Abstract
Histone post-translational modifications (PTMs) are important regulators of chromatin structure and gene expression. Quantitative analysis of histone PTMs by mass spectrometry remains extremely challenging due to the complex and combinatorial nature of histone PTMs. The most commonly used mass spectrometry-based method for high-throughput histone PTM analysis is data-dependent acquisition (DDA). However, stochastic precursor selection and dependence on MS1 ions for quantification impede comprehensive interrogation of histone PTM states using DDA methods. To overcome these limitations, we utilized a data-independent acquisition (DIA) workflow that provides superior run-to-run consistency and post-acquisition flexibility in comparison to DDA methods. In addition, we developed a novel DIA-based methodology to quantify isobaric, co-eluting histone peptides that lack unique MS2 transitions. Our method enabled deconvolution and quantification of histone PTMs that are otherwise refractory to quantitation, including the heavily acetylated tail of histone H4. Using this workflow, we investigated the effects of the histone deacetylase inhibitor SAHA (suberoylanilide hydroxamic acid) on the global histone PTM state of human breast cancer MCF7 cells. A total of 62 unique histone PTMs were quantified, revealing novel SAHA-induced changes in acetylation and methylation of histones H3 and H4.
Keywords: histone PTM, mass spectrometry, data-independent acquisition, DIA, epigenetics, proteomics, SAHA, acetylation, methylation
Introduction
Although the human genome has been sequenced to roughly 99.7% completion just over a decade ago, it has become apparent that significant challenges remain in understanding both gene expression and heredity.1,2 Within eukaryotes, genomic DNA is tightly packed within a dynamic and highly structured polymer of DNA, histones, and non-histone proteins known as chromatin. The basic unit of chromatin is the nucleosome, which consists of approximately two superhelical turns of DNA (roughly 147 bp of DNA) about an octamer of core histone proteins (two copies each of H2A, H2B, H3, and H4). This highly conserved nucleoprotein complex is further assembled into higher order chromatin structure, which ultimately compacts genomic DNA by a factor of 30–40, thus greatly affecting both access to DNA and the orientation and positioning of the DNA molecule itself.3
Histones are small, basic proteins consisting of a globular core domain and a flexible N-terminal tail that is subject to a multitude of covalent modifications.3 The most common of these modifications includes lysine acetylation and mono-, di-, and tri-methylation.4,5 Once regarded as purely structural elements, it has become clear that the modification state of histones has a significant impact on the overall structure of chromatin and ultimately on the many processes that require physical access to DNA. These highly combinatorial modifications have been termed the “histone code” and are thought to contain regulatory information beyond that which is conferred by the nucleotide sequence alone.
Common methods for measurement of histone PTMs involve the use of immunochemistry, either via immunoblot detection or via chromatin-immunoprecipitation (ChIP) coupled to downstream techniques such as quantitative PCR or DNA sequencing. While antibodies to histone PTMs are essential tools for epigenetics research, there are significant drawbacks, including cross-reactivity, epitope occlusion, inherent differences in binding efficiency, and lot-to-lot variation of polyclonal antibodies.5–9 There is also considerable expense associated with both generation and validation of antibodies. By design, all antibody-based methodologies require a priori knowledge of the PTMs of interest, are limited to those for which antibodies are available, and typically do not detect the presence of combinatorial PTMs. In contrast, liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) provides a comprehensive and unbiased method for the identification and quantification of histone PTMs, including combinatorial modifications.5
Histone PTMs are uniquely difficult to analyze via mass spectrometry (MS) due to their high diversity, combinatorial nature, and high concentration in specific domains of the protein. Quantitative histone proteomic experiments typically employ a bottom-up MS approach although top- and middle-down approaches have also been used extensively.10–13 In regards to bottom-up MS, data dependent acquisition (DDA) and targeted proteomics, including selected reaction monitoring (SRM) and parallel reaction monitoring (PRM), are currently the methods of choice to analyze histone PTMs.8,14 In DDA mode, the instrument first performs a survey scan and then selects peptide ions with intensities above a predefined threshold for fragmentation. While DDA is the most commonly used methodology in shotgun proteomics, it exhibits key limitations in regards to histone PTM analysis, including the stochastic selection of precursor ions for fragmentation and the ability to only quantify the MS1 channel.15 In contrast to the DDA methods, targeted methods typically scan the MS2 transition ions of a pre-defined set of peptides across the entire HPLC gradient. This technical difference results in increased specificity, sensitivity, consistency and, most importantly for histone PTMs, discrimination of co-eluting, isobaric peptides. While targeted methods overcome many of the limitations of DDA, targeted methods require scheduling of analytes and optimization of transition selection.16 Finally, neither DDA nor targeted methods have the ability to deconvolute and quantify isobaric, co-eluting peptides that lack unique MS2 transitions, such as the highly acetylated N-terminal tail of histone H4.
In contrast to data-dependent acquisition, data-independent acquisition (DIA) relies on neither the detection of, nor specific knowledge of precursor ions to trigger acquisition of product ions.17,18 In DIA, the instrument cycles through the entire LC retention time range, recording consecutive survey scans and fragment ion spectra for all precursors obtained within a series of pre-defined isolation windows that subdivide a larger m/z region.17–19 Unlike DDA, which produces a large number of missing values, DIA has superior run-to-run sampling efficiency and thus exhibits higher quantitative reproducibility.17,20 Additionally, since quantitative information can be generated from both MS1 and MS2 scans, isobaric and co-eluting peptides can be differentiated from one another. In specific regards to histone PTM analysis, Sidoli et al. have shown that SWATH (an AB Sciex data-independent acquisition method) shows superior precision and repeatability in the analysis of histone H3 peptides.21 Thus DIA provides the ultimate reproducibility and post-acquisition flexibility while being extremely simple and straight forward to optimize.
