Abstract
Background
Childhood cancer survivors treated with cranial or total body irradiation (TBI) are at risk for growth hormone deficiency (GHD). Recombinant growth hormone (rhGH) therapy is associated with slipped capital femoral epiphysis (SCFE). We compared the incidence of SCFE after TBI versus cranial irradiation (CI) in childhood cancer survivors treated with rhGH.
Procedure
Retrospective cohort study (1980–2010) of 119 survivors treated with rhGH for irradiation-induced GHD (56 TBI; 63 CI). SCFE incidence rates were compared in CI and TBI recipients, and compared with national registry SCFE rates in children treated with rhGH for idiopathic GHD.
Results
Median survivor follow-up since rhGH initiation was 4.8 (range 0.2–18.3) years. SCFE was diagnosed in 10 subjects post-TBI and none after CI (P < 0.001). All 10 subjects had atypical valgus SCFE, and 7 were bilateral at presentation. Within TBI recipients, age at cancer diagnosis, sex, race, underlying malignancy, age at radiation, and age at initiation of rhGH did not differ significantly between those with versus without SCFE. The mean (SD) age at SCFE diagnosis was 12.3 (2.7) years and median duration of rhGH therapy to SCFE was 1.8 years. The SCFE incidence rate after TBI exposure was 35.9 per 1,000 person years, representing a 211-fold greater rate than reported in children treated with rhGH for idiopathic GH deficiency.
Conclusions
The markedly greater SCFE incidence rate in childhood cancer survivors with TBI-associated GHD, compared with rates in children with idiopathic GHD, suggests that cancer treatment effects to the proximal femoral physis may contribute to SCFE.
Keywords: childhood cancer survivors, cranial irradiation, growth hormone, SCFE, total body irradiation
INTRODUCTION
Slipped capital femoral epiphysis (SCFE) is a common hip disorder of childhood and adolescence, typically occurring between 8 and 15 years of age [1,2]. SCFE represents a displacement of the proximal femoral metaphysis relative to the epiphysis, with slippage occurring through the growth plate [3]. In most cases the epiphysis is oriented posterior and medial to the metaphysis. The reported prevalence of SCFE in healthy adolescents is 10.8 cases per 100,000 children and more commonly noted in males, blacks, and Pacific Islanders [4]. The etiology of SCFE is often multifactorial, and typically is observed in obese males during a growth spurt. Less commonly, SCFE can be associated with endocrine disorders such as hypothyroidism, hypogonadism, panhypopituitarism [5–7], and treatment with recombinant human growth hormone (rhGH) [2].
Survivors of childhood cancer treated with cranial irradiation (CI) or total body irradiation (TBI) are at risk for radiation-induced growth hormone (GH) deficiency as well as multiple endocrine abnormalities [8]. The hypothalamic–pituitary axis of the young is very radiosensitive and younger children are more susceptible to radiation-induced GH deficiency even after low-doses of CI (1,800–2,400 cGy) or fractionated TBI (1,200 cGy) [9,10]. Thus, treatment with rhGH is often necessary to address growth disorders in childhood survivors with radiation-induced GH deficiency.
SCFE is a recognized orthopedic adverse event reported in survivors of childhood cancer on rhGH [11], as well as in patients treated with pelvic irradiation [12]. However, to date, no study has compared CI or TBI as a risk factor for SCFE in a cohort of patients receiving rhGH. In addition, most studies to date have not specifically focused on SCFE after radiation and chemotherapy in survivors and are limited by very small number of subjects [13]. As SCFE can present with vague symptoms, a delay in diagnosis may be associated with a greater severity of proximal femoral deformity, potentially increasing the risks of complications such as impingement and degenerative hip arthritis [3,14]. Therefore, recognition of specific cancer treatment-related risk factors in survivors of childhood cancer on rhGH is imperative for prompt recognition and treatment of SCFE. The objective of this study was to compare the incidence of SCFE in survivors of childhood cancer therapy after TBI versus CI exposure diagnosed with radiation-induced GH deficiency and treated with rhGH therapy at our institution.
