Abstract
Sleep disturbances not only commonly occur in major depressive disorder, but constitute one of the symptom criteria. Importantly, there is growing inference that sleep disturbances may be more than a symptomatic byproduct of depression; poor sleep may play a role in the development or clinical course of depression, or both. This article reviews the prevalence of the two major classes of sleep disorders, the insomnias and the sleep-disordered breathing disorders, as they pertain to depression. Beyond prevalence, the empirical evidence reviewed suggests that insomnia is a risk factor for depression and that obstructive sleep apnea (OSA) is highly associated with depression. Preliminary evidence indicates that OSA may also be a risk factor for this disorder. The implications of these findings for the treatment of sleep disturbances either prior to or in the context of depression are discussed.
The effect of sleep disturbances on major depressive disorder
There is widespread agreement that sleep and depression are intricately linked; however, the extent to which sleep disturbances play a causative and deleterious role in mood regulation are less established. Although there is a sizable body of literature on this topic, it suffers from a number of deficiencies. To begin with, “sleep disturbance” is a broad term that is not disease-specific but encompasses both a range of diagnostic entities, such as sleep apnea and insomnia, and conceptual constructs, such as poor sleep quality. Studies vary with respect to how sleep disturbance is operationalized, even when a specific disease entity is named (e.g. insomnia). This is especially true for epidemiological studies and those in which depression, and not sleep, is the primary outcome. Findings from such reports are often based on one or more single-item measure of sleep complaints, either specifically developed for the study or embedded within the other instruments applied. This has been validated in some instances. For example, the Hamilton Rating Scale for Depression (HAM-D) has three items related to early-, middle-, and late-night insomnia, respectively [1]. These have been found to load on the same factor of the HAM-D [2], and as a subscale have been shown to detect improvements in insomnia associated with the effective treatment of depression [3], and to have convergent validity with prospective measures of insomnia derived from a week of daily sleep diaries [4].
In addition, the term “risk factor” is often used but not defined in specific reports linking one condition (e.g. sleep apnea) to another (e.g. depression). In particular, cross-sectional studies, by most definitions, cannot be used to support a claim for sleep as a risk factor for depression.
Finally, the symptom criteria for major depressive disorder (MDD), as detailed in the DSM-IV [5], include two symptom sets related to sleep. The first is directly related: “insomnia or hypersomnia”. Both of these are considered diagnostic entities in their own right and each may be induced by medical, psychiatric, or substance abuse conditions as well as by any number of medications. Hypersomnia, in particular, is often associated with sleep-related breathing disorders such as sleep apnea. The second sleep-related criterion for MDD is “fatigue or loss of energy”. This has a more indirectl relationship with sleep as fatigue may arise from numerous causes, but it is important to note that fatigue is itself a frequent consequence of insomnia. Thus, even at this nosological descriptive level, there are a number of overlaps between sleep and depression.
This article will adopt the conventions of stating the specific disease entity in question when sufficiently operationalized, explicitly stating when this is not the case, and will only use the designation of “sleep disturbance” as an all-encompassing term. A risk factor, as defined by Beck [6], is part of the causal chain and requires temporal evidence, which can only be derived from longitudinal studies. Cross-sectional data will be considered as evidence of a “risk indicator”, defined by Burt as a probable or putative risk factor [7]. The article will review those major sleep disorders that have high prevalence rates (the insomnias, sleep-disordered breathing [SDB] disorders, and movement disorders) – which also tend to increase across the lifespan [8–10] – and will discuss the extent to which they are associated with, or contribute to, the development or clinical course of MDD. Furthermore, the term “depression” is used instead of MDD when the data reviewed is based on a depression scale rather than an actual clinical diagnosis or structured clinical interview.
Insomnia and MDD
There is a sizable body of literature on the relationship between insomnia and MDD that suggests insomnia is associated with, as well as a risk factor for, new-onset and recurrent depression [11,12]. Both disorders are highly prevalent and frequently co-occur across all age ranges, and especially in older cohorts [13,14]. Cross-sectional data allow for estimates of prevalence rates as well as estimates of co-occurrence. Longitudinal studies, meanwhile, allow for the direct assessment of risk factors.