Here we developed a label-free DIA workflow for sensitive and accurate quantification of histone modifications and applied this strategy to quantify the alterations in histone PTM states in MCF7 breast cancer cells following treatment with the pharmaceutical histone deacetylase (HDAC) inhibitor, SAHA (suberoylanilide hydroxamic acid, Vorinostat), which has been shown to induce a global increase in histone acetylation.22 We demonstrate superior precision and consistency of DIA relative to DDA. To maximize the amount of biologically relevant information obtainable from our DIA workflow, we developed a novel data analysis methodology that allows for the use of both MS1 and MS2 spectra to calculate percent of total values for isobaric and co-eluting peptides that do not exhibit unique MS2 transitions. This technique is particularly relevant to the N-terminal tail of H4, which has multiple acetylated lysine residues with no unique MS2 transitions. Overall, we successfully quantified 62 acetylated and methylated peptides on histones H2A, H3, and H4, including all possible permutations of acetylated H4. This capacity to resolve and quantify isobaric, co-eluting histone peptides and generate percent of total values of histone PTMs yields valuable insight into site-specific effects of SAHA treatment in the setting of human breast cancer and can be used to compare histone PTMs among samples generated in virtually any experimental setting.
Experimental Procedures
Cell culture and treatment
Human MCF7 breast cancer cells were generously donated by the Patricia Keely laboratory at the University of Wisconsin. Cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum. Cells were treated with either 10, 5, or 2.5 µM SAHA or an equivalent volume of DMSO as a vehicle control (final concentration of 0.026%, 0.013%, or 0.007%, respectively) for 24 hours prior to harvesting. All experiments were performed in biological triplicate.
Cell fractionation and sample preparation
Roughly 20 × 106 cells were trypsinized and pelleted prior to washing twice with ice-cold PBS. Cells were then resuspended in 800 µL ice-cold buffer A (10 mM Tris-HCl, 10 mM NaCl, 3 mM MgCl2, pH 7.4) with histone deacetylase and protease inhibitors (1 mM sodium butyrate, 4 µM trichostatin A, 100 µM phenylmethylsulfonyl fluoride, 10 µg/mL leupeptin, and 10 µg/mL aprotinin). Cells were vortexed at medium speed for 5 seconds prior to being transferred to a pre-chilled 1 mL dounce homogenizer. Cells were homogenized with 40 strokes and centrifuged at 800 × g. The crude nuclear pellet was then resuspended in 200 µL ice-cold PBS and overlaid onto 800 µL of a pre-chilled sucrose cushion (buffer A + 1.5 M sucrose). The nuclear suspension was then centrifuged at 21,100 × g to obtain a purified nuclear pellet. Histones were acid extracted, followed by two rounds of chemical derivatization using propionic anhydride and trypsinized as described.23 After chemical derivatization all N-termini and unmodified or monomethylated lysine residues were propionylated. This labeling method protects all lysine residues from cleavage by trypsin, which cleaves C-terminally to unmodified lysine and arginine residues, enabling consistent generation of histone peptides amenable to MS analysis. The Biognosys HRM Calibration Kit was added to all of the samples according to manufacturer’s instructions (required for the DIA analysis using Biognosys Spectronaut).
Nano-liquid chromatography and electrospray ionization tandem mass spectrometry
For both data dependent acquisition (DDA) and data independent acquisition (DIA), 1 µg of propionylated histone peptides was injected onto a Dionex Ultimate3000 nanoflow HPLC with a Waters NanoEase C18 column (100 µm × 15 cm, 3 µm) coupled to a Thermo Fisher Q-Exactive mass spectrometer at 700 nL/min. Mobile phase consisted of water + 0.1% formic acid (A) and acetonitrile + 0.1% formic acid (B). Histone peptides were resolved with a linear gradient of 2% to 35% mobile phase B over 65 minutes. The mass spectrometer was operated in DDA mode with dynamic exclusion enabled (exclusion duration = 8 seconds), MS1 resolution = 70,000, MS1 automatic gain control target = 1 × 106, MS1 maximum fill time = 100 ms, MS2 resolution = 17,500, MS2 automatic gain control target = 2 × 105, MS2 maximum fill time = 500 ms, and MS2 normalized collision energy = 30. For each cycle, one full MS1 scan range = 300–1100 m/z, was followed by 10 MS2 scans using an isolation window size of 2.0 m/z. An inclusion list was employed to increase the detection efficiency of histone peptides of interest (Supplemental Table 1). In data-independent mode (DIA) the mass spectrometer was operated with a MS1 scan at resolution = 35,000, automatic gain control target = 1×106, and scan range = 390–910 m/z, followed by a DIA scan with a loop count of 10. DIA settings were as follows: window size = 10 m/z, resolution = 17,500, automatic gain control target = 1 × 106, DIA maximum fill time = AUTO, and normalized collision energy = 30. For each cycle, one full MS1 was followed by 10 MS2 scans using an isolation window size of 10 m/z. Total cycle time for a complete scan across the whole scan range was 5.4 seconds.
Database search and spectral library construction
Database searches were performed for each DDA sample using Andromeda (MaxQuant v 1.4.1.2). Spectra were searched against the human SwissProt database (Download: April 2015, containing 20,204 sequences) using a 20 ppm mass tolerance for the first-pass search and a 4.5 ppm mass tolerance for the main search The enzyme was specified as ArgC with zero missed cleavages. No static modifications were set. Variable modifications were set as follows: acetyl(K), monomethyl+propionyl(K), dimethyl(K), propionlyl(K), trimethyl(K) and propionyl(peptide N-terminus). A reverse decoy database was generated within Andromeda and the False Discovery Rate (FDR) was set to <0.01 for peptide spectrum matches (PSMs), proteins, and modification sites. Spectral libraries were generated using Skyline (v 2.5.0.6157) or Spectronaut (v 7.0) using Andromeda search results. All spectral libraries can be found in supplemental data.