METHODS
Study Cohort
We identified 490 children and young adults diagnosed with and treated for a pediatric malignancy between 1980 and 2010, who were currently in remission and followed at the Children’s Hospital of Philadelphia (CHOP) Oncology, Endocrinology, or Survivorship Clinics with available records for review. Subjects were eligible for study inclusion if exposed to CI and/or TBI with subsequent diagnosis of radiation-induced GH deficiency and history of rhGH therapy. Survivors with GH deficiency due to tumor location without radiation treatment (e.g., craniopharyngioma) were not eligible. Other exclusion criteria included underlying diagnosis of Turner syndrome, chronic renal failure, and hematopoietic stem cell transplantation for mucopolysaccharides given associated musculoskeletal complications present in these disorders. Among the 137 survivors with a confirmed diagnosis of GH deficiency, 5 subjects did not receive rhGH therapy and 13 additional subjects were excluded based on the outlined exclusion criteria. SCFE incidence rate in survivors was compared to registry data in children, 6–16 years, treated with rhGH for idiopathic GH deficiency (non-cancer related) based on the National Cooperative Growth Study (NCGS) adverse event reports from 1985 to 2006 [15]. The study protocol was approved by the Institutional Review Board at CHOP.
Cancer and GH Treatment Information
Participant and treatment characteristics were ascertained through abstraction of medical and radiation therapy records, including underlying cancer diagnosis, chemotherapy, radiation therapy, and surgical treatment. GH deficiency was diagnosed based on inadequate growth pattern and confirmatory stimulation testing with arginine and clonidine as per institution standards. All treated survivors received daily rhGH (0.3 mg/kg/week) subcutaneous injection with dose adjustment based on treatment response and monitored growth factor levels.
Definition and Ascertainment of SCFE
SCFE diagnosis was initially ascertained through medical records and surgical diagnostic codes. Subsequent SCFE diagnosis in all subjects was confirmed by review of pre- and post-operative X-ray images by an orthopedic surgeon (D.S.) as well as corresponding operative report.
Endocrine Disorders Following Cancer Treatment
Treatment-associated endocrine disorders such as hypothyroidism and hypogonadism were recorded in the CI and TBI groups. Hypogonadism was defined as need for sex hormone replacement (testosterone in males or estrogen in females) for pubertal initiation, progression, and/or lack of adequate gonadal sex hormone production in post-pubertal survivors.
Statistical Analysis
The incidence rate of SCFE per 1,000 person-years was calculated from the time of initiation of rhGH for the treatment of radiation-induced GH deficiency to the first occurrence of SCFE, with censoring at death or the date of last contact. Standard errors of incidence estimates were calculated and used to evaluate 95% confidence intervals.
SCFE incidence rates in cancer survivors on rhGH therapy following radiation were compared with rates reported by NCGS in 22,696 children treated with rhGH for idiopathic growth hormone deficiency (GHD). The rates were compared as the incidence rate ratio. Stata version 12.0 (Stata Corp., College Station, TX) was used for all analysis. A P-value of < 0.05 was considered significant, and two-sided tests of hypothesis were used throughout. Group differences between CI and TBI survivors were tested using t-test or Wilcoxon rank sum test if indicated.
RESULTS
Subject and Disease Characteristics
A total of 119 survivors with rhGH therapy for radiation-induced GH deficiency were eligible. Characteristics of the cohort with comparisons of TBI and CI are shown in Table I. The mean age at radiation exposure was significantly greater in CI versus TBI recipients (P < 0.001). Sex and race distribution did not differ between the groups. The age at initiation of rhGH was marginally greater in TBI patients (P = 0.06); however, the duration of follow-up was comparable between the groups. Majority of survivors received CI for treatment of a brain tumor (n = 49) followed by acute lymphoblastic leukemia (ALL; n = 7) and nasopharyngeal sarcoma (n = 6). Within the TBI exposed survivors, the most common diagnosis was high risk neuroblastoma (n = 27) followed by ALL (n = 11), acute myelogenous leukemia (n = 10), bone marrow failure (n = 4), juvenile myelomonocytic leukemia (n = 3), hemophagocytic lymphohistiocytosis (HLH; n = 1), and Ewing Sarcoma (n = 1).
TABLE I.
Subject, Disease, and Treatment Characteristics
| Cranial irradiation (n = 62) | Total body irradiation (n = 57) | P-value | |
|---|---|---|---|
| Age at radiation exposure, mean ± SD (range) years | 6.5 ± 3.8 (0.3–17.1) | 3.2 ± 2.6 (0.1–11.1) | <0.01 |
| Sex, n (% male) | 42 (68) | 35 (61) | 0.52 |
| Race, n (%) | |||
| White | 56 (90) | 52 (91) | 0.27 |
| Black | 3 (5) | 3 (5) | |
| Other | 3 (5) | 2 (4) | |
| Age at rhGH start | 11.5 ± 3.7 (4.4–20.2) | 10.3 ± 2.8 (5.2–17.8) | 0.06 |
| Follow-up years since cancer diagnosis, median (range) | 13.6 (3.0–24.2) | 13.0 (3.8–24.3) | 0.40 |
| Follow-up years since rhGH start, median (range) | 7.1 (0.3–18.4) | 4.3 (0.2–14.0) | 0.08 |
| BMI Z-score at rhGH start, median (range) | −0.1 (−3.0–1.8) | 0.3 (−3.1–2.5) | 0.13 |
| Growth rate (cm/yr) on rhGH | 7.6 ± 3.2 (2.5–19.6) | 6.6 ± 2.5 (0.6–13.6) | 0.07 |
| Treated endocrine diagnosis, n (%) | |||
| Hypothyroidism | 36 (60) | 31 (54) | 0.16 |
| Hypogonadisma | 8 (13) | 3 (5) | 0.21 |
rhGH, refers to recombinant human growth hormone.
