Summary
An essential step for therapeutic and research applications of stem cells is the ability to differentiate them into specific cell types. Endodermal cell derivatives, including lung, liver and pancreas, are of interest for regenerative medicine, but efforts to produce these cells have been met with only modest success. In a screen of 4000 compounds, two cell permeable small molecules were indentified that direct differentiation of ESCs into the endodermal lineage. These compounds induce nearly 80% of ESCs to form definitive endoderm, a higher efficiency than that achieved with Activin A or Nodal, commonly used protein inducers of endoderm. The chemically induced endoderm expresses multiple endodermal markers, can participate in normal development when injected into the embryonic gut tube and can form pancreatic progenitors in vitro. The application of small molecules to differentiate mouse and human ESCs into endoderm, and pancreatic progenitors represents a step toward achieving a reproducible and efficient production of desired ES cell derivatives.
Keywords: Chemical screen, embryonic stem cells, endoderm
Introduction
Type I diabetes results from the destruction of insulin producing pancreatic beta cells and therefore there are several approaches aimed at cell-based strategies to replace these cells and rejuvenate the pancreas. The spontaneous or undirected differentiation of ESCs produces very small numbers of insulin producing cells, barely enough for research study and far short of the numbers needed for therapeutic application. One strategy to increase the efficiency of beta cell formation is to mimic embryonic development by exposing ESCs and their derivatives to factors they would normally encounter in vivo. The starting point for this strategy is differentiating ESCs into definitive endoderm.
Our understanding of the developmental pathways that control endoderm formation has so far been guided by studies in Xenopous laevis, zebrafish and mice (reviewed by Wells and Melton, 1999; Lewis and Tam, 2006). Collectively, these studies suggest a conserved mechanism for endoderm/mesoderm commitment utilizing the transforming growth factor-β (TGF-β) family member Activin A and Nodal, fibroblast growth factor (FGF) and Wnt growth factor families. Similarly, in vitro application of Activin A or Nodal to mouse or human ES cell cultures leads to endoderm induction (Kubo et al., 2004; Yasunaga et al., 2005; D'Amour et al., 2005). Other molecules that influence endoderm formation in vitro include WNTs (D'Amour et al., 2005), bone morphogenic proteins (BMPs), and members of the AKT/P13K pathway (McLean et al., 2007).
Permeable small molecules can control cellular processes by modulating signal transduction pathways, gene expression or metabolism and have been effectively used in ES cell differentiation protocols. Small molecules can be synthesized in high quantity and purity as well as conveniently supplied or removed, giving them great potential to be useful for therapeutic applications. High throughput screens have been performed to identify novel small molecules that can support the self renewal of ES cells (Chen et al., 2006; Desbordes et al., 2008), cardiogenic specification of mouse ES (Wu et al., 2004) or neural progenitor cells (Diamandis et al., 2007) as well as inducing specific cell types, notably neuronal and muscle cells (reviewed by (Ding and Schultz, 2004).
We describe here two small molecules, IDE1 and IDE2 that can efficiently induce definitive endoderm (IDE) from mouse and human ES cells. Treatment of mouse ES cell monolayer cultures with either compound yields high quantities of endoderm expressing multiple endodermal marker genes. We show that chemically derived endoderm develops into pancreatic progenitors in vitro in response to the growth factor FGF10, retinoic acid and hedgehog inhibitors, a commonly used combination to induce pancreatic progenitors in vitro. Moreover, we apply a recently identified small molecule, Indolactam V (S. Chen and D.A. Melton, unpublished), that induces pancreatic progenitors in human ES cell culture, to either IDE1 or IDE2 derived endoderm, and induce higher yields of pancreatic progenitors compared to a growth factor based approach. Finally, we show that compound induced endoderm can contribute to gut tube formation in vivo when the cells are injected into the developing gut tube of mouse embryos. All together, we introduce two small molecules which induce a robust differentiation of ESCs into endoderm that has the same or a very similar developmental potential to its in vivo counterpart. The induction of endoderm from ESCs by IDEs is conserved between mouse and human species. While we have yet to achieve complete stepwise differentiation from ESCs to pancreatic beta cells by chemical means, this study focuses on an important first step: formation of endoderm.
Results
Screening with ESCs for endoderm formation
To obtain a reporter for endoderm formation we generated a mouse ES cell line with the red-fluorescent protein dTomato, a variant of DsRed (Shaner et al., 2004) coding sequence under control of the Sox17 promoter (Suppl. Fig. 1). Several lines of evidence show that Sox17 is an endodermal marker, both definitive and extra-embryonic. A null mutation in mice is devoid of foregut endoderm and mid- and hindgut endoderm fails to expand (Kanai-Azuma et al., 2002). Gene expression analysis of isolated endoderm confirms that Sox17 is a marker of endoderm, both definitive and extra-embryonic (Sherwood et al., 2007). However, it is important to note that Sox17 expression is not restricted exclusively to endoderm; genetic lineage tracing shows that Sox17 is expressed in the endodermal lineage as early as E7.5 and but later on marks the gut tube as well as other organs (Park et al. 2006; Kim et al., 2007; Liu et al., 2007; R. Maehr, unpublished data).
In vitro, application of TGF-β family of growth factors, including Activin A or Nodal, to both mouse and human ES cell cultures, leads to the preferential differentiation into endodermal lineages (Kubo et al., 2004; Yasunaga et al., 2005; D'Amour et al., 2005). Consistent with those previous studies, we observe endoderm induction when Sox17-dsRed mouse ESCs are treated with Activin A for 6 days in low serum conditions (see Methods). At 6 days, 45% of cells stain positively for Sox17 and express dsRed (Fig. 1A). All dsRed+ cells also express Sox17 protein as judged by Sox 17 antibody staining, which shows that the reporter line accurately reflects endogenous Sox17 expression. The co-expression of the fluorescent marker and endogenous Sox17 was also confirmed at all time points in vitro as well as in E6.5 and E7.5 embryos (data not shown). There is a minor population of cells (10±3.6%) that stained for Sox17, but did not express dsRed; however, no false positive expression of the transgene (DsRed positive but Sox17 antibody negative) was observed.
Figure 1. High throughput screening.