Histone PTM Quantitation via DDA and DIA
The DDA files were imported into MaxQuant.24 The peptide database search was performed as outlined above. Peptide intensities were determined using the label-free quantitation setting in MaxQuant. The DIA files were imported into either Skyline25 or Spectronaut26 for quantitative analysis. For Skyline, all MS1 and MS2 peaks were selected manually based on retention times and presence of appropriate transitions. Skyline can utilize iRT peptides for automated peak picking, however, in our hands manual selection performed more accurately (data not shown). For Spectronaut, all MS1 and MS2 peaks were selected automatically and then examined manually. Spectronaut performs an automatic calibration of m/z and retention time (iRT) using the detectable signals of the HRM Calibration Kit.27 Spectronaut automatically corrects systematic errors and estimates the precision in these two dimensions from the data. Using a spectral library and the calibration information, targeted extraction of the DIA data is performed, resulting in extracted ion currents on MS1 and MS2 level. Peak detection, peptide identification and quantitation is performed on these extracted ion currents. A number of different scores are calculated, such as scores for mass accuracy, relative fragment ion intensity, correlation of MS1 to MS2 extracted ion current, and expected isotopic patterns. These scores are combined into a discriminant score and a false discovery rate is estimated as described in reference.28 Peak areas for all selected transitions in Skyline and Spectronaut were combined for quantitation. In general, either three precursor ions or no less than four MS2 transition ions were used for quantitation to ensure accurate, unbiased measurement. All histone peptide MS1 peaks and their integration bounds were verified using XCalibur Qual Browser (v2.2).
For quantifying the percent of total for each peptide species, all peptide areas belonging to a peptide “family” were summed to obtain the total area for that “family.” A peptide “family” is defined as a group of peptides spanning the same residues within a histone protein, e.g. H3 residues 18–26 (KQLATKAAR), which contains lysines 18 and 23 is a family of peptides with 6 members: K18ac+K23un, K18un+K23ac, K18ac+K23ac, K18me1+K23un, K18un+K23me1, and K18un+K23un. The proportion of the total for each peptide member of the family was then obtained by dividing the individual peptide area by the total family area. In cases of isobaric and co-eluting peptides, the summed area of precursor peaks was averaged among all co-eluting, isobaric members since all such members are represented by the same set of precursor peaks. MS2 areas from unique transitions were then used to differentiate and quantify the relative proportion of each co-eluting, isobaric peptide as described in Figure 4 and the supplemental methods.
Figure 4. MS2-level resolution and quantification of isobaric and co-eluting singly acetylated histone H4 4–17 peptides.
(A) Raw MS1 spectra of the unmodified and singly acetylated forms of H4 4–17, which contains lysines 5, 8, 12, and 16. (B) Transition ions of H4 4–17 (top) and unique b and y ions that can be used for quantitation (middle and bottom). (C) Quantitation of singly acetylated H4 4–17 peptides using a combination of MS1 and MS2 ions.
Data normalization and statistics
Exported area or intensity values from Skyline and Spectronaut or MaxQuant, respectively, were normalized prior to calculations of fold changes and statistics. We utilized two normalization schemes, including normalization: (1) to the total area or signal intensity of all histone peptides observed, (2) normalization within peptide families to the total area or intensity (Supplemental Figure 1). Normalization scheme 1 was implemented for all data used for all comparisons between MS1 and MS2 quantitation and for comparisons between acquisition modes. Normalization scheme 2 was used for analysis of SAHA treatment on MCF7 cells, since this manner of comparison facilitated our use of MS2 spectra to calculate relative proportions of peptide family totals for individual PTMs.
All p-values were generated using a Welch’s t-test (biological replicates, n = 3). Statistical significance was determined by p < 0.05. Pearson’s correlation, correlation matrices, and plots were generated using the corr( ) and plotmatrix( ) functions of MatLab v2015a. The HeatMap was generated using log10 transformed area or intensity values with the HeatMap( ) function of MatLab v2015a. Boxplots were generated using the boxplot( ) function of MatLab v2015a. All functions are part of the Statistics and Bioinformatics toolboxes of MatLab v2015a.
Western blot
Lysine acetylation was detected using a pan-acetylated lysine antibody (Cell Signaling #9814). Total Histone H3, as a loading control, was detected using a Histone H3 antibody (Abcam #ab46765). Both antibodies were used at 1:5000 and incubated at 4°C overnight.
Results
Experimental design and validation
To assess the applicability of our DIA workflow for the analysis of histone PTMs in a biologically relevant system, we used human breast cancer MCF7 cells treated with the HDAC inhibitor SAHA. We observed a dose-dependent increase in histone acetylation at all concentrations of SAHA tested (Supplemental Figure 2). The most dramatic response was elicited in cells treated with 10 µM SAHA, and accordingly, we chose this concentration for all subsequent work. SAHA-induced changes in histone PTMs were assessed according to the workflow outlined in Figure 1. Briefly, histones were acid extracted, propionylated at all unmodified and monomethylated lysines residues, and then trypsin digested. Propionylation chemically protects lysine residues from cleavage by trypsin, generating uniform histone peptides, irrespective of modification state, that are of suitable size and charge for MS detection. After a second round of propionylation to label newly generated N-termini on tryptic peptides, histone peptides were analyzed by LC-MS/MS via data-dependent acquisition (DDA). Hereafter, although unmodified and monomethylated lysine residues are propionylated, for simplicity of results interpretation these residues will be referred to by their endogenous state, either unmodified or monomethylated. Peptides were identified using the Andromeda search engine and the results were used to generate spectral libraries using either Skyline or Spectronaut. The propionylated histones were then re-injected and acquired via data-independent acquisition (DIA). These results were then matched to spectral libraries and quantified with Skyline or Spectronaut (Supplemental Tables 2 and 3, respectively).