hypogonadism is defined as hormone replacement for treatment of gonadal failure.
All survivors received radiation as part of cancer treatment. For TBI subjects, fractionated radiation dose ranged from 990 to 1,200 cGy, while CI exposure ranged from 1,200 to 5,400 cGy, depending on primary diagnosis (ALL vs. brain tumor). The median age (range) in patients with neuroblastoma at the time of diagnosis and cancer treatment was 1.8 (1.0–4.4) years, compared to 4.5 (0.1–11.1) years in patients with other cancer diagnosis in the TBI exposure group. For TBI-treated survivors, mean age at the time of rhGH start was 10.3 ± 2.8 years compared with 11.5 ± 3.2 years for CI (P = 0.06). Median survivor follow-up time for the cohort since initiation of rhGH was 4.8 (range 0.2–18.4) years. The median BMI Z-scores in the CI and TBI cohorts at the time of rhGH initiation were −0.1 and 0.3, respectively. There were no significant differences in post-treatment associated endocrine diagnoses and treatment (Table I).
SCFE Characteristics
Among the 119 survivors, SCFE was diagnosed in 10 subjects post-TBI and none after CI (P < 0.001). Seven subjects were diagnosed with bilateral SCFE at the time of presentation. Review of X-ray images revealed atypical valgus SCFE or posterior and lateral slippage for all 10 subjects with SCFE diagnosis after TBI (Fig. 1). The participant and disease characteristics among the TBI recipients with and without SCFE are compared in Table II. Age at cancer diagnosis, sex, race, underlying cancer, age at radiation, and age at initiation of rhGH did not differ according to SCFE status (P > NS for all). None had untreated endocrinopathies. Five subjects were on thyroid hormone replacement and four required Lupron to mitigate pubertal advancement while on rhGH therapy to maximize final height potential. The mean age at SCFE diagnosis was 12.3 ± 2.7 (range 8.0–16.0) years and the median duration of rhGH therapy prior to SCFE diagnosis was 1.8 (range 0.1–6.5) years. The SCFE subjects were Tanner 1 (n = 6) or early Tanner 2 (n = 4) at the time of SCFE diagnosis and the age at the time of TBI exposure, initiation of rhGH therapy or SCFE diagnosis did not differ according to sex. Median BMI Z-score at the time of SCFE diagnosis was 0.3 (−1.6 to 1.5) and mean growth velocity during rhGH therapy prior to SCFE diagnosis was 5.0 (range 2.4–10.6) cm/year. Mean annualized growth velocity during rhGH therapy in SCFE subjects was not significantly different compared to CI or TBI without SCFE cohort (Tables I and II).
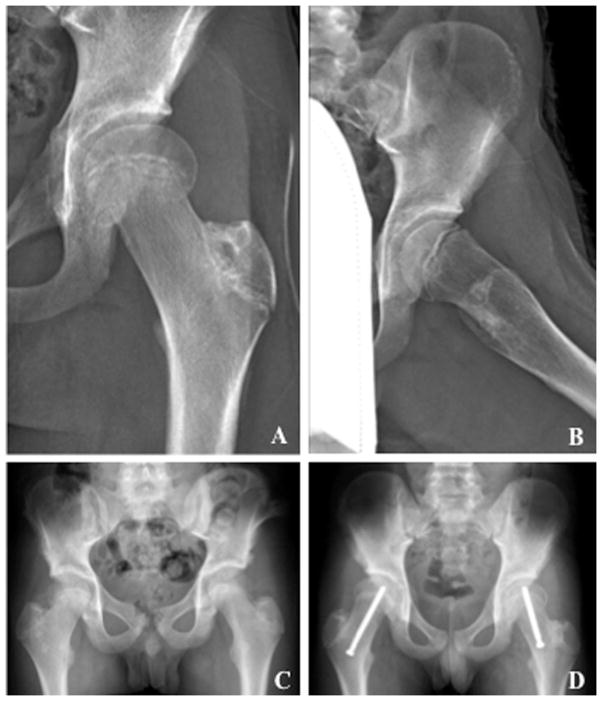
Fig. 1.