(A) Scheme of differentiation into endoderm and evaluation of an endoderm reporter line. Treatment with either Activin A or Nodal induces endoderm in mouse ESC cultures and at day 6 of treatment 45% of total cells are Sox17/dsRed double positive. Every dsRed+ cell stains positively for Sox17 antibody. (B) Overview of the identification of endoderm inducers from small molecule collection. Out of > 4000 screened compounds, 27 primary hits were selected and further evaluated for specificity and toxicity. Markers for definitive endoderm (DE) and extra-embryonic endoderm (EE) were tested by Q-RT-PCR and immunohistochemistry and 2 compounds that induced high levels of DE were indentified. ≥ 3 s.d = more than 3 standard deviations.
The endoderm differentiation protocol used in this screen is based on previously published protocols (Kubo et al., 2004; Yasunaga et al., 2005; D'Amour et al., 2005) with several modifications to allow endoderm lineage induction in a high throughput format. Specifically, we culture the mouse ESCs as a monolayer (in contrast to embryoid bodies) in gelatin-coated 384 well plates without any feeder cell layer. We also adjusted the cell density and media composition to promote better survival of mouse ESCs (see Methods for details).
Twelve hours after plating, Sox17-dsRed mouse ESCs were supplied with differentiation media (low serum content) and a single chemical compound was added by pin transfer. At this time point, (x=0) no dsRed + and/or Sox17 antibody reactive cells were detected. In contrast, nearly all cells expressed Oct4, a pluripotency marker (data not shown). After day 6 of culture in the presence of compounds, we evaluated Sox17/DsRed expression and total cell number by flow cytometry.
Over 4,000 compounds were tested from a small molecule collection consisting of known compounds that influence stem cell fate (such as retinoic acid and 5-azacytidine), bioactive compounds, compounds with known activity in signaling pathways, US Food and Drug Administration approved drugs, and a histone deacetylase (HDAC) inhibitors biased small molecule library resulting from diversity oriented synthesis.
Positive hits were defined as compounds that induce expression of Sox17/dsRed at three standard deviations above the DMSO (vehicle) control and were not auto-fluorescent or cytotoxic (Fig. 1B). Effects on cell viability or toxicity were set by requiring that the number of cells after 6 days of culture doubled i.e. reaching at least 1600 cells/well. Activin A treatment was used as a positive control. The effects of various treatments on differentiation were first evaluated by flow cytometric analysis for dsRed expressing cells and later confirmed by Sox17 and FoxA2 immunofluorescence and quantative-RT-PCR (Q-RT-PCR) analysis of endodermal markers including Sox17, FoxA2, Gata4, Gata6, alpha-fetoprotein (Afp) and Sox7. Twenty seven compounds, ˜0.7% of total screened compounds, were selected as primary hits and further characterized. We also tested for the expression of ectodermal genes including Sox1, Pax6, Zic1 and mesodermal markers Pdgfr-α, Pdgfr-β and Meox1 by Q-RT-PCR (data not shown) to ensure specificity. Finally, since propensity to form different germ layer cell types varies between different mouse and human ES cell lines (Burridge et al., 2007; Osafune et al., 2008), we tested primary hits using three mouse ES cell lines of two different genetic backgrounds (129 and 129/C57BL6 hybrid). Considering all these criteria, two small molecules, IDE1 and IDE2, were identified, out of the 27 primary hits, as inducers of endoderm and selected for further studies (Fig. 1B).
Induction of definitive and extra-embryonic endoderm
During evaluation of primary hit compounds, we observed that small molecules induced Sox17+ cells with two distinct morphologies. One class, including IDE1 and IDE2, induced clustered populations of Sox17+ cells, whereas other compounds led to the formation of a dispersed population of Sox17+ cells (Suppl. Fig. 2A). A third class of compounds induced cells with a mixed morphology. Sox17 is expressed in definitive endoderm, but also in extra-embryonic endoderm, and in other germ layer derivatives at later stages of development including vascular endothelium (Kanai-Azuma et al., 2002; Matsui et al., 2006). Since the positive identification of definitive endoderm is hindered by the lack of unique markers that are expressed exclusively there, and not present in other types of endoderm, we performed a negative selection and tested for markers of extra-embryonic endoderm. We found, that the vast majority (>95%) of the dispersed Sox17+ cells (class II) also expressed extra-embryonic endoderm markers including Gata4 (Morrisey et al., 1996), SPARC (Mason et al., 1986), AFP (Dziadek, 1979) and Dab2 (Yang et al., 2002) (Suppl. Fig. 2B). Conversely, Sox17+ cells induced by treatment with either IDE2 (Suppl. Fig. 2B) or IDE1 (data not shown) formed clustered, epithelial like populations and contained no or a negligible number of cells positive for extra-embryonic markers (Suppl. Fig. 2B).
Optimization of definitive endoderm induction by active compounds
IDE1 and IDE2 are products of de novo chemical synthesis and come from a library of putative HDAC inhibitors (Fig. 2A). Titration of IDE1 and IDE2 from 50 nM to 5 μM showed that they function in a dose-dependent manner (EC50=125 nM for IDE1 and EC50= 223 nM for IDE2, Fig. 2A) with the highest efficiency, and no toxicity, in the 250-800 nM range. The optimal concentration of IDE1 induces Sox17 expression in 80% and IDE2 in 72% of total ES cells at day 6 of treatment (Fig. 2B). We also tested for the expression of FoxA2 (also known as HNF3β), as an essential gene for the development of the definitive endoderm in mouse, (Ang et al., 1993; Monaghan et al., 1993; Sasaki and Hogan, 1993) and observed that over 95% of compound induced Sox17+ cells co-express FoxA2 (Fig. 2B).
Figure 2. Two small molecule inducers of endoderm in mouse and human ESC cultures.