Prevalence estimates for insomnia and depression
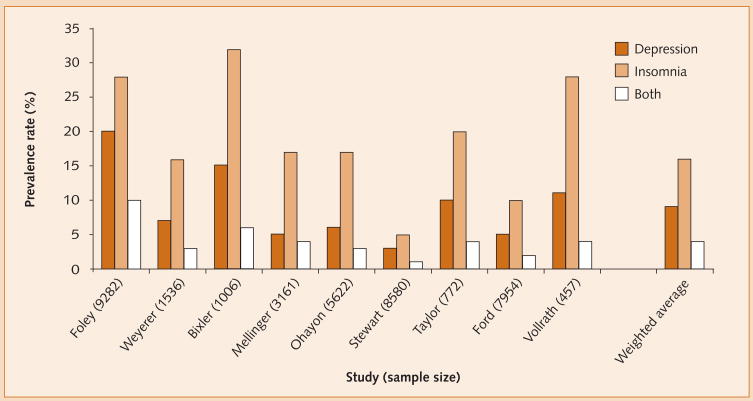
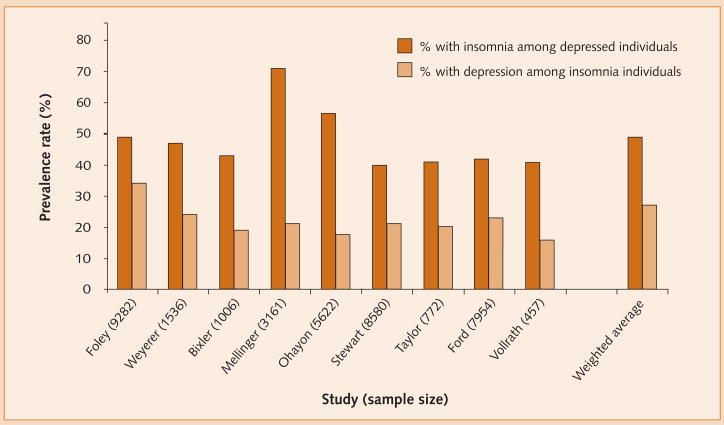
Although variably defined across community and epidemiological studies, the prevalence of insomnia is approximately 16% while that of depression is approximately 9%. Notably, rates partly vary in accordance with the stringency of diagnostic criteria used in the investigations in question. Prevalence data from nine such studies, comprising a total of 38 370 participants, are summarized in Figure 1, and show that these disorders co-occur in approximately 4% of the general population. In addition, Figure 2 illustrates that the prevalence of insomnia in the context of depression (49%) is approximately twice that of depression in the context of insomnia (27%). Such data suggest that insomnia is a risk indicator for depression.
Figure 1.
Prevalence rates of depression and insomnia.
Figure 2.
Prevalence of insomnia among depressed individuals and vice versa.
Longitudinal studies of insomnia and depression
Longitudinal studies provide additional insight into the relationship between insomnia and depression. A study by Perlis et al. addressed the development of recurrent episodes of depression [15]. Subjects who experienced a recurrence of depression during a 42-week period were matched with controls with no recurrence over the same time period [15]; both groups were assessed weekly using the Beck Depression Inventory (BDI) [16]. The recurrent depression group exhibited an elevated level of insomnia, commencing 5 weeks prior to recurrence, compared with the non-recurrent group. Moreover, the insomnia symptom cluster was the most prominent depressive symptom cluster leading up to the depressive episodes in the recurrent group, and it reached its peak in the week of recurrence. Although from a single study, the findings suggest that insomnia is both a risk factor for, and a prodromal sign of, a recurrent depressive episode.
The majority of longitudinal studies on this subject have assessed whether insomnia occurring at certain time points predicts depression at a succeeding time point. These include several studies assessing the onset of new depression over a 1-year period. Three such studies investigated mixed-age populations, and found that insomnia posed an increased risk of developing depression with odds ratios (OR) ranging from 5.4 to 40 [17–19]. Based on epidemiological study data from Zürich, Switzerland, which included assessment of insomnia and depression across six time-points over a 20-year span, Buyssee and colleagues found that the presence of insomnia absent depression at each time point was strongly associated with the presence of co-occurring insomnia and depression at the subsequent time point [20]. Similar although less robust findings were observed for the presence of depression absent insomnia, which was associated with subsequent co-occurring insomnia and depression.
Studies conducted in adolescents and young adults have reported similar findings. In a large sample of adolescents, the presence of insomnia was associated with the occurrence of depression in young adulthood, 6–7 years later [21]. In a sample of 21–30-year-olds, and controlling for prior depressive symptoms, those reporting ≥2 weeks of insomnia occurring nearly every night at any point in their lives were at an increased risk of developing new-onset depression at a 3-year follow-up compared with those with no history of insomnia (OR 2.4, 95% confidence interval [CI] 1.2–4.8) [22]. In a study that began with college-aged men, insomnia while at college conferred a relative risk of 2.0 (95% CI 1.2–3.3) for the development of depression at some point during the following 30 years [23].
Studies conducted in older-aged cohorts have produced similar, but slightly mixed, findings. In 147 patients with no prior history of depression, the presence of persistent insomnia at baseline was associated with new-onset depression 1 year later, but only in women [24]. In 524 community-dwelling elderly subjects, insomnia at baseline and 3 years later compared with no insomnia or insomnia at only one time point was associated with depression at 3-year follow-up [25]. In a re-analysis of this dataset including only subjects with activity limitations and no psychiatric morbidity at baseline, baseline insomnia was not associated with depression at 3 years [26]. Roberts et al. found that insomnia at baseline was associated with an increased risk of depression 1 year later (OR 2.5 95% CI 1.7–3.7) and that insomnia at both time-points carried an eight-fold increased risk of depression, although other factors were more significant predictors [27]. In older adults followed for 12 years [28], baseline insomnia was an independent predictor of depression in women only (OR 4.1 95% CI 2.1–7.2). In 771 elderly subjects, only initial insomnia (not middle of the night or early morning awakening) that occurred both at baseline and at 3 years was associated with the development of new depression at 3 years [29]. Finally, a meta-analysis of studies conducted in older adults found that sleep disturbance (OR 2.6) was second only to recent bereavement (OR 3.3) as the major risk factor for late-life depression [30]. Across all age groups, the evidence suggests that both incident and persistent insomnia predict new-onset depression, although the findings are not unanimous and insomnia is not the sole risk factor. Taken as a whole, these longitudinal studies support insomnia as a risk factor for depression development [31]. It would appear that insomnia is part of the causal chain for many individuals who develop depression.