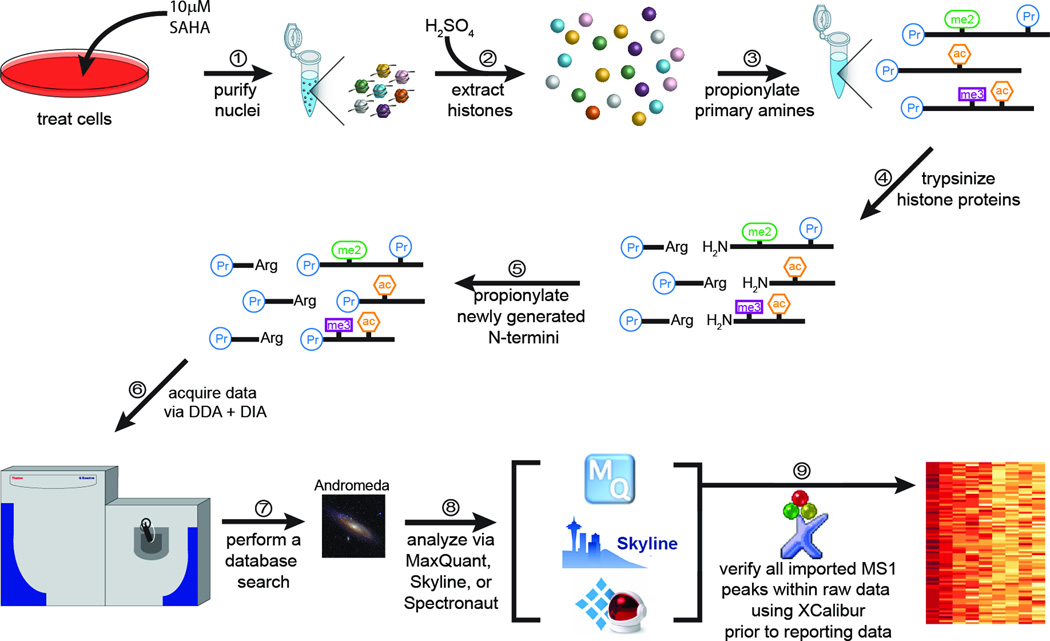
Figure 1. Histone DIA mass spectrometry workflow.
Cells are treated with either 10 µM SAHA or DMSO as a vehicle control for 24 hours. Cells are then harvested and the nuclear fraction is collected (1). Histones are acid extracted from nuclei (2) and are then subjected to propionyl labeling (3). Histone proteins are digested with trypsin, which cleaves C-terminally to arginine, but not labeled or modified lysine (4). Newly generated N-termini are then propionylated (5), and each sample is injected twice. The first injection is acquired using a DDA method, and the second is acquired using a DIA method (6). Peptides are identified via the Andromeda search engine (7), and spectral libraries are generated in Skyline or Spectronaut (8). All histone peptides are verified manually using raw MS1 spectra (9).
Precision and run-to-run consistency of DIA data
To examine whether precision among biological replicates in data obtained via DDA differed from data obtained using DIA, we first plotted log2-transformed fold-change data and calculated correlation matrices among all possible combinations of SAHA-treated and control samples (Figure 2A–C). To allow for accurate comparison across DDA and DIA data sets, only peptides appearing in all three data sets (MaxQuant, Skyline, and Spectronaut) were plotted. Fold changes among DDA data displayed good correlation between replicates (median correlation coefficient = 0.59; Figures 2A, D). DIA data had higher precision among biological replicates as evidenced by less scatter and higher median correlation coefficients in DIA data quantified using MS1 or MS2 (median correlation coefficient = 0.76 and 0.69, respectively; Figures 2B–D). Notably, correlation did not significantly differ among DIA data quantified using only MS1 when compared to the same data quantified using only MS2 fragments (Figure 2D–E).
Figure 2. Comparison of precision across biological replicates in DDA and DIA data.
(A–C) Correlation matrix of all possible combinations of SAHA and DMSO treated samples acquired and quantified using (A) DDA, (B) DIA using MS1 ions for quantitation, (C) DIA using MS2 ions for quantitation. Sample pairings are listed along top and right axes, where S = SAHA, D = DMSO, 1–3 = biological replicate number. Scatter plots of log2-transformed histone PTM fold changes (SAHA/DMSO) are displayed in the bottom left half of the matrix and corresponding Pearson’s correlation coefficients are printed in mirroring boxes in the upper right half of the matrix. Histograms of sample distributions are displayed along the diagonal. (D) Boxplot displays of Pearson’s correlation coefficients for all possible combinations of log2-transformed histone PTM fold change data. Upper and lower bounds of boxes signify the 25th (q1) and 75th (q3) percentiles, respectively, and the bar displays the median (q2 or 50th percentile). Whiskers extend to +/− [q3 + 1.5(q3 − q1)] and any points outside this range are displayed as open circles. (E) Correlation between histone PTM fold changes (log2) measured using MS1 ions vs. MS2 ions from DIA data. Data were fit using least squares linear regression and Pearson’s correlation coefficient was calculated. ** p < 0.01, *** p < 0.001 (Welch’s T-test)
To examine run-to-run consistency between DDA and DIA data, we next plotted log10-transformed normalized areas (Skyline and Spectronaut) or intensities (MaxQuant) of quantified peptides in a heat map (Figure 3). Not only are there more missing values in DDA data relative to DIA data quantified either with Skyline or Spectronaut, but the missing values are stochastic among different injections (Figure 3). There were 71 total missing values in DDA data, while there were only 18 missing values in DIA data processed with Spectronaut and no missing values in DIA data processed with Skyline (Table 1). Notably, the missing values reported in the Spectronaut data set are due to consistent differences in spectrum-to-peptide matches for three peptides between Skyline and Spectronaut rather than stochastic acquisition (Figure 3, Table 1). As an additional measure of variation, mean %CV values across biological replicates are also lower in DIA data relative to DDA data (Table 1). Together, these data suggest that quantitation of histone PTMs using DIA has both higher precision and consistency across runs in comparison to DDA.