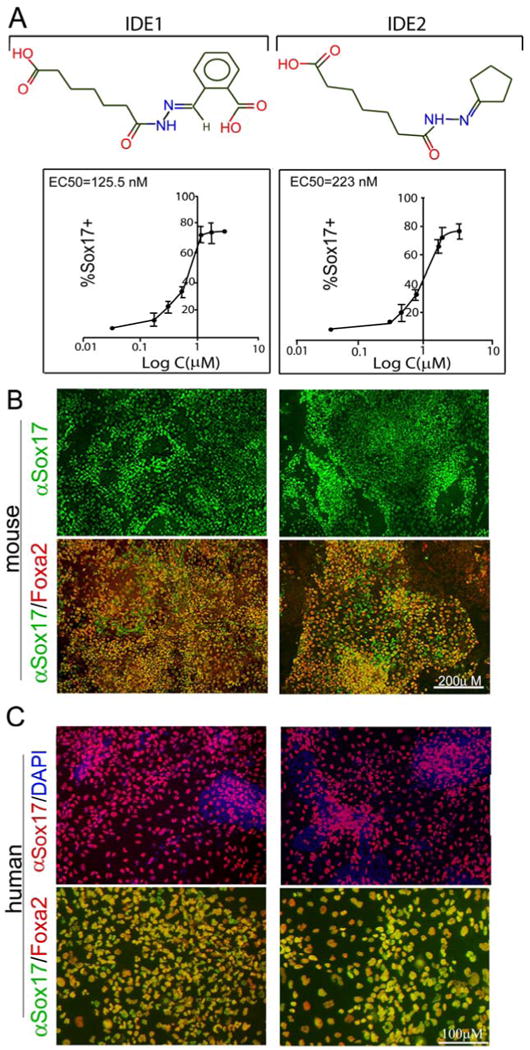
(A) Chemical structure of IDE1 and IDE2 that induce endoderm from mouse ESCs. The lower panel shows dose response curves of Sox17 expression (based on immunofluorescence) following treatment with compound for 6 days. The EC50 values and curve fitting were performed with Graph Prism software. Data presented as mean ± s.d. n=4 (B) Representative images of αSox17 immunofluorescence show the highest Sox17 induction in mouse ESCs by compound at day 6 of treatment, quantified as the percent of cells expressing Sox17 (upper panel) out of the total cells. The majority of Sox17+ cells (≥95%) induced by chemical treatment co-express another definitive endoderm marker, FoxA2. (C) HUES cells cultures were treated for 6 days with IDE1, IDE2 and endoderm markers, Sox17 and FoxA2 were analyzed by immunofluorescence. Either of compound treatment leads to Sox17 expression in 55-65% of total cells and ≥ 95% of Sox17+ cells co-expresses also FoxA2.
IDE1 and 2 induce endoderm from human embryonic stem cells
Then, we tested whether the hit compounds, have the ability to direct also human ES cells (HUES) into endodermal fate. Two HUES lines, HUES 4 and 8, treated with IDE compounds show propensity to differentiate into endodermal lineage {Osafune, 2008 #23} as judged by Sox17 expression. Both compounds induced Sox17 expression in dose-depended manner. Treatment with IDE1 (100 nM) for 4 days leads to Sox17 expression in 62±8.1% of cells (Fig. 2C), an efficiency similar to Activin A treatment in these culture condition (64±6.3%) (Suppl. Fig. 3). IDE2 (200 nM) induces Sox17 expression in 57±6.7% of total cell number, (Fig. 2C) and the effect of IDE2 is significant different (p=0.00255) compared to the mock treatment (16%±3.6), albeit slightly below the Activin A efficiency (Suppl. Fig. 3). The majority (≥90%) of Sox17+ cells co-expressed also other endodermal marker, FoxA2 (Fig. 2C). Co-treatment of HUES cells with Activin A and Wnt3a increases the efficiency of endoderm induction only by an additional 3-5%. A similar efficiency of endoderm induction was observed when HUES were culture in presence of MEF layer or on gelatin coated plates.
Time course and synergy between active compound and growth factors
Endoderm induction from mouse ESCs peaks at day 6 of treatment with small molecules, when 81±14% (for IDE1) treatment and 76±14% (for IDE2) out of total cell number were Sox17+ (Fig. 3A). In the course of the next 8 days, the efficiency of endodermal induction does not significantly change. Compared to Activin A treatment both small molecules, under the conditions reported here, induced more cells to express Sox17, and at earlier time points. The percentage of mouse cells expressing Sox17 at day 2 of treatment is 34% for IDE1 (Fig. 3A) and 32% for IDE2, whereas Activin A yielded 13% Sox17+ cells (Fig. 3A); at day 4, 40-50% of cells treated with either of the compounds are Sox17+, whereas only 28% of Activin A treated cells are Sox17+.
Figure 3. Time course of endoderm induction and synergy between compound and growth factors.

(A) The effect of compounds on the number of Sox17+ and total cells during 14 days of treatment is shown. Endoderm induction by IDE1 and IDE2 peaks at about day 6 and as little as 12hrs treatment with either compound is sufficient to induce Sox17 expression in ˜ 40% of the cells. Activin A treatment induces significant but lower % of Sox17 + cells at all tested time points. Cells were analysed at day 6 (for earlier time points) or 14 of culture. (B) The combined effect of compounds and growth factors on Sox17 expression. Co-treatment of mouse ESCs with compounds and Nodal enables shortening of the treatment time and leads to the induction of Sox17 with a slightly high efficiency (55.6%) expression at day 4. No synergy was observed between IDE1 and IDE2 or for the combination of either compound with Wnt3a. All quantifications were based on the percentage of cells stained by Sox17 antibody out of total cell. Data presented as mean ±s.d. n=4 experiments.
Simultaneous treatment of mouse ESCs with both IDE1 and IDE2 did not produce any synergistic effect on Sox17+ induction (Fig. 3B). Co-treatment with IDE2 and Nodal, significantly (p≤0.001) increases the Sox17 induction at day 4 to 55.8±6.49%, compared to 42±3.83% with the compound alone. Wnt3a has no significant effect on Sox17 expression when combined with compounds.
Gene expression analysis of endoderm induced by active compounds
To determine whether other genes that compose part of an endodermal signature are induced in mouse ESC cultures treated with active compounds. Sox17-DsRed+ cells were isolated by flow cytometry after day 6 of compound stimulation and profiled by gene expression analysis. Of nineteen genes previously defined as a definitive endoderm signature in the mouse (Sherwood et al., 2007), fourteen were induced more than two fold in the compound treated samples compared to mock treatment (Fig. 4A). We also compared in vitro derived endoderm with its in vivo counterparts sorted-out from Sox17-DsRed E7.5-E8.5 embryos. Of these nineteen endoderm signature genes, only two genes, Spink3 and Tmprss2, were significantly different and were expressed at higher levels in the E7.5 endoderm (Fig. 4A). Furthermore, we did not observe any significant changes in the expression of markers characteristic for other cell lineages, such as the ectoderm markers Zic1, Pax6, and mesoderm markers Flk1, CD31 (data not shown) after compound treatment. The r2 value (square of linear correlation coefficient) between chemically induced endoderm and Sox17/dsRed+ endoderm isolated from mouse E7.5-E8.0 embryos was 0.94-0.97 in three independent experiments (Fig. 4B). In contrast, the r2 value for non-treated mouse ESC and naïve endoderm isolated form E.75-8.0 embryos was 0.5-0.56 (Fig. 4B). These data suggest that in vitro derived endoderm by hit compound treatment is essentially equivalent to E7.5-E8.0 endoderm with respect to the expression of key endodermal markers.