Antero-posterior (AP) (A) and frog-leg lateral radiographs (B) showing left lateral and posterior (valgus) slip of the capital femoral epiphysis in a patient on rhGH therapy after total body irradiation (TBI). C: Pre-operative AP radiograph of the pelvis showing bilateral valgus slipped capital femoral epiphysis (SCFE) in a childhood cancer survivor on rhGH after TBI. D: Post-operative AP radiograph of the same patient demonstrating screw fixation of the bilateral SCFE.
TABLE II.
Subject Characteristics in Total Body Irradiation Group (n = 57)
| SCFE (n = 10) | No. SCFE (n = 47) | |
|---|---|---|
| Age at cancer diagnosis, mean ± SD (range) years | 2.7 ± 1.6 (0.6–5.5) | 3.3 ± 2.8 (0.1–11.1) |
| Sex, n (%) | ||
| Male | 7 (70) | 28 (60) |
| Female | 3 (30) | 19 (40) |
| Race, n (%) | ||
| White | 10 (100) | 42 (89) |
| Black | 0 (0) | 3 (6) |
| Other | 0 (0) | 2 (4) |
| Cancer diagnosis | ||
| Neuroblastoma | 7 (70) | 20 (43) |
| Acute leukemia | 2 (20) | 22 (47) |
| HLH | 1 (10) | 0 (0) |
| Other | 0 (0) | 5 (11) |
| Age at rhGH start, mean ± SD (range) yrs | 10.0 ± 2.5 (6.4–13.0) | 10.3 ± 2.9 (5.2–17.8) |
| BMI Z-score at rhGH start | 0.3 (−1.6–1.5) | 0.4 (−3.1–2.4) |
| Growth rate (cm/yr) on rhGH | 5.9 ± 0.9 (4.4–7.8) | 6.7 ± 2.7 (0.6–13.6) |
| Treated endocrine diagnosis, n (%) hypothyroidism | 5 (50) | 26 (55) |
yrs, refers to years; HLH, hemophagocytic lymphohistiocytosis; BMI, body mass index; rhGH, recombinant human growth hormone. The Other cancer diagnosis includes aplastic anemia, Ewing sarcoma, myelodysplastic syndrome, and bone marrow failure.
SCFE Incidence Rate
The SCFE incidence rate after TBI exposure during treatment of childhood malignancy was 35.9 per 1,000 person years (95% CI 0.02, 0.07) (Table III). The incidence rate ratio in the TBI group compared with the rate reported in children treated with rhGH for idiopathic GH deficiency was 211 (95% CI 53.7, 287.4).
TABLE III.
Slipped Capital Femoral Epiphysis Incidence Rates in Cancer Survivors Treated With Recombinant Human Growth Hormone for Radiation-Induced Growth Hormone Deficiency After Total Body Irradiation Compared to Patients With Idiopathic Growth Hormone Deficiency
| No. patients | No. SCFE | Person yrs | Incidence rate (per 1,000 person yrs) | Incidence rate ratio | |
|---|---|---|---|---|---|
| TBI | 57 | 10 | 279 | 35.9 (95% CI 20.0, 70.0) | 211.2 (95% CI 53.7, 287.4) |
| Idiopathic GHDa | 22,696 | 12 | 71,570 | 0.17 | — |
GHD, refers to growth hormone deficiency; Person yrs, person years on rhGH treatment.
National cooperative growth study May 2009 Pediatric Endocrinology Lawson Wilkins meeting reported results for subjects treated with recombinant human growth hormone (rhGH) for idiopathic growth hormone deficiency.
DISCUSSION
This study assessed the risk of SCFE in children and adolescents treated with rhGH for radiation-induced GH deficiency after childhood cancer therapy. Childhood TBI exposure after chemotherapy was a significant risk factor for the development of SCFE during rhGH treatment. In comparison to the reported SCFE epidemiology data in the general population [4], cancer survivors diagnosed with SCFE were younger, more likely to be pre-pubertal and had a lower BMI Z-score at presentation.