Figure 3. Consistency of peptide identification in DDA and DIA data.
Heatmap displaying log10-transformed areas for 62 unmodified, acetylated, and methylated histone H2, H3, and H4 peptides. Green indicates higher abundance and blue indicates lower abundance. Grey boxes indicate a missing value. 1–3 = SAHA-treated biological triplicates, 4–6 = DMSO-treated biological triplicates. *Area under the chromatograph are reported by Skyline and Spectronaut as a measure of peptide abundance, whereas intensities are reported by MaxQuant.
Table 1. Comparison of DDA and DIA.
Instrument acquisition mode, software used for quantification, sample identification, aggregate missing values, and aggregate %CV are listed.
| Acquisition Mode | DDA | DIA | DIA |
|---|---|---|---|
| Software | MaxQuant | Skyline | Spectronaut |
| Missing Values | 71 | 0 | 18 |
| Mean %CV | 63.335 | 42.38 | 49.42 |
Quantitation of isobaric, co-eluting peptides with MS2 data
To determine whether quantitation differed markedly when using MS1 or MS2 data, we plotted mean log2-transformed fold changes from MS1 and MS2 DIA data sets (Figure 2E). Quantitation using either MS1 or MS2 alone yielded highly correlated results (Pearson’s correlation = 0.96). This similarity between ratios obtained from MS1 and MS2 supports that accurate quantitation can be performed using either quantitation level in isolation or in combination. To deconvolute and quantify a number of isobaric, co-eluting histone peptides, we used unique MS2 fragments from the DIA analyses. To illustrate how MS2 fragments can be used to deconvolute such peptides, we consider the H3 peptide containing K18 and K23 (Supplemental Figure 3). The singly acetylated form of this peptide may be acetylated either at K18 or K23, causing a monoisotopic mass shift of 42.01 Da in either case. These two peptide species co-elute and thus, if measurements are based solely on MS1 data, it is impossible to distinguish between these two species (Supplemental Figure 3A). To address this issue, we utilized unique transitions to not only determine which residue is acetylated, but also quantify the relative PTM abundance using the MS2 XICs (Supplemental Figure 3B–C, Supplemental Methods).
Deconvolution and quantification of H4 peptides lacking unique transitions
There are a significant number of isobaric and co-eluting histone H4 peptides that remain impossible to directly quantify by either MS1 or MS2 spectra due to a lack of unique transition ions. To quantitatively assess changes in these peptides, we developed a technique to calculate their relative amounts as a percentage of the whole (Supplemental Methods). Briefly, average extracted areas from MS1 scans of all isobaric, co-eluting members were determined. These peptides were then further resolved and quantified using unique sets of b and y ions. Finally, when no unique b or y ions existed for a particular permutation of singly, doubly, or triply acetylated H4 peptide, we calculated the relative contribution of those peptides to the total area.
To illustrate this methodology, we consider the peptide H4 4–17, which has 4 lysines (K5, K8, K12, and K16) that can be acetylated. Similar to K18ac/K23ac of histone H3, the majority of the peptides in this family with the same number of acetylated lysines (e.g.: 1, 2, or 3 acetylated lysines of 4 total possible residues) co-elute and are indistinguishable by precursor m/z alone. As an example, we present our quantitation method applied to the 4 peptides containing a single acetylated lysine in Figure 4. While H4 K5ac, K8ac, and K12ac co-elute, there is a slight delay in elution time for K16ac. Precursor XICs were used to first obtain relative quantitation of K16ac and the co-eluting group of peptides K5ac, K8ac, and K12ac (Figure 4A). To further differentiate and quantify the K5ac, K8ac, and K12ac peptides, we first used transitions y10–12 and b2–4 to determine what proportion of the total area was contributed by K5ac relative to K8ac and K12ac combined (Figure 4B–C). We next used y6–9 and b5–8 to determine the proportion of the observed area that was contributed by K12ac (Figure 4B–C). There exist no unique y or b ions to describe K8ac. However, since both the total area of K5ac, K8ac, and K12ac peptides combined and the individual proportions contributed by K5ac and K12ac are known, we can calculate the proportion contributed by K8ac (Figure 4C). We applied this methodology to the families of doubly and triply acetylated H4 4–17 peptides and were able to accurately differentiate and quantify each individual PTM using MS2 XICs (Supplemental Methods). In this manner, we were able to use MS2 XICs from DIA data to distinguish and quantify all 14 permutations of acetylated H4 4–17.
Application of DIA methodology reveals robust and site-specific effects of SAHA treatment in human breast cancer cells
Using the methodology described above, we performed an in-depth analysis of the effects of SAHA treatment on human breast cancer MCF7 cells (Supplemental Table 2). Consistent with immunoblot results, there was a robust increase in the total acetylation on H4 (Figure 5A–B). There was little difference in singly acetylated H4 4–17 (Figure 5C); however the doubly, triply, and quadruply acetylated forms of this peptide increased 3.0-, 5.6-, and 43.3-fold, respectively, with SAHA treatment (Figure 5B, D–E, p < 0.001 to < 0.05). There was also a concomitant 4.1-fold decrease in the unmodified form of this peptide (Figure 5B, p < 0.05).
Figure 5. SAHA treatment induces changes in histone H4 acetylation.
(A) Peptide sequence of Histone H4 4–17. Lysine residues are shown in green and numbered below. (B–D) Acetylation of H4 4–17 in control samples (DMSO) and SAHA-treated samples. (B) Gross changes in H4 acetylation. (C) Singly acetylated H4 4–17 peptides. (D) Doubly acetylated H4 4–17 peptides. (E) Triply acetylated H4 4–17 peptides. All peptides are graphed as a % of total. n=3 biological replicates per treatment, * p < 0.05, ** p < 0.01, *** p < 0.001 (Welch’s t-test). Error bars represent standard deviation.