Figure 4. Gene expression analysis of chemically induced endoderm.

(A) Expression of definitive endodermal markers in Sox17+ cells induced by compound treatment or isolated form E7.5-8.0 embryos. Sox17/dsRed+ cells were sorted by FACS and expression of endoderm genes was analysed by Illumina microarray. Expression of “endoderm signature” genes normalized to the DMSO treated mouse ESCs is shown. Out of 17 genes, only 2, Spink3 and Tmprss2 (marked by grey circle) were expressed at significantly higher levels (>2 fold change) in Sox17+ cells isolated from E7.75 embryos (endoderm). Each bar represents an average of 3 biological replicates and mean ± s.d. is shown (B) Scatter plots comparing the global gene expression in Sox17/dsRed+ populations sorted out from Sox17/dsRed E7.75 embryos and derived either in vitro by treatment with IDE2 (day 6 of treatment) or with non-treated mouse ESC cultures. Red line in the middle visualizes the equivalent levels in gene expression; two side red lines show two-fold change in gene expression levels between both samples.
Activation of Nodal/ Smad signaling in Sox17+ cells produced by small molecules IDE1 and IDE2 treatment
Genetic and biochemical studies point to Smad proteins as the intracellular transducers of TGF-β signaling, including Activin A and Nodal (Whitman, 1998) and a high level of Smad2 phosphorylation is detected in cells lysates of ESCs that have been treated with Activin A or Nodal treatment. Both IDE1 and IDE2 induce phosphorylation of Smad2 after 24 hrs or longer at levels comparable to that induced by Activin A treatment (Fig. 5A). This phosphorylation of Smad2 is strongly attenuated by co-treatment of mouse ESCs with either of compounds and the Activin receptor-like kinase 4/5/7 (ALK) inhibitor, SB43125. Under these conditions, induction of Sox17 protein is also reduced to 5.6 ±1.3% (data not shown). These data indicate that both IDE1 and IDE2 function by activating the TGFβ signaling pathway, however the specific biochemical targets of these small molecules are unknown.
Figure 5. Small molecule inducers of endoderm activate TGF-β signaling.

(A) Phosphorylation of Smad2 was analysed in lysates of ESCs treated with IDE1, IDE2, DMSO, Activin A or Nodal or in the presence of the ALK4/5/7 inhibitor, SB431542. Treatment with IDE1 or IDE2 leads to activation of the TGF-β pathway after 24 hrs, similar to either Nodal or Activin A treatment. Phosphorylation of Smad2 by either of the two compounds is significantly attenuated in the presence of SB431542. (B) Increase in Nodal expression after treatment with small molecules IDE2, IDE1 and Nodal. Relative expression over DMSO treatment is shown as a mean of triplicate experiments ± s. d.
Treatment with either of the compounds for 6 hrs leads to upregulation of Nodal transcripts by 6-8-fold compared to the control (Fig. 5B) and the levels of Nodal expression increase further with time of compound exposure. Nodal treatment increases its own expression at similar levels and time schedule. This may reflect an autoregulatory mechanism for maintaining and upregulating Nodal expression through a Smad2/FAST-1-dependent autoregulatory loop that feeds on Nodal transcription (Agius et al., 2000; Pogoda et al., 2000).
In vivo competency of chemically derived endoderm
For chemically derived endoderm to be useful, it is important to determine its developmental potential, beyond the expression of endodermal markers. The ultimate goal is to direct the cells to form functional beta cells. To begin to address this possibility and functional potential of derived populations, mouse ESCs ubiquitously expressing enhanced yellow fluorescent protein (EYFP) (Hadjantonakis et al., 2002) were induced to form endoderm with IDE1, IDE2, or DMSO (control). At day 6 of treatment, we dissociated and injected the cells into the gut tube (a derivative of endoderm) of live E8.75 embryos. The lumen of the gut tube can be accessed prior to the completion of embryonic turning and gut tube closure and therefore provides a developmental window for functional assessment of ES-derived endoderm cells. Chemically derived endoderm was injected into the primitive gut tube at the anterior and posterior intestinal portals. At this time the gut tube is still open and anterior intestinal portal is accessible (A.E. Chen and D.A. Melton, in preparation, and Fig. 6A). We culture the embryos ex vivo for 24-30 hrs during which time the lateral walls of the embryonic gut fold ventrally and the gut tube closes, around E9.5. ES derived endodermal cells induced by small molecules integrated into the developing gut tube, whereas control treated ESCs did not. Moreover, ESC derived endoderm showed the characteristic morphology of gut cells and expressed markers of gut tube markers including FoxA2 and Claudin6 (Anderson et al., 2008) (Fig. 6B). Cells induced by IDE1 treatment integrated into the gut, in 8 out of 35 cases, and in 7 out of 29 embryos for IDE2. Conversely, DMSO (control) treated cells never integrate into the developing gut tube (0/10 and 0/11, respectively) and instead remain in the lumen (Fig. 6B).
Figure 6. Functional evaluation of chemically derived endoderm.

(A) Scheme of in vivo assay to assess the functional potential of compound induced endoderm. Mouse ESCs treated with chemical inducers incorporate into the developing host gut tube. Cultures of mouse ESC reporter lines expressing constitutive YFP were differentiated into endoderm with IDE1 or IDE2, producing 60-70% Sox17+ cells, then trypsinized and injected into the nascent gut lumen of E8.75 mouse embryos. Dashed line shows an approximate plane of section (B) After 24-30 hours ex vivo culture, mouse embryos were fixed, transversally sectioned and stained with antibodies against FoxA2 and Cldn6 to detect gut epithelial cells and anti-YFP antibodies to visualize injected cells. IDE1 and IDE2 induced endodermal cells incorporate into gut tube and show expression of gut tube markers. In contrast, DMSO treated cells remain clustered in the gut tube lumen 30 hrs after injection and do not incorporate into the gut epithelia nor express gut tube markers.
In vitro potential of compound induced endoderm
To further evaluate the developmental potential of compound induced endoderm, we tested whether it can differentiate in vitro into pancreatic progenitors. Pancreatic progenitors appear in the pancreatic bud at E9.5 and are marked by the expression of the transcription factor Pdx1. Pdx1 is required for pancreas development and β-cell formation, as a null mutation of Pdx1 in mice results in a failure to form a pancreas (Jonsson et al., 1994; Offield et al., 1996) and lineage tracing studies show that Pdx1 marks progenitors that give rise to all pancreatic cell types (Gu et al., 2003). Using Pdx1-GFP knock-in (Micallef et al., 2005) mouse ESCs we induced definitive endoderm using either of the hit compounds and cultured cells in various conditions for additional six days.