Since the 1970s, SCFE has been recognized as an orthopedic adverse event (AE) during rhGH therapy [16,17]. To date, the NCGS, established by Genentech, Inc. (South San Franciso, CA) as an open-label, multicenter, post-marketing surveillance study in the United States, has published the largest safety and efficacy results for rhGH products used in children with growth disorders. These data characterized rhGH’s safety profile and captured the rare AEs that were not detected in large clinical trials [18–21]. The most recent NCGS report included 54,996 patients (65% male) and 195,419 patient years of rhGH therapy from December 1985 to January 1, 2006. The overall SCFE standardized incidence rate (per 100,000 patient-years) was 0.1%. The disease specific rates were 0.2% for idiopathic GH deficiency, 0.2% for Turner syndrome (TS), 0.3% for chronic kidney disease, and 0.4% for organic GH deficiency—the latter category including the childhood cancer survivors [15]. SCFE was reported in 93 patients and associated with rapid growth, obesity, trauma, or prior cancer diagnosis. Patients with Turner syndrome, chronic kidney disease, and organic GH deficiency had the greatest proportionate risk compared with other etiologies [15]. As previously recognized, Turner syndrome is a risk factor for SCFE regardless of rhGH therapy [22,23]. In addition, patients with chronic kidney disease demonstrate an increased risk of SCFE with a reported incidence rate of 0.4% for dialysis patients treated with rhGH versus 0.2% for untreated patients [24]. The risk factors for SCFE in survivors of childhood cancer therapy, including the impact of the type of radiation (cranial or TBI), chemotherapy, or surgical treatment have not been established.
SCFE is typically encountered during adolescence and the accompanied physiologic growth spurt [23]. Stimulation of cartilage cells by somatomedin occurs during the pubertal growth spurt, [25] with the increased thickness of the hypertrophied cartilage layer in the epiphyseal plate contributing to reduced shear strength and subsequent increased risk of SCFE [25]. In addition, GH-deficient rats treated with GH demonstrate hyperplasia and hypertrophy of growth plates at the epiphysis with increased stiffness posing a risk for both slippage at the epiphysis and fractures, leading to speculation that treatment with rhGH reduces the resistance to shear stresses at the physis. These observations have been proposed as potential factors contributing to enhanced weakness of the growth plate in rhGH-treated children and an explanation for the increased recognized incidence of SCFE during rhGH treatment [26,27].
The few prior publications reporting SCFE after radiation therapy [12,28,29] are largely limited to small case series. The first reported association between radiation and SCFE was in 1968, [30] with a subsequent report also including a possible association with chemotherapy [31]. In 1985, Barrett reported two cases of SCFE in children following pelvic radiation for rhabdomyosarcoma and summarized the 18 described cases in the literature with SCFE following radiotherapy to the hip, including the femoral head and neck region [32]. The overall peak incidence of post-radiotherapy cases described were significantly lower at 11 years of age compared to the peak incidence for SCFE at 13.5 years in males in the general population [32,33]. None of the reported patients were receiving GH therapy at the time of SCFE diagnosis. Furthermore, 50% of the SCFE were bilateral after radiation exposure compared with 20–25% incidence in the general population [32,33]. While radiotherapy is a plausible attributable cause for SCFE, a direct cause-and-effect relationship between radiation therapy and subsequent slipping of the epiphysis has been difficult to prove. However, previous studies have implicated several mechanisms by which SCFE can occur following radiation: (1) successive radiosensitivity of the growing cartilage and proliferating cells within the growth plate along with the germinal layer [30,32], (2) indirect endocrine effects (reduced estrogen resulting in decreased physeal shear strength), and (3) chemotherapy-associated radiosensitization.
SCFE in the general population is typically associated with obesity [34–36] and pubertal age [4], with majority of SCFE patients exhibiting BMI above the 95th percentile [35]. In a study by Poussa et al. [36], SCFE patients in Finland demonstrated higher BMI during growth based on annual height and weight evaluations from birth to the onset of slippage in patients compared to the normal population. Furthermore, population-based studies in the United States reveal higher SCFE incidence rates in blacks and Hispanics with a correlation between average weight and the incidence of SCFE [4]. In addition, the average age of SCFE presentation in the general population is 12.7 versus 11.2 years in males and females, respectively [4] with majority of patients pubertal at the time of diagnosis. In contrast, cancer survivors with SCFE post-TBI were more likely to exhibit significantly lower BMI Z-scores and pre-pubertal at the time of presentation further highlighting that increased BMI Z-scores and pubertal age are not predisposing risk factors for development of SCFE in cancer survivors after radiation exposure (Table I).