SAHA induced site-specific changes in H4 acetylation (Figure 5C–E). Among singly acetylated peptides, SAHA treatment caused a 1.8-fold increase in H4 K5 acetylation (Figure 5C, p < 0.05). While there are no statistically significant changes at other lysine residues among singly acetylated peptides, it is noteworthy that there is a high level of H4 K16 acetylation, regardless of treatment (Figure 5C). Among doubly acetylated H4 peptides, there was a large decrease in the amount of H4 K5ac+K8ac peptide (17.9-fold, p < 0.01), and a 2.4 to 3.1-fold increase in doubly acetylated peptides containing acetylated H4 K16 (Figure 5D, p < 0.01). Interestingly, the triply acetylated peptide H4 K5ac+K8ac+K16ac decreased significantly (Figure 5E, 9.6-fold, p < 0.001), while the relative amounts of H4 K5ac+K12ac+K16ac and H4 K8ac+K12ac+K16ac increased 4.5- and 2.8-fold (Figure 5E, p < 0.001).
SAHA treatment also induced site-specific changes in acetylation and methylation on histone H3 (Figure 6, Supplemental Table 2). Although both H3 K9un+K14ac and the doubly acetylated peptide H3 K9ac+K14ac increased in abundance with SAHA treatment (Figure 6A; 27.4-, and 3.0-fold, respectively; p < 0.01 to < 0.05), there was a concomitant decrease in singly acetylated H3 K9ac+K14un (Figure 6A, 1.9-fold, p < 0.05). Doubly acetylated H3 K18ac+K23ac increased 15.9-fold and the singly acetylated peptides H3 K18ac+K23un and H3 K18un+K23ac increased 4.4- and 2.6-fold, respectively (Figure 6B, p < 0.001 to < 0.01). There were no significant changes in acetylation or methylation of K27 and K36 on histone H3.2, but K27ac on histone variant H3.3 increased 4.9-fold (Figure 6D, p < 0.05). SAHA did not induce any significant changes in H3 K79 methylation (Figure 6E). However, there was a 1.84-fold decrease in H3 K9me1 (Figure 6A, p < 0.01) and a 1.9-fold decrease in H3 K18me1 (Figure 5B, p < 0.05). Finally, H3 K18me1 accounts for less than 0.01% of the total area observed for this peptide family in both SAHA and DMSO treated samples, yet we were able to detect a significant change in abundance using DIA.
Figure 6. SAHA induces changes in acetylation and methylation of histone H3 and H2A.Z.
Histone acetylation and methylation of H3 in SAHA- and DMSO-treated samples. (A) H3 K9 and K14, (B) H3 K18 and K23, (C) H3.2 K27 and K36, (D) H3.3 K27 and K36, (E) H3 K79, and (F) H2A.Z K4, K7, and K11. All peptides are graphed as a % of total. n = 3 biological replicates per treatment, * p < 0.05, ** p < 0.01, *** p < 0.001 (Welch’s t-test). Error bars represent standard deviation.
We observed a number of H2A peptides, including members of canonical H2A and histone variants H2A.X, H2A.Y (macro H2A), and H2A.Z (Supplemental Tables 2–3). Similar to observations on histone H4, SAHA induced robust increases in acetylation of Histone H2A.Z (Figure 6F). Singly, doubly, and triply acetylated forms of H2A.Z 1–19 increased 2.7-, 13.0-, and 27.2-fold, respectively (Figure 6F; p < 0.01 to < 0.001). These data suggest that SAHA robustly affects histone acetylation on histones H2A, H3, and H4 in addition to affecting histone H3 methylation.
Discussion
In the current study, we have developed a data-independent acquisition (DIA) workflow that allows for in-depth and reproducible analysis of histone PTMs. This label-free DIA method offers maximal flexibility and robustness by enabling quantitation in both the MS1 and MS2 channels. Garcia and colleagues recently published a similar method supporting the utility of SWATH in histone PTM quantitation, wherein MS2 signal was used to quantify 41 acetylated, methylated, and phosphorylated histone H3 peptides.21 In our workflow, we have used a combination of MS1 and MS2 spectra to deconvolute and quantify 62 unique acetylated and methylated histone peptides from H2A, H3, and H4 (Supplemental Tables 2–3, Supplemental Methods). The post-acquisition flexibility of our DIA workflow is essential for the quantification of the large number of isobaric, co-eluting peptides that exist in histones. This unique workflow has yielded valuable mechanistic insight into the effects of SAHA on histone modifications.