Published protocols for differentiation of ESCs into pancreatic progenitors are based on studies of the factors that modulate signaling during pancreatic organogenesis (Stafford and Prince, 2002; Lau et al., 2006; Bhushan et al., 2001). For example, in one protocol, endoderm is induced with Activin A, followed by treatment with FGF10 and the Hedgehog signaling inhibitor KAAD-cyclopamine for 3 days, and then exposed to a posteriorizing factor, retinoic acid, for an additional 3 days to induce Pdx1 expression (D'Amour et al., 2006). Using this regimen, 12.1±4% of the mouse ESCs differentiate further into pancreatic progenitors, defined by Pdx1+ expression. By comparison, when compound induced endoderm was used as a starting population, instead of Activin A induced endoderm, 25±6% of cells expressed Pdx1. Spontaneous differentiation, in the absence of hit compounds, additional growth factors or signaling modulators, occurs at a lower level, producing GFP expression in 4.6±1.1% of cells (Fig. 7).
Figure 7. Developmental potential of chemically derived endoderm.
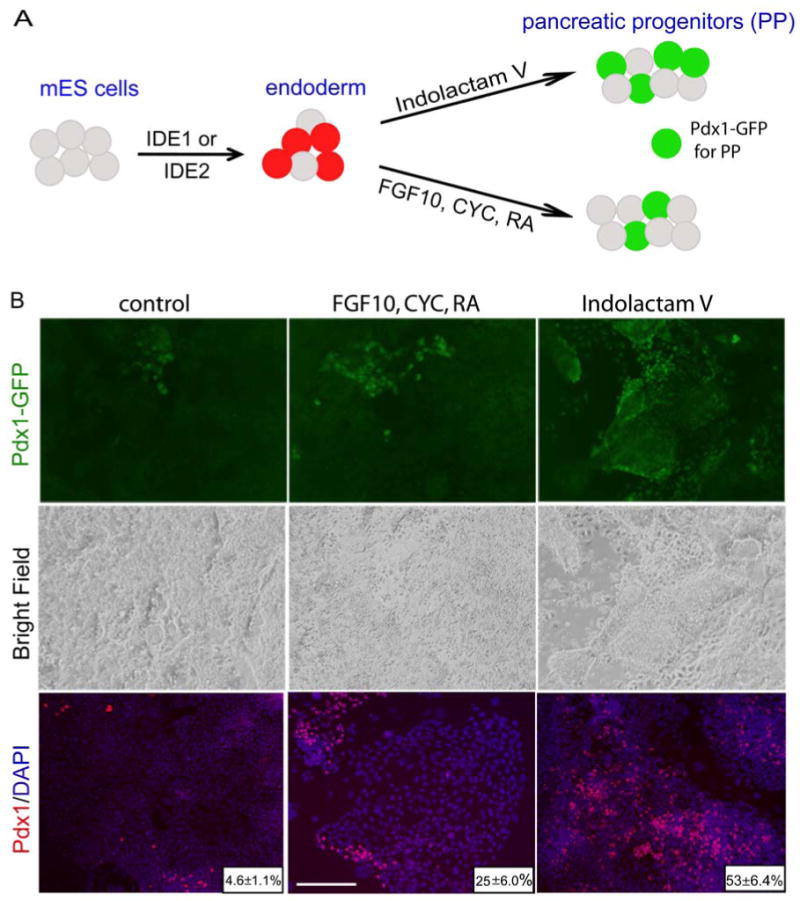
(A) Scheme of mouse ESC differentiation into Pdx1 + pancreatic progenitors. Endoderm was first derived through treatment with either IDE1 or IDE2 and then formation of pancreatic progenitors was monitored using a Pdx1-GFP reporter line. (B) Endoderm enriched cultures were grown for another 6 days in chemically defined media containing: DMSO without any additional growth factors or compounds (control), in presence of growth factors (FGF10, CYC, RA) or in the presence of Indolactam V to induce the expression of Pdx1. At day 12, in cultures treated initially with IDE1 or IDE2 and followed by ILV, 50% of the total cells were Pdx1+, a 10-fold increase above control treatment.
Finally, a separate chemical screen performed using human cells has identified a compound, Indolactam V (Chen et al, submitted), that can induce pancreatic progenitors from endoderm that has been produced from HUES. Indolactam V is also able to efficiently induce pancreatic progenitors (Pdx1+ cells) in endoderm derived by chemical treatment of mouse ESC. Treatment of IDE1 or IDE2 induced mouse endoderm yields 51±7.4% Pdx1-GFP expressing pancreatic progenitors at day 6 (Fig. 7). When cultures were stained with Pdx1 antibodies we observed similar number (53±6.4%) of positive cells after two-step treatment with small molecules. Notably, this is a 10-fold increase in cell number compared to the control DMSO treated and a 4-fold increase compared to published protocols. The majority of Pdx1+ (92±5%) cells also express other pancreatic progenitor markers, including HNF6 (Suppl. Fig. 4). Together, these compounds provide a two-step protocol for in vitro generation of pancreatic progenitors that utilizes the small molecules at each step, first as inducers of endoderm and later to generate pancreatic progenitors.
Discussion
The directed differentiation of ESCs into therapeutically useful cell types has received much attention because of the potential to study and possibly treat a number of diseases. One promising differentiation strategy is to recapitulate, in vitro, the developmental signals that guide cells down specific lineages during development. A complementary approach is to identify cell permeable small molecules that direct differentiation toward specific cell fates. As an example of this latter approach, the chemical screen described here shows that it is possible to induce definitive endoderm formation from mouse ESCs in vitro.
We found two potent small molecules, IDE1 and IDE2 that can direct mouse ESCs differentiation such that 70-80% of cells are endoderm cells. Both compounds are products of de novo synthesis and their biological activity has not been previously reported. This efficiency of induction compares favorably with published protocols employing TGF-β family members, e.g. Activin A or Nodal, which produce about 45% endoderm. In addition, both IDEs induce endoderm formation also in HUES cultures therefore providing new tools for ES cells directed differentiation. In the long term, the potential benefits of finding chemicals like IDE1 or IDE2 include the prospect of minimizing the risk of animal disease infections and a cost reduction for materials and the temporal control that can be achieved using small molecules which can be easily delivered and removed. Application of either of the two small molecules reported here does not eliminate the necessity of serum presence in the differentiation protocols. Our results enhance the repertoire of chemical compounds for manipulating ESC fate and encourage the high throughput screening of small molecules to direct differentiation of ESCs under chemically defined conditions.