Treatment studies of insomnia and depression
Departing from the epidemiological data, findings from treatment trials of depression have shown that insomnia is often a residual symptom following antidepressant medication treatment [32–35]. This has been demonstrated in a fluoxetine trial in which disturbed sleep and fatigue were the most common residual symptoms among depression remitters (present in 44% and 38%, respectively) [34]. In a trial of nortriptyline, depression remitters demonstrated significant reductions in sleep disturbance scores, but their mean score remained above clinical cutoff levels and was higher than that of healthy controls [36]. Similar observations have been made in studies of cognitive–behavioral therapy (CBT) for depression [37,38]. In two separate randomized depression treatmet trials comparing CBT with antidepressant medication, approximately 50% of those with remitted depression in each treatment arm had residual insomnia [39,40]. Of course, this also means that approximately half of treatment responders did not have residual insomnia; however, it is notable that for some individuals insomnia persists despite successful treatment of depression.
In an informative analysis of data from a stepped-care depression management trial for older adults in primary care, patients with insomnia that persisted between baseline and 3-month assessments had a diminished depression treatment response at 6 and 12 months compared with patients with insomnia at one or neither of the initial assessments [41]. This finding expands the notion of insomnia as a risk factor for depression to include comorbid insomnia as a risk factor for unremitting depression.
Equally informative data come from treatment trials of insomnia in which depression is reported as an outcome. In two uncontrolled studies of patients presenting with insomnia and depression who received CBT for insomnia, improvements were seen in both sleep and depression [42,43]. In two randomized, controlled trials, simultaneous treatment of insomnia and depression improved both conditions to a greater effect than treatment of the depression alone [44,45]. One of these studies found that concomitant treatment with fluoxetine and eszopiclone was superior to fluoxetine alone in reducing depression severity and time to response [44]. The other found that concomitant treatment with escitalopram and CBT for insomnia produced a higher rate of remission for depression and insomnia than escitalopram alone [45]. This particular set of studies highlights insomnia as a risk factor for depression that can be modified to the benefit of both conditions.
In summary, insomnia represents a true risk factor for the development of new-onset and recurrent depression. It presages a recurrent episode and serves as a treatment barrier to full recovery from antidepressant therapy. While not tested, we can hypothesize that left untreated, residual insomnia represents a risk factor for relapse. When it is treated, the available data suggest that insomnia can resolve in the context of ongoing depression, and that by targeting the insomnia, additional gains can be derived.
Theoretical basis for how insomnia affects depression
While speculative, the effect of insomnia on the clinical course of depression most likely results from an interaction between psychological and biological factors [11]. Insomnia, when chronic, is associated with increased fatigue and a diminished ability to cope with life stressors, and can be perceived as a loss of control. These factors, in turn, may initiate the classic depression schema of helplessness and hopelessness. In essence, insomnia becomes a consistent life stressor. This sets the stage for neuroendocrine imbalances such as limbic hyperarousal, increases in aminergic tone, and serotonin deficiency, producing a set of symptoms common to both insomnia and depression (anhedonia, fatigue, and memory and concentration problems). In its worst form, this set of psychological and biological changes may give rise to interpersonal problems, social withdrawal, and/or cognitive distortions that underlie MDD.
An interesting piece of evidence comes from the paradoxical finding that acute sleep deprivation has acute antidepressant effects (e.g. [46]). While acute sleep deprivation differs substantially from chronic insomnia, it has been proposed that insomnia initially occurs as a compensatory phenomenon in MDD to increase serotonergic tone but, unlike its sleep deprivation counterpart, cannot reach a level that exerts an antidepressant effect [47–49]. Even so, if insomnia represents a failed attempt at homeostatic regulation then the regulated sleep deprivation that is a cornerstone of cognitive–behavioral interventions may succeed where the endogenous regulatory response fails. Behavioral treatment of insomnia, therefore, might exert direct antidepressant effects.
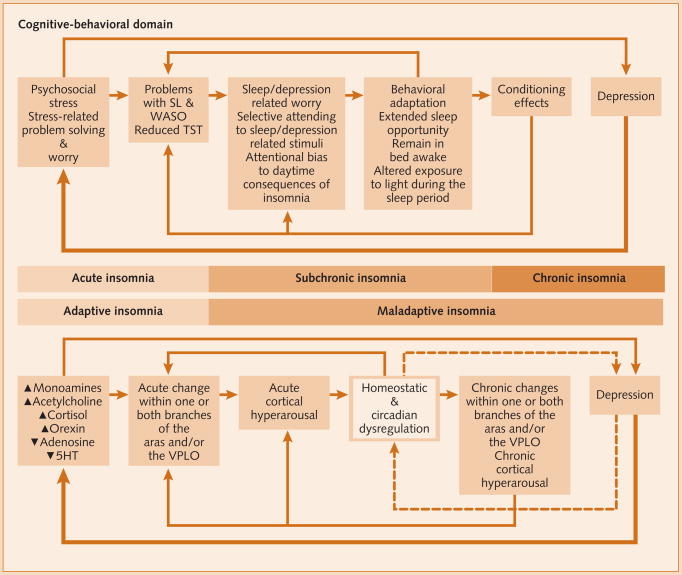
This model proposes that insomnia may be a normative initial response to biopsychosocial stress. However, when insomnia persists beyond the life of the stressor, it becomes a risk factor for the development of new-onset depression (Fig. 3). As has been reviewed elsewhere, other candidate mechanisms or models for the role of insomnia-related sleep disturbances in the pathophysiology of depression include opiod, serotonergic, adenosine, and psychostimulant hypotheses as well as homeostatic models for re-regulation of circadian disturbances and sleep rebound following sleep loss [46]. What is clear from theoretical reviews is that the relationship between sleep and depression is strong, but multifactorially determined.
