Kunz TH. Responses of Myotis lucifugus (Chiroptera: Vespertilionidae) to calls of conspecifics and to other sounds. Can J Zool. 1976;54: 1443–1448. [Google Scholar]
- 11. August PV. Distress calls in Artibeus jamaicensis: ecology and evolutionary implications In: Eisenberg JF, editor. Vertebrate ecology in the northern Neotropics. Washington, DC: Smithsonian Institution Press; 1979. pp. 151–159. [Google Scholar]
- 12. August PV. Acoustical properties of the distress calls of Artibeus jamaicensis and Phyllostomus hastatus (Chiroptera: Phyllostomidae). Southwest Nat. 1985;30: 371–375. [Google Scholar]
- 13. Ryan JM, Clark DB, Lackey JA. Response of Artibeus lituratus (Chiroptera: Phyllostomidae) to distress calls of conspecifics. J Mamm. 1985;66: 179–181. [Google Scholar]
- 14. Russ J, Racey P, Jones G. Intraspecific responses to distress calls of the pipistrelle bat, Pipistrellus pipistrellus . Anim Behav. 1998;55: 705–713. 10.1006/anbe.1997.0665 [DOI] [PubMed] [Google Scholar]
- 15. Russ JM, Jones G, Mackie IJ, Racey PA. Interspecific responses to distress calls in bats (Chiroptera: Vespertilionidae): a function for convergence in call design? Anim Behav. 2004; 67: 1005–1014. 10.1016/j.anbehav.2003.09.003 [DOI] [Google Scholar]
- 16. Russ JM, Jones G, Racey PA. Responses of soprano pipistrelles, Pipistrellus pygmaeus, to their experimentally modified distress calls. Anim Behav. 2005;70: 397–404. [Google Scholar]
- 17.Nagel J. Variation in distress calls of New World bats. M.Sc. Thesis. University of Western Ontario. 2006.
- 18. Lima SL, O'Keefe JM. Do predators influence the behaviour of bats?. Biol Rev. 2013;88: 626–644. 10.1111/brv.12021 [DOI] [PubMed] [Google Scholar]
- 19. Caro T. Antipredator defenses in birds and mammals Chicago: University of Chicago Press; 2005. [Google Scholar]
- 20. Hauser MD. Male responsiveness to infant distress calls in free-ranging vervet monkeys. Behav Ecol Sociobiol. 1986;19: 65–71. [Google Scholar]
- 21. Lemasson A, Palombit RA, Jubin R. Friendships between males and lactating females in a free-ranging group of olive baboons (Papio hamadryas anubis): evidence from playback experiments. Behav Ecol Sociobiol. 2008;62: 1027–1035. [Google Scholar]
- 22. Rendall D, Notman H, Owren MJ. Asymmetries in the individual distinctiveness and maternal recognition of infant contact calls and distress screams in baboons. J Acous Soc Amer. 2009;125: 1792–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hogstedt G. Adaptation unto death: function of fear screams. Am Nat. 1983;121: 562–570. [Google Scholar]
- 24. Naguib M, Mundry R, Ostreiher R, Hultsch H, Schrader L, Todt D. Cooperatively breeding Arabian babblers call differently when mobbing in different predator-induced situations. Behav Ecol. 1999;10:636–640. 10.1093/beheco/10.6.636 [DOI] [Google Scholar]
- 25. Manser MB. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc R Soc B. 2001;268: 2315–2324. 10.1098/rspb.2001.1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Templeton CN, Greene E, Davis K. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science. 2005;308: 1934–1937. 10.1126/science.1108841 [DOI] [PubMed] [Google Scholar]
- 27. Zuberbühler K. Predator-specific alarm calls in Campbell's monkeys, Cercopithecus campbelli . Behav Ecol Sociobiol. 2001;50: 414–422. [Google Scholar]
- 28. Griesser M. Referential calls signal predator behavior in a group-living bird species. Curr Biol. 2008;18: 69–73. 10.1016/j.cub.2007.11.069 [DOI] [PubMed] [Google Scholar]
- 29. Griesser M. Mobbing calls signal predator category in a kin group-living bird species. Proc Roy Soc B. 2009;276: 2887–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knörnschild M, Tschapka M. Predator mobbing behaviour in the Greater Spear-Nosed Bat, Phyllostomus hastatus . Chiroptera Neotropical 2012; 18: 1132–1135. [Google Scholar]