With respect to their potential to induce endoderm, IDE1 and IDE2 appear to be interchangeable as we did not observe any substantial differences as far as efficiency, gene expression or functional potential between populations induced by the two compounds. Both compounds are novel and their specific biological targets remain to be identified, but a strong hint comes from the fact that they activate part of a TGF-β pathway as evidenced by Smad2 phosphorylation.
In the experiments reported here, mouse ES were cultured in the absence of feeders or other supportive cell types. However, extrinsic signaling could well improve the efficiency of the differentiation and maturation of cells. Interactions between endoderm and different mesodermal cell types pattern the gut epithelium into progenitor domains and promote local organ outgrowth (Horb and Slack, 2001; Deutsch et al., 2001; Kumar et al., 2003). One source for prospective inductive signals is pancreatic mesenchyme which supports budding of dorsal pancreatic tissue into the stroma (Golosow and Grobstein, 1962; Wells and Melton, 2000). Another important signal comes from vessel endothelium which provides inductive signals for islet development (Lammert et al., 2001). Future studies may identify small molecule substitutes for these developmental signals.
If in vitro differentiation of ESCs is to be used for treating human diseases, including diabetes, it will likely require derivation of large quantities of cells with high purity in chemically defined conditions. This study shows the feasibility of chemical screening to identify molecules that may achieve this effect, in this case direct ESCs into endoderm, which is relevant for liver, lung, stomach, intestine, and thymus as well as the pancreas.
Experimental Procedures
Mouse ESC culture and differentiation
Mouse ESCs were routinely cultured on irradiated CF-1 MEF feeder cells in DMEM (Gibco) media supplemented with 15% Fetal Bovine Serum (HyClone FBS, Invitrogen), 2 mM L-glutamine (L-Glu, Gibco), 1.1 mM 2-mercaptoethanol (Gibco), 1 mM nonessential amino acids (Gibco), 1×penicillin/streptomycin (P/S, Gibco) and 5×105 units LIF (Chemicon). Cells were passaged at the ratio of 1:6-1:12 every 2-3 days using 0.25% trypsin. To generate the starting population, mouse ESCs were cultured on MEF feeder cells until they reached 80-90% confluence. Prior to differentiation, ESCs were passaged onto gelatin coated plates for 30 min to remove MEFs and afterwards ESCs were seeded at 2500 cells per cm2 on gelatinized plates. After overnight culture, cells were exposed to 25ng/ml Wnt3a (R&D Systems) + 50ng/ml Activin A (R&D Systems) or 500ng/ml Nodal (R&D Systems) in DMEM (Gibco) supplemented with 1×L-Glu and 0.2% FBS (Gibco) for 1 day, then Activin A or Nodal in the same media and cultured for 4-6 days to induce endoderm differentiation. For the chemical inductions, IDE1 and IDE2 (provided by Stuart L. Schreiber) were added at 5μM concentration in the differentiation media. For pancreatic progenitors induction, the cells were transferred to 50 ng/ml FGF10 (R&D Systems), 0.75 μM KAAD-cyclopamine (Calbiochem) and 2 μM RA (Sigma) or Indolactam V 330 nM (Axxora) in DMEM supplemented with 1×L-Glu, 1×PS, 1× B27 (Invitrogen) for 4 days. SB431542 was purchased from Sigma. All stock compounds were made with either DMSO or PBS.
High throughput screen
To carry out the screen, mouse Sox17/dsRed ESCs (passage 16-20) were trypsinized, MEF depleted, and plated on gelatin-coated 384-well plates at density 800 cells/well using Biotek μFill. After overnight incubation in regular mouse ES media, the media was changed to low serum containing differentiating media (2% FBS) and compounds were added by pin transfer at final concentration 5 μM, in a volume 50 μl per well containing 1% DMSO (v/v). After an additional 6 days of culture, cells were washed with PBS, trypsinized for 3 min, suspended in FACS buffer (PBS, 5% FBS) and dsRed expression was detected by high throughput FACS analysis (Aria, Becton Dickinson).
Chemical libraries
The compound libraries used for this study included: the MicroSource library consisting of 2,000 bioactive compounds and known drugs, 1,000 synthetic compounds biased for HDAC inhibition (obtained from Stuart L. Schreiber laboratory) and a selection of hand-picked known modulators of stem cell fate (20 compounds), small molecule microarray consisting of approximately 400 compounds, including bioactives, natural products, and 400 compounds that are known modulators of development or signaling pathways (both prepared by Stuart L. Schreiber laboratory).
HUES cells culture and differentiation
HUES cell lines were cultured essentially as described {Cowan, 2004 #6}. Briefly, HUES4, HUES8 and HUES9 cells were routinely cultured on irradiated CF-1 MEF feeder cells in KnockOut DMEM (Gibco) supplemented with 10% KnockOut Serum Replacement (Gibco), 10% human plasma (Invitrogen), 2 mM L-Glu, (Gibco), 1.1 mM 2-mercaptoethanol (Gibco), 1 mM nonessential amino acids (Gibco), 1× P/S (Gibco) and 10 ng/ml bFGF (Invitrogen). Cells were split at the ratio of 1:10-1:12 every 4–5 days using 1 mg/ml collagenase type IV. To induce endoderm formation, HUES cells were cultured on MEF feeder cells till 80-90% confluent, then treated with 100 ng/ml Activin A in advanced RPMI (Gibco) supplemented with 1×L-Glu and 0.2% FBS (Gibco), or with combination of 25 ng/ml Wnt3a (R&D systems) + 100 ng/ml Activin A (R&D systems) or were exposed to compounds in the same media. At days 4 or 6 of culture cells were analysed for endodermal marker expression, Sox17.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde (PFA, Sigma) in PBS for 20 min at 4°C followed by a wash with PBS. Cells were blocked with 10% donkey serum (Jackson Immunoresearch) in PBS/0.1% Triton X and incubated with primary antibodies overnight at 4°C. Secondary antibodies were incubated for 1 h at room temperature. The following antibodies and dilutions were used: goat anti-SOX17, (1:500 R&D systems); rabbit anti-PDX1 (1:200, Chemicon), rabbit anti-FoxA2 (1:500; Upstate), rabbit anti-Dab2 (1:200, Santa Cruz), rabbit anti-SPARC (1:200, Santa Cruz,), mouse anti-AFP (1:100, Sigma), anti-Gata6 (1:50, Santa Cruz Biotech), goat anti-HNF6 (1:200, Santa Cruz Biotech), rabbit anti-RFP/DsRed (1:300, MBL), rabbit anti-GFP (1:200, Molecular Probes). Secondary antibodies were: rhodamine Red-X-conjugated donkey anti-goat antibody, 1:200 (JIRL), Alexa-488-conjugated goat anti-mouse and Alexa-594-conjugated goat anti-rabbit antibodies (1:300, Molecular Probes). Nuclei were visualized by Hoechst 33342 (1:1000, Molecular Probes). Images were taken using an Olympus IX70 Microscope. For quantification images were analyzed for the frequency of Sox17+, FoxA2+ or Pdx1+ cells using Metamorph image analysis software (Molecular Devices) and at least 6 images per well were collected. Data were confirmed in four independent experiments.