Figure 3.
Etiology and pathology of insomnia and depression.
N.B. The box delineating the homeostatic and circadian factors is highlighted because the neurobiological control mechanisms are not detailed.5-HT: serotonin; SL: sleep latency; TST: total sleep time; VLPO: ventrolateral preoptic area of the hypothalamus; WASO: wake after sleep onset.
Sleep-disordered breathing
SDB encompasses a number of conditions including obstructive sleep apnea (OSA), central sleep apnea (CSA), and upper airway resistance syndrome (UARS). OSA, which involves obstruction of the airway during sleep, is the most common form of this family of disorders and has a prevalence rate of approximately 8% in men and 4% in women, although the prevalence increases dramatically over the lifespan rising to as high as 20–30% in middle-age and 50–70% in the elderly [50–52]. UARS is a relatively more recently recognized condition in which limited or no obstructions are observed, but in which increased respiratory effort is associated with frequent arousals from sleep. CSA is a cessation of airflow that occurs due to centrally mediated interruptions in respiratory control. Breathing alterations may include apneas (nearly full to full cessation of breathing), hypopneas (partial cessation of breathing), and respiratory event-related arousals. The severity of the conditions is measured in terms of the number of breathing disturbances that occur per hour of sleep and reported as either a respiratory disturbance index (RDI) that includes all breathing events) or an apnea–hypopnea index (AHI) that includes apneas and hypopneas only. The most conservative conventional clinical cutoff is an AHI or RDI ≥5 (i.e. 5 events per h of sleep). Values in this range are indicative of SDB and an AHI of 5–15 is considered to represent mild apnea. There is unanimous agreement that an AHI ≥15 represents a clinically meaningful level of SDB. The vast majority of the extant literature on SDB currently focuses on OSA, and this is also the case in terms of SDB and depression.
There is one additional clinical SDB presentation worthy of mention and that is hypersomnia. This is a very common symptom of OSA, and is typically referred to as excessive daytime sleepiness (EDS) in the sleep literature; this convention will be adopted here.
SDB and depression
SDB has been shown to have an association with depression. For instance, in a large epidemiological study of 18 980 individuals from the general population, Ohayon reported that approximately 18% of those with a sleep-related breathing disorder also met criteria for MDD; conversely (and perhaps coincidentally), approximately 18% of subjects with MDD also met criteria for a sleep-related breathing disorder [53]. These findings were corroborated by Sharafkhaneh et al., who identified over 100 000 patients from a Veterans Health Administration database with diagnostic codes related to sleep apnea; of these approximately 22% also had MDD (including depression NOS but excluding dysthymia) [54].
In OSA specifically, a number of smaller studies with variable assessment strategies have shown that the rates of depression (or depressive symptoms) among OSA patients can range approximately 25–50% [55–62]. In a larger study (n=2271) of predominantly male patients assessed for OSA, no associations between SDB severity and depression were observed although among the women (n=294), those with severe OSA had higher depression scores than those with mild OSA [63]. In a separate community study of 427 older adults that included overnight polysomnography recordings, observer-rated depression significantly correlated with apnea severity [64], while in one small sample of older adults, 18% of those with depression had OSA compared with 4% of healthy elderly controls [65]. In addition, other factors have been found to partially account for the severity of depression among OSA patients such as age, body mass index, hypertension, and fatigue [66,67].
A seminal prospective study of SDB was recently accomplished by Peppard and colleagues [68]. In this novel epidemiological study, 1408 adults completed a depression inventory and underwent overnight polysomnography at baseline and at one or more other time points over a 4-year period. Apnea severity was categorized as none, minimal (AHI <5), mild (AHI ≥5 and <15), and moderate–severe (AHI ≥15). Compared with patients with no SDB at baseline, there was an increased risk of developing depression for each level of SDB severity, with the OR increasing in an exposure–response pattern from an OR of 1.6 (95% CI 1.2–2.1) for mild SDB to an OR of 2.6 (95% CI 1.7–3.9) for moderate–severe SDB. Similarly, an increase in severity category from baseline assessment to subsequent polysomnography recording was associated with a 2.6-fold (95% CI 1.7–3.9) increase in the risk of developing depression [68].
Studies evaluating the effect of treatment of SDB with administration of continuous positive airway pressure (CPAP) on depression are somewhat less illustrative than the insomnia treatment literature. This is mainly due to methodological issues and mixed findings. Nonetheless, CPAP therapy has been shown to reverse depression associated with sleep apnea in most, but not all, studies undertaken [62,69–81]. In general, there is a suggestion that CPAP treatment improves mood in some patients with sleep apnea, but larger, well-controlled studies are needed to fully address this question. Combined, these data provide reasonable evidence to consider SDB as a risk factor for MDD, and provide several theoretical hypotheses as to why this may be the case.
EDS and depression
EDS (i.e. hypersomnia) is not often assessed in epidemiological studies, but when included has been found to have a prevalence rate of 5–20%. For example, in a large epidemiological study conducted in Pennsylvania, USA, the prevalence of EDS was found to be 9% in the general population [82]. In an elderly cohort of 4578 Medicare enrollees, approximately 20% reported EDS [83]. In another older-aged cohort of 1050 rural community dwellers in the US, 19% reported EDS [84].