- 31. Lučan RK, Šálek M. Observation of successful mobbing of an insectivorous bat, Taphozous nudiventris (Emballonuridae), on an avian predator, Tyto alba (Tytonidae). Mammalia. 2013;77: 235–236. [Google Scholar]
- 32. Dugatkin LA, Godin JJ. Prey approaching predators: a cost-benefit perspective. Ann Zool Fennici. 1992;29: 233–252. [Google Scholar]
- 33. Pitcher TJ, Green D A, Magurran AE. Dicing with death: predator inspection behaviour in minnow shoals. J Fish Biol. 1986;28: 439–448. [Google Scholar]
- 34. FitzGibbon CD. The costs and benefits of predator inspection behaviour in Thomson's gazelles. Behav Ecol Sociobiol. 1994;34: 139–148. [Google Scholar]
- 35. Dechmann DK, Kranstauber B, Gibbs D, Wikelski M. Group hunting—a reason for sociality in molossid bats? PLOS One. 2010;5: e9012 10.1371/journal.pone.0009012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Norberg UM, Rayner JM. Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc B. 1987;316: 335–427. [Google Scholar]
- 37. Voigt CC, Holderied MW. High manoeuvring costs force narrow-winged molossid bats to forage in open space. J Comp Phys B. 2012; 182: 415–424. [DOI] [PubMed] [Google Scholar]
- 38. Mora EC, Macías S, Vater M, Coro F, Kössl M. Specializations for aerial hawking in the echolocation system of Molossus molossus (Molossidae, Chiroptera). J Comp Physiol A 2004; 190: 561–574. 10.1007/s00359-004-0519-2 [DOI] [PubMed] [Google Scholar]
- 39. Denzinger A, Schnitzler HU. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front Physiol. 2013;4: 1–15. 10.3389/fphys.2013.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung K, Molinari J, Kalko EKV. Driving factors for the evolution of species-specific echolocation call design in New World free-tailed bats (Molossidae). PLOS One. 2014;9: e85279 10.1371/journal.pone.0085279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knörnschild M, Fulmer AG, Von Helversen O. Duration of courtship displays corresponds to social status in male greater sac-winged bats (Saccopteryx bilineata). Can J Zool. 2010;88: 589–594. [Google Scholar]
- 42. Holland RA, Meyer CF, Kalko EKV, Kays R, Wikelski M. Emergence time and foraging activity in Pallas' mastiff bat, Molossus molossus (Chiroptera: Molossidae) in relation to sunset/sunrise and phase of the moon. Acta Chiropterol. 2011;13: 399–404. [Google Scholar]
- 43. Frommolt KH. Sidebands- facts and artefacts. Bioacoustics, 1999;10: 219–224. [Google Scholar]
- 44.JMP, Version 12. SAS Institute Inc., Cary, NC, 1989–2007.
- 45. Carter G, Wilkinson G. Cooperation and conflict in the social lives of bats In: Adams R, Pedersen S, editors. Bat evolution, ecology, and conservation. New York: Springer Science Press; 2013. pp. 225–242. [Google Scholar]
- 46. Blumstein DT, Récapet C. The sound of arousal: The addition of novel non‐linearities increases responsiveness in marmot alarm calls. Ethol. 2009;115: 1074–1081. [Google Scholar]
- 47. Townsend SW, Manser MB. The function of nonlinear phenomena in meerkat alarm calls. Biol Lett. 2011;7: 47–49. 10.1098/rsbl.2010.0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slaughter EI, Berlin ER, Bower JT, Blumstein DT. A test of the nonlinearity hypothesis in great‐tailed grackles (Quiscalus mexicanus). Ethol. 2013;119: 309–315. [Google Scholar]
- 49. Karp D, Manser MB, Wiley EM, Townsend SW. Nonlinearities in meerkat alarm calls prevent receivers from habituating. Ethol. 2014;120: 189–196. [Google Scholar]
- 50. Arnal LH, Flinker A, Kleinschmidt A, Giraud AL, Poeppel D. Human screams occupy a privileged niche in the communication soundscape. Curr Biol. 2015. 10.1016/j.cub.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Breviglieri CPB., Piccoli GCO, Uieda W, Romero GQ. Predation-risk effects of predator identity on the foraging behaviors of frugivorous bats. Oecologia. 2013;173: 905–912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Playback response data can be found at Figshare: http://dx.doi.org/10.6084/m9.figshare.1454597.