Flow cytometry
Cells were dissociated using 0.25% trypsin for 3 min followed by quenching of trypsin and further dissociation in PBS with 5% FBS. Suspension was filtered through nylon and cells were analyzed and sorted out by MoFlo (Dako Cytomation, Ft. Collins, CO).
Quantification of endoderm formation
Endoderm formation was monitored by Sox17 expression by either flow cytometry detection of Sox17/DsRed or immunofluorescence using anti-Sox17 antibodies. Cells labeled by antibody staining were quantified using Metamorph software as percentage of total cell number (based on Hoechst 33342 nuclei staining). All conditions were tested in either tri- or quadruplicates.
Global gene expression analysis
Sox17/DsRed+ cells were sorted out by FACS from mouse ESC cultures treated either with growth factors or compounds. Total RNA was isolated using Qiashredder and RNAeasy Mini Kit (both from Qiagen). Biotinylated cRNA was prepared from ≥100ng of isolated RNA using Illumina TotalPrep RNA Amplification Kit (Ambion) and hybridized to the Illumina mouse genome Bead Chips (MouseRef8). All samples were prepared as three biological replicates. Data were acquired with Illumina Beadstation 500 and were evaluated using BeadStudio Data Analysis Software (Illumina)
Western Blot analysis
Cells were lysed in 1x RIPA lysis buffer in the presence of protease inhibitor mixture (Roche)/1% phosphatase inhibitor mixture (Roche). Proteins were separated by 10% Tris-Glycine SDS/PAGE (Bio-rad) under denaturing conditions and transferred to a nitrocellulose membrane. After blocking with 5% skim milk in PBS/0.1%Triton X, the membrane was incubated with antibodies against phospho-Smad2 (1:1000, Cell Signaling) or β-actin for overnight at 4°C. The membrane was then washed, incubated with anti-mouse/rabbit peroxidase-conjugated secondary antibody (1:1000, Cell Signaling) at room temperature for 1h, and developed by SuperSignal chemiluminescence (Pierce).
Injections into gut tube and embryo culture
Mouse E8.5 ICR embryos were dissected in Hanks Balanced Salt Solution and cultured in the DMEM/F12 (Invitrogen) media supplemented with 50% rat serum (Valley Biomedical), 1xP/S, L-Glu, at 37°C for 20-24 h. The YFP mouse ESCs were cultured in the presence of IDEs for 6 days to induce endoderm formation and then cells were dissociated with trypsin and approx. 100 000 cells were injected into gut tube of E8.75 embryos. Following injections, embryos were transferred to rotating bottle culture unit and were then cultured in media (1.5-2 ml per embryo) as above under humidified conditions at 40% O2, 5% CO2, 55% N2, and 37°C. After 30 h culture, embryos were fixed in 4% PFA/PBS, embedded in tissue-tek and cryosections were stained with antibodies against Cld6, FoxA2 and YFP.
Supplementary Material
Supplementary Figure 1. Generation of Sox17-dsRed reporter mouse ES line (A) Gene targeting strategy used to target the dsRed variant (Shaner et al., 2004) dTomato to the mouse Sox17 locus. Indicated are the targeting vector with exons as grey boxes, the wild type locus and the targeted locus after homologous recombination. EcoRV and HindIII digest were used for Southern blot analysis with an external probe and an internal probe to identify targeted ES cell clones. (B) Positive clones were confirmed by Southern blot analysis with an external 5′ probe (upper panel) and a probe against the neomycin selection cassette (lower panel). Parental untargeted cell line, AV3, and targeted Sox17 dsRed clones are indicated. The wild type allele migrates at 10.1kb and the targeted allele at 6.1kb as identified with the 5′ probe. A neomycin cassette can be identified at 6.1kb with a neo probe. (C) Day 4 embryoid bodies of wild type control (upper panel) and Sox17 targeted cells (lower panel) are shown with respect to their red fluorescence, green fluorescence and bright field appearance. The left column represents an overlay of the bright field picture with the red fluorescence.
Supplementary Figure 2: Immunofluorescent analysis of endodermal markers induced by treatment of mouse ESCs with chemical compounds.(A) The Sox17+ cells induced by IDE2 or IDE1 form a compact, epithelial sheet as shown in the left panel (αSox17 represent antibody staining for Sox17). Cells are more dispersed following treatment with a different small molecule (XE09), one that also induces Sox17 (right panel). (B) Expression of extra-embryonic endoderm markers (EE). EE markers such as GATA4, SPARC, AFP and Dab2 are present in the dispersed population of Sox17+ cells but only rarely observed in the compact population of Sox17+ cells induced by the IDE2 compound. Similarly, treatment with IDE1 leads to marginal expression of EE markers (data not shown). Scale bars=200μM.
Supplementary Figure 3. Endoderm induction in human ES cells cultures. Human ES cells cultures were treated for 6 days with Activin A or vehicle (DMSO) only. Activin A treatment leads to Sox17 expression in 55-65% of total cells, which is 4-fold increase as compared to the vehicle treatment. Scale bars=100μM
Supplementary Figure 4. Induction of pancreatic progenitors by treatment of mouse ESCs with two small molecules. Co-expression of pancreatic markers, Pdx1 and HFN6 in cells induced by a two-step small molecule protocol. During the first step, mouse ESCs were treated with IDE2 for 6 days to induce definitive endoderm and then, in the second step, cells were treated with Indolactam V for an additional 6 days to allow pancreatic progenitor specification. Cells stained for both Pdx1 (red) and HNF6 (green) are yellow in the left panel.