Some epidemiological data also highlight the relationship of EDS with depression. Two separate studies found depression to be more strongly associated with EDS scores than other variables consistent with sleep apnea (e.g. snoring, gasping/snorting, and trouble breathing) [82,83]. One of these studies also found a significant relationship between EDS and the use of antidepressant medication (OR 3.3, 95% CI 2.9–3.8; p<0.0001) [83]. In fact, undergoing treatment for depression was the most significant risk factor for EDS, while the presence of sleep apnea was the weakest. EDS has been found to be associated with the development of depression in longitudinal studies, although it was a less robust predictor than insomnia [17,27]. EDS was also associated with all-cause mortality in one elderly community sample [84].
Overall, there is a fairly high prevalence of EDS in the general population and there is limited, but positive, evidence that the presence of EDS is a risk factor for subsequent depression.
Theoretical bases for how SDB affects MDD
As is the case for the role of insomnia in the development of depression, there are several ways by which SDB may contribute to the clinical course of MDD. Firstly, and not unlike the case with insomnia, there is symptom overlap between SDB and MDD, including the presence of cognitive/memory impairments, fatigue/anhedonia, and – as reviewed above –EDS. To the extent that these are a consequence of SDB, an individual with this set of symptoms will have already endorsed a number of depressive criteria. Beyond these shared symptoms, SDB and MDD are also both associated with medical conditions such as obesity, hypertension, cardiovascular disease, and insulin resistance and diabetes. This suggests the possibility of a common biological substrate or at least shared pathways. Several authors have suggested that because depression is associated with reductions in serotonin (or serotonin transmission) [57] and as serotonin appears to affect airway rigidity and collapsibility [58], the directional relationship arrow is such that depression may contribute to the development or severity of OSA [85].
On the other hand, the results of a neuroimaging study suggest that this directional arrow may point from OSA towards depression [86]. Untreated OSA patients with and without elevated depressive symptoms were compared with each other and with healthy controls. Increased neural damage was found in OSA patients compared with controls, and the degree and location of these damages differed between depressed and non-depressed OSA patients. As the authors suggest, “depressive symptoms may exacerbate injury accompanying OSA, or introduce additional damage in affective, cognitive, respiratory, and autonomic control regions” [86]. Other imaging studies have found that the acute but repetitive hypoxemia that occurs during and following apneic events is associated with additional cerebral changes, including white matter damage [87], which are similar to those seen in depressed subjects [88,89]. Moreover, the severity of the hypoxemic events in OSA correlate with the degree of neurocognitive impairment [90].
Finally, in addition to hypoxemia, respiratory events cause both brief awakenings from sleep and microarousals of a few seconds, which do not lead to full awakenings. Combined, these cause sleep fragmentation, which has been linked very closely (more closely than apnea severity) to EDS. In summary, this evidence suggests a somewhat complex series of potentially bidirectional interactions of some or all of the putative mechanisms linking SDB to depression, including the effect of such interactions on psychosocial factors that may then contribute to depression.
Other sleep-related disorders and MDD
Narcolepsy, a condition characterized by EDS, may also be associated with depression in the elderly. In a postal survey of 305 narcolepsy patients in the UK, 57% of respondents had some degree of depression (BDI score ≥10) and 15.1% had BDI scores ≥20, suggesting the presence of moderate to severe depression [91].
Restless legs syndrome and periodic limb movement disorder have not been associated with depression in the few studies that addressed this question. Among a sample of 22 elderly subjects undergoing polysomnography, all complained of disturbed sleep and 12 reported low mood [92], but no significant associations were found between movement indices and depression or EDS. These findings were confirmed in a sample of 39 elderly subjects [93].
Polysomnographic findings in depression
While not directly germane to how sleep affects depression, there is a sleep profile associated with MDD. As has been reviewed by several authors (e.g. [11]), polysomnography recordings from patients with MDD reliably exhibit sleep continuity abnormalities, such as increased sleep latency, increased time awake, and reduced sleep efficiency, which are similar to, albeit lesser in magnitude, than those observed in primary insomnia. Sleep architecture abnormalities, including slow wave sleep deficits, have also been noted in MDD, although this finding has been inconsistently observed. REM sleep findings in MDD such as short REM latencies, increased REM density, and increased total time in REM sleep (20–30%) have been more consistently recorded. However, interestingly, REM latency and total REM sleep findings have been less robust in studies published in the past decade. Nonetheless, to the extent that any of these abnormalities precede the development of MDD, they might have a causative role in depression; however, this has yet to be assessed.
Conclusion
The common assumption that sleep and depression are related is supported by epidemiological data showing a high degree of comorbidity between MDD in particular and both insomnia and SDB. Data from longitudinal epidemiological studies, and treatment trials of both depression and insomnia, make a convincing case that insomnia is a risk factor for the development and recurrence of MDD and may serve as a barrier to fully effective depression management. There are also data supporting several theoretical underpinnings for a causative role of insomnia in the etiology of depression.
EDS is more often considered a symptom or complaint than a diagnostic entity. It is highly associated with the presence of MDD, although there is only limited evidence to suggest it may serve as a risk factor for depression, and it is therefore best viewed as an indicator of an underlying disorder. MDD frequently co-occurs with OSA and there are several well-founded hypotheses relating OSA to the development of MDD. A prospective investigation and several treatment studies for OSA in which MDD was improved provide preliminary empirical support for considering OSA as a risk factor for MDD. From a clinical perspective, it is quite reasonable for any patient with MDD to be screened for the following:
Insomnia, which if diagnosed should receive targeted intervention.