DIA enables increased depth of coverage and flexibility for histone PTM quantification
Several advantages of DIA have been demonstrated over both DDA and targeted methodologies. Unlike DDA, DIA continuously samples all MS1 and MS2 signals across the entire HPLC gradient without bias. This unique aspect of DIA yields both reproducibility and the flexibility to use MS1, MS2, or a combination of the two channels for accurate quantitation. In addition, targeted acquisition requires scheduling of analytes and optimization of transition selection whereas DIA allows for the post hoc selection of transitions for quantitation of any peptide in either MS1 or MS2 channels in isolation or in combination. DIA data can also be re-mined at any point for additional histone modifications, since all ions are indiscriminately fragmented and acquired in DIA
Inferred quantification of peptides lacking unique transitions
Due to the highly modified nature of histone peptides, many of which are isobaric and co-elute, direct differentiation and quantification of histone peptides using MS1 scans alone is not possible. Even with the use of MS2 transitions, there remain a number of peptides which lack unique transitions, thus precluding their differentiation and quantification. To solve this issue, we developed a method using simple algebra to calculate the respective contributions of these peptides to the overall abundance of a given peptide family (Figure 4, Supplemental Methods). Combined use of MS1 and MS2 scans from DIA data for histone PTM quantitation offered detailed insight into the effects of SAHA on H4 acetylation (Figure 5). Since unique transitions are used for quantitation, differences in ionization efficiency could affect the accuracy of relative quantitation. However, the average effect of differences in histone peptide ionization has been shown to be small, not predicted by the chemical or physical properties, and is limited to low-abundant peptides.29
Increased precision and consistency with DIA
DIA has been demonstrated to improve sensitivity, precision, coverage, and repeatability in a number of studies.20,21,30,31 Here, we compared precision across biological replicates and demonstrated that histone PTM quantitation with DIA data using either the MS1 or MS2 channel had superior precision relative to DDA data from the same set of samples (Figure 2A–D). We also demonstrated uniform identification of peptides across all sample injections with DIA data, which contrasts significantly with the stochastic nature of DDA data (Figure 3, Table 1). Finally, we were able to detect significant changes in histone PTMs that account for less than 0.01% of the total peptide family area, which demonstrates high sensitivity of DIA for histone PTM quantification. In agreement with our results, Sidoli et al. have recently demonstrated similar improvements in performance using SWATH acquisition, including high precision, sensitivity, and repeatability.21 There was also strong correlation between the fold change values measured from DIA derived MS1 and MS2 quantitation (Figure 2E). Thus, we conclude that both MS1 and MS2 signals yield accurate quantitation and can be used together or separately to obtain the greatest depth of analysis.
Potential disadvantages of DIA for histone PTM quantification
Although DIA offers many advantages, there are also potential disadvantages. In DIA methods, all precursor ions within a given retention time and m/z window are subjected to fragmentation, resulting in the precursor m/z being uncoupled from the MS2 fragment m/z. This unique characteristic of DIA precludes the use of traditional database searching algorithms for peptide identification and thus requires the use of spectral libraries for spectrum-to-peptide matching. Typically, the generation of these libraries requires parallel analysis of the same samples via DDA; these additional analyses consume more sample and require more instrument time than typical DDA workflows.19,32 However, while this manuscript was being prepared, Nesvizhskii and colleagues released open-source software that uses chromatographic features of precursor and fragment ions within DIA data to generate pseudo-tandem MS spectra.33 These spectra can then be searched with traditional database software and other data pre-processing applications, thus eliminating the necessity for an additional set of DDA runs.
SAHA treatment of MCF7 cells induces changes in acetylation and methylation
SAHA chelates zinc at the active site of HDACs and has been shown to induce histone acetylation, cell cycle arrest, and differentiation in human breast cancer cells.22,34 The effects of SAHA in neuroblastoma and non-small cell lung cancer (NSCLC) cell lines have also been demonstrated and generally agree with the results presented here. Xu and colleagues quantified 46 single histone acetylation or butyrylation sites in SH-SY5Y neuroblastoma cells.35 Wu and colleagues used affinity enrichment to quantify 29 histone acetylation sites in A549 NSCLC cells.36 Our results expand upon existent SAHA data, providing not only increased coverage, but also combinatorial and site-specific changes in acetylation and methylation of histones H2A, H3, and H4 in a breast cancer cell line (Figures 5–6). Interestingly, Histone H4 K16 acetylation has previously been associated with highly proliferative cells and increased tumor growth, and was also detected in 300 human breast, lung, and prostate tumors.37 Our data, which demonstrates high levels of H4 K16ac in human breast cancer cells is consistent with these findings (Figure 5). This high level of H4 K16 acetylation is maintained by the histone acetyltransferase MOF, and K16 is a known target of Sir2, a class III HDAC that is not a target of SAHA.37,38 This is consistent with our data, wherein H4 K16 acetylation is not significantly altered with SAHA treatment.
Conclusions
In summary, we have developed a global histone PTM quantification workflow using DIA. Our in-depth analysis revealed changes in histone acetylation and methylation, and suggests site-specificity of SAHA effects that extend beyond direct HDAC inhibition. We conclude that the increased capacity of this methodology for flexible, precise, and highly consistent quantification of histone PTMs expands the scope of mass spectrometric interrogation of chromatin states.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the EpiMotif Group for support in establishing the workflow. The authors acknowledge the support of NIH grant GM059785-15/P250VA. K.A. Krautkramer is supported by the Molecular and Applied Nutrition Training Program (NIH grant 5T32DK007665-22) and the University of Wisconsin Medical Scientist Training Program (NIH grant 5T32GM008692-15).