Acknowledgments
D. A. M and S. L. S. are investigators of the Howard Hughes Medical Institute. Edouard G. Stanley (Pdx1-GFP) and Andreas Nagy and Kat Hadjantonakis (EYFP) kindly provided the mouse ESCs. We also thank Anastasie Kweudjeu, Calvin Balatbat and Brian Tilton for their excellent technical support, Renate Hellmiss for her expertise in figure design, Qiao Zhou and Peter Carolan for valuable comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WJ, Zhou Q, Alcalde V, Kaneko OF, Blank LJ, Sherwood RI, Guseh JS, Rajagopal J, Melton DA. Genetic targeting of the endoderm with claudin-6(CreER) Dev Dyn. 2008;237:504–512. doi: 10.1002/dvdy.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- Burridge PW, Anderson D, Priddle H, Barbadillo Munoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- Chen S, Do JT, Zhang Q, Yao S, Yan F, Peters EC, Scholer HR, Schultz PG, Ding S. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci USA. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Desbordes SC, Placantonakis DG, Ciro A, Socci ND, Lee G, Djaballah H, Studer L. High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell. 2008;2:602–612. doi: 10.1016/j.stem.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Diamandis P, Wildenhain J, Clarke ID, Sacher AG, Graham J, Bellows DS, Ling EK, Ward RJ, Jamieson LG, Tyers M, Dirks PB. Chemical genetics reveals a complex functional ground state of neural stem cells. Nat Chem Biol. 2007;3:268–273. doi: 10.1038/nchembio873. [DOI] [PubMed] [Google Scholar]
- Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- Dziadek M. Cell differentiation in isolated inner cell masses of mouse blastocysts in vitro: onset of specific gene expression. J Embryol Exp Morphol. 1979;53:367–379. [PubMed] [Google Scholar]
- Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Macmaster S, Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. doi: 10.1186/1472-6750-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Endoderm specification and differentiation in Xenopus embryos. Dev Biol. 2001;236:330–343. doi: 10.1006/dbio.2001.0347. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lau J, Kawahira H, Hebrok M. Hedgehog signaling in pancreas development and disease. Cell Mol Life Sci. 2006;63:642–652. doi: 10.1007/s00018-005-5357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SL, Tam PP. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev Dyn. 2006;235:2315–2329. doi: 10.1002/dvdy.20846. [DOI] [PubMed] [Google Scholar]
- Liu Y, Asakura M, Inoue H, Nakamura T, Sano M, Niu Z, Chen M, Schwartz RJ, Schneider MD. Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:3859–3864. doi: 10.1073/pnas.0609100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason IJ, Murphy D, Munke M, Francke U, Elliott RW, Hogan BL. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. Embo J. 1986;5:1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- McLean AB, D'Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE, Dalton S. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. doi: 10.2337/diabetes.54.2.301. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, Wert SE, Whitsett JA. Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol. 2006;294:192–202. doi: 10.1016/j.ydbio.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Pogoda HM, Solnica-Krezel L, Driever W, Meyer D. The zebrafish forkhead transcription factor FoxH1/Fast1 is a modulator of nodal signaling required for organizer formation. Curr Biol. 2000;10:1041–1049. doi: 10.1016/s0960-9822(00)00669-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Hara K, Kanai-Azuma M, Matsui T, Miura Y, Tsunekawa N, Kurohmaru M, Saijoh Y, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Jitianu C, Cleaver O, Shaywitz DA, Lamenzo JO, Chen AE, Golub TR, Melton DA. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol. 2007;304:541–555. doi: 10.1016/j.ydbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Wu X, Ding S, Ding Q, Gray NS, Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. 2004;126:1590–1591. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- Yang DH, Smith ER, Roland IH, Sheng Z, He J, Martin WD, Hamilton TC, Lambeth JD, Xu XX. Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev Biol. 2002;251:27–44. doi: 10.1006/dbio.2002.0810. [DOI] [PubMed] [Google Scholar]
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Generation of Sox17-dsRed reporter mouse ES line (A) Gene targeting strategy used to target the dsRed variant (Shaner et al., 2004) dTomato to the mouse Sox17 locus. Indicated are the targeting vector with exons as grey boxes, the wild type locus and the targeted locus after homologous recombination. EcoRV and HindIII digest were used for Southern blot analysis with an external probe and an internal probe to identify targeted ES cell clones. (B) Positive clones were confirmed by Southern blot analysis with an external 5′ probe (upper panel) and a probe against the neomycin selection cassette (lower panel). Parental untargeted cell line, AV3, and targeted Sox17 dsRed clones are indicated. The wild type allele migrates at 10.1kb and the targeted allele at 6.1kb as identified with the 5′ probe. A neomycin cassette can be identified at 6.1kb with a neo probe. (C) Day 4 embryoid bodies of wild type control (upper panel) and Sox17 targeted cells (lower panel) are shown with respect to their red fluorescence, green fluorescence and bright field appearance. The left column represents an overlay of the bright field picture with the red fluorescence.
Supplementary Figure 2: Immunofluorescent analysis of endodermal markers induced by treatment of mouse ESCs with chemical compounds.(A) The Sox17+ cells induced by IDE2 or IDE1 form a compact, epithelial sheet as shown in the left panel (αSox17 represent antibody staining for Sox17). Cells are more dispersed following treatment with a different small molecule (XE09), one that also induces Sox17 (right panel). (B) Expression of extra-embryonic endoderm markers (EE). EE markers such as GATA4, SPARC, AFP and Dab2 are present in the dispersed population of Sox17+ cells but only rarely observed in the compact population of Sox17+ cells induced by the IDE2 compound. Similarly, treatment with IDE1 leads to marginal expression of EE markers (data not shown). Scale bars=200μM.
Supplementary Figure 3. Endoderm induction in human ES cells cultures. Human ES cells cultures were treated for 6 days with Activin A or vehicle (DMSO) only. Activin A treatment leads to Sox17 expression in 55-65% of total cells, which is 4-fold increase as compared to the vehicle treatment. Scale bars=100μM
Supplementary Figure 4. Induction of pancreatic progenitors by treatment of mouse ESCs with two small molecules. Co-expression of pancreatic markers, Pdx1 and HFN6 in cells induced by a two-step small molecule protocol. During the first step, mouse ESCs were treated with IDE2 for 6 days to induce definitive endoderm and then, in the second step, cells were treated with Indolactam V for an additional 6 days to allow pancreatic progenitor specification. Cells stained for both Pdx1 (red) and HNF6 (green) are yellow in the left panel.