OSA risk factors (e.g. large neck circumference, elevated BMI, snoring, EDS); when present patients should be referred for a full overnight sleep evaluation.
Insomnia that presents on its own or in the context of another disorder should also be aggressively treated, as doing so may prevent or forestall a depressive episode. In the case of SDB, any of these disorders should be aggressively treated due to the wide range of consequences associated with their presence and the possibility that their management may ameliorate depression. A variety of sleep disturbances have been reported to negatively impact on depression, and these effects can be reversible.
Footnotes
Disclosures
The author has received research support from Merck, Inc. and Sanofi-Aventis in the past 12 months.
References
- 1.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 2.Fleck MPA, Poirierlittre MF, Guelfi JD, et al. Factorial structure of the 17-item Hamilton Depression Rating Scale. Acta Psychiatr Scand. 1995;92:168–72. doi: 10.1111/j.1600-0447.1995.tb09562.x. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME, Rush AJ, Manber R, et al. Differential effects of nefazodone and cognitive behavioral analysis system of psychotherapy on insomnia associated with chronic forms of major depression. J Clin Psychiatry. 2002;63:493–500. doi: 10.4088/jcp.v63n0605. [DOI] [PubMed] [Google Scholar]
- 4.Manber R, Blasey C, Arnow B, et al. Assessing insomnia severity in depression: comparison of depression rating scales and sleep diaries. J Psychiatr Res. 2005;39:481–8. doi: 10.1016/j.jpsychires.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Revised (DSM-IV-TR) Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 6.Beck JD. Risk revisited. Community Dent Oral Epidemiol. 1998;26:220–5. doi: 10.1111/j.1600-0528.1998.tb01954.x. [DOI] [PubMed] [Google Scholar]
- 7.Burt BA. Risk factors, risk markers, and risk indicators. Community Dent Oral Epidemiol. 1998;26:219. doi: 10.1111/j.1600-0528.1998.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16:349–59. doi: 10.1007/BF00999989. [DOI] [PubMed] [Google Scholar]
- 9.Carskadon MA, van den Hoed J, Dement WC. Insomnia and sleep disturbances in the aged: sleep and daytime sleepiness in the elderly. J Geriatr Psychiatry. 1980;13:135–51. [PubMed] [Google Scholar]
- 10.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 11.Pigeon WR, Perlis ML. Insomnia and depression: birds of a feather? Int J Sleep Disorders. 2007;1:82–91. [Google Scholar]
- 12.Taylor DJ. Insomnia and depression. Sleep. 2008;31:447–8. doi: 10.1093/sleep/31.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 14.Lichstein KL, Durrence HH, Riedel BW, et al. Epidemiology of sleep: age, gender, and ethnicity. Mahwah, NJ: Erlbaum; 2002. [Google Scholar]
- 15.Perlis ML, Buysse D, Giles DE, et al. Sleep disturbance may be a prodromal symptom of depression. J Affect Disord. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psych Rev. 1988;8:77–100. [Google Scholar]
- 17.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 18.Weissman MM, Greenwald S, Nino-Murcia G, et al. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–50. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 19.Dryman A, Eaton WW. Affective symptoms associated with the onset of major depression in the community: findings from the US National Institute of Mental Health Epidemiologic Catchment Area Program. Acta Psychiatr Scand. 1991;84:1–5. doi: 10.1111/j.1600-0447.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Angst J, Gamma A, et al. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2006;31:1351–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 23.Chang PP, Ford DE, Mead LA, et al. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 24.Perlis M, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 25.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br J Gen Pract. 1993;43:445–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Livingston G, Watkin V, Milne B, et al. Who becomes depressed? The Islington community study of older people. J Affect Disord. 2000;58:125–33. doi: 10.1016/s0165-0327(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 27.Roberts RE, Shema SJ, Kaplan GA, et al. Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry. 2000;157:81–8. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Mallon L, Broman JE, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12:295–306. doi: 10.1017/s1041610200006414. [DOI] [PubMed] [Google Scholar]
- 29.Brabbins CJ, Dewey ME, Copeland JR, et al. Insomnia in the elderly: prevalence, gender differences and relationships with morbidity and mortality. Int J Geriatr Psychiatry. 1993;8:473–80. [Google Scholar]
- 30.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–56. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 31.Taylor DJ. Commentary on insomnia and depression. Sleep. 2008;31:447–8. doi: 10.1093/sleep/31.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karp JF, Buysse DJ, Houck PR, et al. Relationship of variability in residual symptoms with recurrence of major depressive disorder during maintenance treatment. Am J Psychiatry. 2004;161:1877–84. doi: 10.1176/ajp.161.10.1877. [DOI] [PubMed] [Google Scholar]