The SCFE cases in this study after TBI were all atypical or valgus SCFE, defined as lateral and superior displacement of the epiphysis related to the metaphysis [37]. Compared to the typical or varus SCFE, a valgus slip is much less common with reported estimated prevalence of 1.9% and a higher incidence in females [38]. While the exact cause of valgus slip is not known, a preexisting coxa valga resulting in an increased femoral neck-shaft angle has been implicated as a plausible risk factor due to the relative horizontal position of the physis [38]. Awareness of valgus SCFE is not only important for an accurate diagnosis but also for appropriate pre-operative surgical planning [39]. When evaluating children for valgus SCFE, lateral radiographs are essential as the Klein line, an early feature present in typical SCFE radiographs, will always be normal [40]. Therefore, identifying valgus SCFE is important for the appropriate surgical approach given the immediate proximity of the femoral neurovascular bundle in the valgus slip to the medial screw entry point often employed in typical SCFE repair [38,41].
The substantially greater incidence of SCFE in childhood cancer survivors treated with rhGH after TBI and intense chemotherapy implies that in the context of different cancer therapies rhGH alone does not predict the risk of developing SCFE. Furthermore, these data suggest that reported risk factors such as obesity, pubertal growth spurt, glucocorticoid exposure, and endocrine disorders, such as hypothyroidism or hypogonadism, did not contribute to the excess risk in the patients treated with TBI.
The primary limitation of this study is the retrospective design with potential selection bias and unmeasured confounders. However, the lack of evidence for confounding by obesity, glucocorticoid exposure, growth velocity, or endocrine diagnoses supports our conclusions. It is also unlikely that selection bias contributed to the very high incidence rates observed in the TBI group. While the participant heterogeneity is an additional limitation, this is the largest study to address SCFE in patients treated with rhGH following radiation therapy. The incidence of SCFE was greatest among survivors treated for neuroblastoma. The younger age at the time of diagnosis and cancer treatment in these patients, as well as the intensive chemotherapy, may have contributed to their greater risk. However, given the small number of SCFE cases and the fact that all subjects with TBI also received multi-agent chemotherapy, we are unable to evaluate age or independent chemotherapy effects contributing to SCFE risk. Last, these findings represent a single center and may not be generalizable. Future multicenter studies are imperative to identify additional and unique risk factors for orthopedic complications in survivors of childhood cancer.
In summary, this study identified that chemotherapy and radiation in childhood cancer increases the risk for the development of SCFE during rhGH therapy. All patients exhibited an atypical valgus SCFE. The comparison with children treated for idiopathic GH deficiency with rhGH illustrates that the excess SCFE risk in childhood cancer survivors is not due to rhGH alone. The markedly greater SCFE incidence rate in childhood cancer survivors with TBI-associated GHD compared with rates in children treated with CI suggests that treatment effects to the proximal femoral physis, combined with intensive chemotherapy may contribute to this increased risk. Therefore, provider and parental education on presenting symptoms is key to allow for early diagnosis and prompt surgical treatment and to further minimize long-term musculoskeletal complications in the growing at-risk population of childhood cancer survivors.
Acknowledgments
Grant sponsor: NIH; Grant numbers: K24; DK076808
Footnotes
Conflict of interest: Nothing to declare.
References
- 1.Loder RT. Slipped capital femoral epiphysis. Am Fam Physician. 1998;57:2135–2142. 2148–2150. [PubMed] [Google Scholar]
- 2.Gholve PA, Cameron DB, Millis MB. Slipped capital femoral epiphysis update. Curr Opin Pediatr. 2009;21:39–45. doi: 10.1097/MOP.0b013e328320acea. [DOI] [PubMed] [Google Scholar]
- 3.Peck D. Slipped capital femoral epiphysis: Diagnosis and management. Am Fam Physician. 2010;82:258–262. [PubMed] [Google Scholar]
- 4.Lehmann CL, Arons RR, Loder RT, et al. The epidemiology of slipped capital femoral epiphysis: An update. J Pediatr Orthop. 2006;26:286–290. doi: 10.1097/01.bpo.0000217718.10728.70. [DOI] [PubMed] [Google Scholar]
- 5.Papavasiliou KA, Kirkos JM, Kapetanos GA, et al. Potential influence of hormones in the development of slipped capital femoral epiphysis: A preliminary study. J Pediatr Orthop B. 2007;16:1–5. doi: 10.1097/BPB.0b013e328010b73d. [DOI] [PubMed] [Google Scholar]
- 6.Wabitsch M, Horn M, Esch U, et al. Silent slipped capital femoral epiphysis in overweight and obese children and adolescents. Eur J Pediatr. 2012;171:1461–1465. doi: 10.1007/s00431-012-1743-3. [DOI] [PubMed] [Google Scholar]
- 7.Shank CF, Thiel EJ, Klingele KE. Valgus slipped capital femoral epiphysis: Prevalence, presentation, and treatment options. J Pediatr Orthop. 2010;30:140–146. doi: 10.1097/BPO.0b013e3181d076b6. [DOI] [PubMed] [Google Scholar]
- 8.Felicetti F, Manicone R, Corrias A, et al. Endocrine late effects after total body irradiation in patients who received hematopoietic cell transplantation during childhood: A retrospective study from a single institution. J Cancer Res Clin Oncol. 2011;137:1343–1348. doi: 10.1007/s00432-011-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow EJ, Liu W, Srivastava K, et al. Differential effects of radiotherapy on growth and endocrine function among acute leukemia survivors: A childhood cancer survivor study report. Pediatr Blood Cancer. 2013;60:110–115. doi: 10.1002/pbc.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isfan F, Kanold J, Merlin E, et al. Growth hormone treatment impact on growth rate and final height of patients who received HSCT with TBI or/and cranial irradiation in childhood: A report from the French Leukaemia long-term follow-up study (LEA) Bone Marrow Transplant. 2012;47:684–693. doi: 10.1038/bmt.2011.139. [DOI] [PubMed] [Google Scholar]
- 11.Darendeliler F, Karagiannis G, Wilton P. Headache, idiopathic intracranial hypertension, and slipped capital femoral epiphysis during growth hormone treatment: A safety update from the KIGS database. Horm Res. 2007;68:41–47. doi: 10.1159/000110474. [DOI] [PubMed] [Google Scholar]
- 12.Loder RT, Hensinger RN, Alburger PD, et al. Slipped capital femoral epiphysis associated with radiation therapy. J Pediatr Orthop. 1998;18:630–636. doi: 10.1097/00004694-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher BD, Crom DB, Krance RA, et al. Radiation-induced bone abnormalities after bone marrow transplantation for childhood leukemia. Radiology. 1994;191:231–235. doi: 10.1148/radiology.191.1.8134578. [DOI] [PubMed] [Google Scholar]
- 14.Boero S, Brunenghi GM, Carbone M, et al. Pinning in slipped capital femoral epiphysis: Long-term follow-up study. J Pediatr Orthop B. 2003;12:372–379. doi: 10.1097/01.bpb.0000079202.23239.64. [DOI] [PubMed] [Google Scholar]
- 15.Bell J, Parker KL, Swinford RD, et al. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95:167–177. doi: 10.1210/jc.2009-0178. [DOI] [PubMed] [Google Scholar]
- 16.Rennie W, Mitchell N. Slipped femoral capital epiphysis occurring during growth hormone therapy. Report of a case. J Bone Joint Surg Br. 1974;56-B:703–705. doi: 10.1302/0301-620X.56B4.703. [DOI] [PubMed] [Google Scholar]
- 17.Fidler MW, Brook CG. Slipped upper femoral epiphysis following treatment with human growth hormone. J Bone Joint Surg Am. 1974;56:1719–1722. [PubMed] [Google Scholar]
- 18.August GP, Lippe BM, Blethen SL, et al. Growth hormone treatment in the United States: Demographic and diagnostic features of 2331 children. J Pediatr. 1990;116:899–903. doi: 10.1016/s0022-3476(05)80647-x. [DOI] [PubMed] [Google Scholar]
- 19.Blethen SL, Allen DB, Graves D, et al. Safety of recombinant deoxyribonucleic acid-derived growth hormone: The National cooperative growth study experience. J Clin Endocrinol Metab. 1996;81:1704–1710. doi: 10.1210/jcem.81.5.8626820. [DOI] [PubMed] [Google Scholar]
- 20.Maneatis T, Baptista J, Connelly K, et al. Growth hormone safety update from the National cooperative growth study. J Pediatr Endocrinol Metab. 2000;13:1035–1044. [PubMed] [Google Scholar]