- 33.Opdyke KS, Reynolds CF, 3rd, Frank E, et al. Effect of continuation treatment on residual symptoms in late-life depression: how well is “well”. Depress Anxiety. 1996;4:312–9. doi: 10.1002/(SICI)1520-6394(1996)4:6<312::AID-DA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 34.Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60:221–5. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- 35.Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–80. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds CF, Hoch CC, Buysse DJ, et al. Sleep in late-life recurrent depression. Changes during early continuation therapy with nortriptyline. Neuropsychopharmacology. 1991;5:85–96. [PubMed] [Google Scholar]
- 37.Simons AD, Murphy GE, Levine JL, et al. Cognitive therapy and pharmacotherapy for depression: sustained improvement over on year. Arch Gen Psychiatry. 1986;43:43–8. doi: 10.1001/archpsyc.1986.01800010045006. [DOI] [PubMed] [Google Scholar]
- 38.Thase ME, Simons AD, Cahalane JF, et al. Cognitive behavior therapy of endogenous depression: part 1: an outpatient clinical replication series. Behav Ther. 1991;22:457–67. [Google Scholar]
- 39.Manber R, Rush J, Thase ME, et al. The effects of psychotherapy, nefazodone, and their combination on subjective assessment of disturbed sleep in chronic depression. Sleep. 2003;26:130–36. doi: 10.1093/sleep/26.2.130. [DOI] [PubMed] [Google Scholar]
- 40.Carney CE, Segal ZV, Edinger JD, et al. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68:254–60. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- 41.Pigeon WR, Hegel MT, Unutzer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;3:481–8. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor DJ, Lichstein KL, Weinstock J, et al. A pilot study of cognitive-behavioral therapy of insomnia in people with mild depression. Behav Ther. 2007;38:49–57. doi: 10.1016/j.beth.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Morawetz D. Behavioral self-help treatment for insomnia: a controlled evaluation. Behav Ther. 1989;20:365–79. [Google Scholar]
- 44.Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–77. [PubMed] [Google Scholar]
- 47.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6:341–51. [PubMed] [Google Scholar]
- 48.Adrien J. Reply to Commentary. Sleep Med Rev. 2002;6:359. [Google Scholar]
- 49.Perlis M, Smith MT, Orff H. Major Depressive Disorder (MDD) is associated with a primary defect within the serotonergic system. Sleep Med Rev. 2002;6:353–7. [PubMed] [Google Scholar]
- 50.Bixler EO, Kales A, Soldatos CR, et al. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–62. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 51.Shapiro CM, Dement WC. ABC of sleep disorders. Impact and epidemiology of sleep disorders. BMJ. 1993;306:1604–07. doi: 10.1136/bmj.306.6892.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 53.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–200. doi: 10.4088/jcp.v64n1009. [DOI] [PubMed] [Google Scholar]
- 54.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 55.Beutler LE, Ware JC, Karacan I, et al. Differentiating psychological characteristics of patients with sleep-apnea and narcolepsy. Sleep. 1981;4:39–47. doi: 10.1093/sleep/4.1.39. [DOI] [PubMed] [Google Scholar]
- 56.Kales A, Caldwell AB, Cadieux RJ, et al. Severe obstructive sleep apnea—II: associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38:427–34. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 57.Aikens JE, Mendelson WB. A matched comparison of MMPI responses in patients with primary snoring or obstructive sleep apnea. Sleep. 1999;22:355–9. doi: 10.1093/sleep/22.3.355. [DOI] [PubMed] [Google Scholar]
- 58.Aikens JE, Caruana-Montaldo B, Vanable PA, et al. MMPI correlates of sleep and respiratory disturbance in obstructive sleep apnea. Sleep. 1999;22:362–9. doi: 10.1093/sleep/22.3.362. [DOI] [PubMed] [Google Scholar]
- 59.Mosko S, Zetin M, Glen S, et al. Self-reported depressive symptomatology, mood ratings, and treatment outcome in sleep disorders patients. J Clin Psychol. 1989;45:51–60. doi: 10.1002/1097-4679(198901)45:1<51::aid-jclp2270450107>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 60.Ramos-Platon MJ, Espinar-Sierra J. Changes in psychopathological symptoms in sleep apnea patients after treatment with nasal continuous positive airway pressure. Int J Neurosci. 1992;62:173–95. doi: 10.3109/00207459108999770. [DOI] [PubMed] [Google Scholar]
- 61.Reynolds CF, Kupfer DJ, McEachran AB, et al. Depressive psychopathology in male sleep apneics. J Clin Psychiatry. 1984;45:287–90. [PubMed] [Google Scholar]
- 62.Millman RP, Fogel BS, McNamara ME, et al. Depression as a manifestation of obstructive sleep apnea: reversal with nasal continuous positive airway pressure. J Clin Psychiatry. 1989;50:348–51. [PubMed] [Google Scholar]
- 63.Pillar G, Lavie P. Psychiatric symptoms in sleep apnea syndrome: effects of gender and respiratory disturbance index. Chest. 1998;114:697–703. doi: 10.1378/chest.114.3.697. [DOI] [PubMed] [Google Scholar]
- 64.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynolds CF. Sleep apnea in Alzheimer’s dementia: correlation with mental deterioration. J Clin Psychiatry. 1985;46:257–61. [PubMed] [Google Scholar]
- 66.Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47:583–96. doi: 10.1016/s0022-3999(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 67.Bardwell WA, Moore P, Ancoli-Israel S, et al. Fatigue in obstructive sleep apnea: driven by depressive symptoms instead of apnea severity? Am J Psychiatry. 2003;160:350–5. doi: 10.1176/appi.ajp.160.2.350. [DOI] [PubMed] [Google Scholar]