- 21.Kemp SF, Kuntze J, Attie KM, et al. Efficacy and safety results of long-term growth hormone treatment of idiopathic short stature. J Clin Endocrinol Metab. 2005;90:5247–5253. doi: 10.1210/jc.2004-2513. [DOI] [PubMed] [Google Scholar]
- 22.Bolar K, Hoffman AR, Maneatis T, et al. Long-term safety of recombinant human growth hormone in turner syndrome. J Clin Endocrinol Metab. 2008;93:344–351. doi: 10.1210/jc.2007-1723. [DOI] [PubMed] [Google Scholar]
- 23.Haidar RK, Nasrallah MP, Der-Boghossian AH, et al. Orthopedic complications related to growth hormone therapy in a pediatric population. J Pediatr Orthop Part B. 2011;20:57–61. doi: 10.1097/BPB.0b013e32833ed967. [DOI] [PubMed] [Google Scholar]
- 24.Fine RN, Ho M, Tejani A, et al. Adverse events with rhGH treatment of patients with chronic renal insufficiency and end-stage renal disease. J Pediatr. 2003;142:539–545. doi: 10.1067/mpd.2003.189. [DOI] [PubMed] [Google Scholar]
- 25.Harris WR. The endocrine basis for slipping of the upper femoral epiphysis. An experimental study. J Bone Joint Surg. 1950;32-B:5–11. [Google Scholar]
- 26.Clayton PE, Cowell CT. Safety issues in children and adolescents during growth hormone therapy—A review. Growth Horm IGF Res. 2000;10:306–317. doi: 10.1054/ghir.2000.0175. [DOI] [PubMed] [Google Scholar]
- 27.Rappaport EB, Snoy P, Habig WH, et al. Effects of exogenous growth hormone on growth plate cartilage in rats. Am J Dis Child. 1987;141:497–501. doi: 10.1001/archpedi.1987.04460050039026. [DOI] [PubMed] [Google Scholar]
- 28.Dickerman JD, Newberg AH, Moreland MD. Slipped capital femoral epiphysis (SCFE) following pelvic irradiation for rhabdomyosarcoma. Cancer. 1979;44:480–482. doi: 10.1002/1097-0142(197908)44:2<480::aid-cncr2820440216>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Sabio H, Sussman M, Levien M. Postradiation slipped capital femoral epiphyses (SCFE) J Surg Oncol. 1987;36:45–47. doi: 10.1002/jso.2930360110. [DOI] [PubMed] [Google Scholar]
- 30.Rubin P, Casarett GW. Clinical radiation pathology as applied to curative radiotherapy. Cancer. 1968;22:767–778. doi: 10.1002/1097-0142(196810)22:4<767::aid-cncr2820220412>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Ryan BR, Walters TR. Slipped capital femoral epiphysis following radiotherapy and chemotherapy. Med Paediatr Oncol. 1979;6:279–283. doi: 10.1002/mpo.2950060402. [DOI] [PubMed] [Google Scholar]
- 32.Barrett IR. Slipped capital femoral epiphysis following radiotherapy. J Pediatr Orthop. 1985;5:268–273. doi: 10.1097/01241398-198505000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Kelsey JL. Epidemiology of slipped capital femoral epiphysis: A review of the literature. Pediatrics. 1973;51:1042–1050. [PubMed] [Google Scholar]
- 34.Loder RT, Starnes T, Dikos G, et al. Demographic predictors of severity of stable slipped capital femoral epiphyses. J Bone Joint Surg Am. 2006;88:97–105. doi: 10.2106/JBJS.E.00069. [DOI] [PubMed] [Google Scholar]
- 35.Mannof E, Banffy M, Winell J. Relationship between body mass index and slipped capital femoral epiphysis. J Pediatr Orthop. 2005;25:744–746. doi: 10.1097/01.bpo.0000184651.34475.8e. [DOI] [PubMed] [Google Scholar]
- 36.Poussa M, Schlenzka D, Yrjonen T. Body mass index and slipped capital femoral epiphysis. J Pediatr Orthop Part B. 2003;12:369–371. doi: 10.1097/01.bpb.0000079201.23239.bf. [DOI] [PubMed] [Google Scholar]
- 37.Muller W. Die entstehung von coxa valga durch epiphysenverschiebung. Beitr Klin Chir. 1926;28:869–872. [Google Scholar]
- 38.Loder RT, O’Donnell PW, Didelot WP, et al. Valgus slipped capital femoral epiphysis. J Pediatr Orthop. 2006;26:594–600. doi: 10.1097/01.bpo.0000230331.96157.14. [DOI] [PubMed] [Google Scholar]
- 39.Venkatadass K, Shetty AP, Rajasekaran S. Valgus slipped capital femoral epiphysis: Report of two cases and a comprehensive review of literature. J Pediatr Orthop Part B. 2011;20:291–294. doi: 10.1097/BPB.0b013e328346d2ec. [DOI] [PubMed] [Google Scholar]
- 40.Klein A, Joplin RJ, Reidy JA, et al. Slipped capital femoral epiphysis; early diagnosis; and treatment facilitated by normal roentgenograms. J Bone Joint Surg Am. 1952;34-A:233–239. [PubMed] [Google Scholar]
- 41.Segal LS, Weitzel PP, Davidson RS. Valgus slipped capital femoral epiphysis. Fact or fiction? Clin Orthop Relat Res. 1996;322:91–98. [PubMed] [Google Scholar]