- 68.Peppard PE, Szklo-Coxe M, Hla KM, et al. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 69.Engleman HM, Cheshire KE, Deary IJ, et al. Daytime sleepiness, cognitive performance and mood after continuous positive airway pressure for the sleep apnoea/hypopnoea syndrome. Thorax. 1993;48:911–4. doi: 10.1136/thx.48.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu BH, Ancoli-Israel S, Dimsdale JE. Effect of CPAP treatment on mood states in patients with sleep apnea. J Psychiatr Res. 1999;33:427–32. doi: 10.1016/s0022-3956(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 71.Cooke JR, Amador X, Lawton S, et al. Long-term CPAP may improve cognition, sleep, and mood in patients with Alzheimer’s disease and SDB. Sleep. 2006;29:A103–4. [Google Scholar]
- 72.Schwartz DJ, Kohler WC, Karatinos G. Symptoms of depression in individuals with, obstructive sleep apnea may be amenable to treatment with continuous positive airway pressure. Chest. 2005;128:1304–9. doi: 10.1378/chest.128.3.1304. [DOI] [PubMed] [Google Scholar]
- 73.Kawahara S, Akashiba T, Akahoshi T, et al. Nasal CPAP improves the quality of life and lessens the depressive symptoms in patients with obstructive sleep apnea syndrome. Intern Med. 2005;44:422–7. doi: 10.2169/internalmedicine.44.422. [DOI] [PubMed] [Google Scholar]
- 74.Engleman HM, Asgari-Jirhandeh N, McLeod AL, et al. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest. 1996;109:1470–6. doi: 10.1378/chest.109.6.1470. [DOI] [PubMed] [Google Scholar]
- 75.Borak J, Cieslicki JK, Koziej M, et al. Effects of CPAP treatment on psychological status in patients with severe obstructive sleep apnoea. J Sleep Res. 1996;5:123–7. doi: 10.1046/j.1365-2869.1996.d01-60.x. [DOI] [PubMed] [Google Scholar]
- 76.Derderian SS, Bridenbaugh RH, Rajagopal KR. Neuropsychologic symptoms in obstructive sleep apnea improve after treatment with nasal continuous positive airway pressure. Chest. 1988;94:1023–7. doi: 10.1378/chest.94.5.1023. [DOI] [PubMed] [Google Scholar]
- 77.Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome – a randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2001;163:911–7. doi: 10.1164/ajrccm.163.4.9910025. [DOI] [PubMed] [Google Scholar]
- 78.Munoz A, Mayoralas LR, Barbe F, et al. Long-term effects of CPAP on daytime functioning in patients with sleep apnoea syndrome. Eur Respir J. 2000;15:676–81. doi: 10.1034/j.1399-3003.2000.15d09.x. [DOI] [PubMed] [Google Scholar]
- 79.Edinger JD, Carwile S, Miller P, et al. Psychological status, syndromatic measures, and compliance with nasal CPAP therapy for sleep apnea. Percept Mot Skills. 1994;78:1116–8. doi: 10.2466/pms.1994.78.3c.1116. [DOI] [PubMed] [Google Scholar]
- 80.Lewis KE, Seale L, Bartle IE, et al. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27:134–8. doi: 10.1093/sleep/27.1.134. [DOI] [PubMed] [Google Scholar]
- 81.Means MK, Lichstein KL, Edinger JD, et al. Changes in Beck depression inventory (BDI) symptoms after continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea (OSA) Sleep. 2002;25:A218–9. [Google Scholar]
- 82.Bixler EO, Vgontzas AN, Lin HM, et al. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 83.Whitney CW, Enright PL, Newman AB, et al. Correlates of daytime sleepiness in 4578 elderly persons: the cardiovascular health study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 84.Ganguli M, Reynolds CF, Gilby JE. Prevalence and persistence of sleep complaints in a rural elderly community sample: the MoVIES Project. J Am Geriatr Soc. 1996;44:778–84. doi: 10.1111/j.1532-5415.1996.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 85.Schroder CM, O’Hara R. Depression and obstructive sleep apnea (OSA) Ann Gen Psychiatry. 2005;4:1–8. doi: 10.1186/1744-859X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31:1103–9. [PMC free article] [PubMed] [Google Scholar]
- 87.Kamba M, Inoue Y, Higami S, et al. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry. 2001;71:334–9. doi: 10.1136/jnnp.71.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas AJ, Perry R, Kalaria RN, et al. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry. 2003;18:7–13. doi: 10.1002/gps.720. [DOI] [PubMed] [Google Scholar]
- 89.Thomas AJ, O’Brien JT, Barber R, et al. A neuropathological study of periventricular white matter hyperintensities in major depression. J Affect Disord. 2003;76:49–54. doi: 10.1016/s0165-0327(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 90.Engleman HM, Kingshott RN, Martin SE, et al. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23:S102–8. [PubMed] [Google Scholar]
- 91.Daniels E, King MA, Smith IE, et al. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10:75–81. doi: 10.1046/j.1365-2869.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 92.Youngstedt SD, Kripke DF, Klauber MR, et al. Periodic Leg movements during sleep and sleep disturbances in elders. J Gerontol A Biol Sci Med Sci. 1998;53:391–4. doi: 10.1093/gerona/53a.5.m391. [DOI] [PubMed] [Google Scholar]
- 93.Cuellar NG, Strumpf NE, Ratcliffe SJ. Symptoms of restless leg syndrome in older adults: outcomes on sleep quality, sleepiness, fatigue, depression, and quality of life. JAGS. 2007;55:1387–92. doi: 10.1111/j.1532-5415.2007.01294.x. [DOI] [PubMed] [Google Scholar]