Lukas Reiter serves as Head of Research and Development at Biognosys AG.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Supplemental Figures 1–3: Supporting figures referenced within manuscript text
Supplemental Methods: Methods used to quantify histone PTMs from DIA data using MS1 and MS2 XICs
Supplemental Table 1: Inclusion list used for DDA injections
Suppemental Table 2: Histone PTM quantitation using DIA data, normalized to peptide family
Supplemental Table 3: Histone PTM quantitation using DDA and DIA data, normalized to total signal
Supplemental Table 4: Quantitation method tutorial, to accompany supplemental methods
This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187–197. doi: 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- 2.Lander ES, Consortium IHGS, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 3.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 4.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 5.Britton L-MP, Gonzales-Cope M, Zee BM, Garcia BA. Breaking the histone code with quantitative mass spectrometry. Expert Rev Proteomics. 2011;8:631–643. doi: 10.1586/epr.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs SM, Krajewski K, Baker RW, Miller VL, Strahl BD. Influence of Combinatorial Histone Modifications on Antibody and Effector Protein Recognition. Curr. Biol. 2011;21:53–58. doi: 10.1016/j.cub.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori T, Taft JM, Swist KM, Luo H, Witt H, Slattery M, Koide A, Ruthenburg AJ, Krajewski K, Strahl BD, et al. Recombinant antibodies to histone post-translational modifications. Nat Meth. 2013;10:992–995. doi: 10.1038/nmeth.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peach SE, Rudomin EL, Udeshi ND, Carr SA, Jaffe JD. Quantitative assessment of chromatin immunoprecipitation grade antibodies directed against histone modifications reveals patterns of co-occurring marks on histone protein molecules. Mol. Cell Proteomics. 2012;11:128–137. doi: 10.1074/mcp.M111.015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikori S, Hattori T, Fuchs SM, Yasui N, Wojcik J, Koide A, Strahl BD, Koide S. Broad Ranges of Affinity and Specificity of Anti-Histone Antibodies Revealed by a Quantitative Peptide Immunoprecipitation Assay. Journal of Molecular Biology. 2012;424:391–399. doi: 10.1016/j.jmb.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moradian A, Kalli A, Sweredoski MJ, Hess S. The top-down, middle-down, and bottom-up mass spectrometry approaches for characterization of histone variants and their post-translational modifications. Proteomics. 2014;14:489–497. doi: 10.1002/pmic.201300256. [DOI] [PubMed] [Google Scholar]
- 11.Nicklay JJ, Shechter D, Chitta RK, Garcia BA, Shabanowitz J, Allis CD, Hunt DF. Analysis of histones in Xenopus laevis. II. mass spectrometry reveals an index of cell type-specific modifications on H3 and H4. Journal of Biological Chemistry. 2009;284:1075–1085. doi: 10.1074/jbc.M807274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesavento JJ, Bullock CR, LeDuc RD, Mizzen CA, Kelleher NL. Combinatorial modification of human histone H4 quantitated by two-dimensional liquid chromatography coupled with top down mass spectrometry. Journal of Biological Chemistry. 2008;283:14927–14937. doi: 10.1074/jbc.M709796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ntai I, Kim K, Fellers RT, Skinner OS, Smith AD, Early BP, Savaryn JP, LeDuc RD, Thomas PM, Kelleher NL. Applying label-free quantitation to top down proteomics. Anal. Chem. 2014;86:4961–4968. doi: 10.1021/ac500395k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell Proteomics. 2012;11:1475–1488. doi: 10.1074/mcp.O112.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Law KP, Lim YP. Recent advances in mass spectrometry: data independent analysis and hyper reaction monitoring. Expert Rev Proteomics. 2013;10:551–566. doi: 10.1586/14789450.2013.858022. [DOI] [PubMed] [Google Scholar]
- 16.Picotti P, Rinner O, Stallmach R, Dautel F, Farrah T, Domon B, Wenschuh H, Aebersold R. High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nat Meth. 2010;7:43–46. doi: 10.1038/nmeth.1408. [DOI] [PubMed] [Google Scholar]
- 17.Venable JD, Dong M-Q, Wohlschlegel J, Dillin A, Yates JR. Automated approach for quantitative analysis of complex peptide mixtures from tandem mass spectra. Nat Meth. 2004;1:39–45. doi: 10.1038/nmeth705. [DOI] [PubMed] [Google Scholar]
- 18.Bern M, Finney G, Hoopmann MR, Merrihew G, Toth MJ, MacCoss MJ. Deconvolution of mixture spectra from ion-trap data-independent-acquisition tandem mass spectrometry. Anal. Chem. 2010;82:833–841. doi: 10.1021/ac901801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillet LC, Navarro P, Tate S, Röst H, Selevsek N, Reiter L, Bonner R, Aebersold R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell Proteomics. 2012;11 doi: 10.1074/mcp.O111.016717. O111.016717–O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruderer R, Bernhardt OM, Gandhi T, Miladinovic SM, Cheng L-Y, Messner S, Ehrenberger T, Zanotelli V, Butscheid Y, Escher C, et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen treated 3D liver microtissues. Mol. Cell Proteomics. 2015 doi: 10.1074/mcp.M114.044305. mcp.M114.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidoli S, Lin S, Xiong L, Bhanu NV, Karch KR, Johansen E, Hunter C, Mollah S, Garcia BA. SWATH Analysis for Characterization and Quantification of Histone Post-translational Modifications. Mol. Cell Proteomics. 2015 doi: 10.1074/mcp.O114.046102. mcp.O114.046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001;61:8492–8497. [PubMed] [Google Scholar]
- 23.Lin S, Garcia BA. Examining histone posttranslational modification patterns by high-resolution mass spectrometry. Meth. Enzymol. 2012;512:3–28. doi: 10.1016/B978-0-12-391940-3.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 25.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernhardt OM, et al. Spectronaut: a fast and efficient algorithm for MRMlike processing of data independent acquisition (SWATH-MS) data.. Proceedings of 60th American Society for Mass Spectometry Conference American Society for Mass Spectrometry; Vancouver. 2012.2012. [Google Scholar]
- 27.Escher C, Reiter L, MacLean B, Ossola R, Herzog F, Chilton J, MacCoss MJ, Rinner O. Using iRT, a normalized retention time for more targeted measurement of peptides. Proteomics. 2012;12:1111–1121. doi: 10.1002/pmic.201100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiter L, Rinner O, Picotti P, Hüttenhain R, Beck M, Brusniak M-Y, Hengartner MO, Aebersold R. mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nat Meth. 2011;8:430–435. doi: 10.1038/nmeth.1584. [DOI] [PubMed] [Google Scholar]
- 29.Lin S, Wein S, Gonzales-Cope M, Otte GL, Yuan Z-F, Afjehi-Sadat L, Maile T, Berger SL, Rush J, Lill JR, et al. Stable-isotope-labeled histone peptide library for histone post-translational modification and variant quantification by mass spectrometry. Mol. Cell Proteomics. 2014;13:2450–2466. doi: 10.1074/mcp.O113.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Distler U, Kuharev J, Navarro P, Levin Y, Schild H, Tenzer S. Drift time-specific collision energies enable deep-coverage data-independent acquisition proteomics. Nat Chem Biol. 2014;11:167–170. doi: 10.1038/nmeth.2767. [DOI] [PubMed] [Google Scholar]
- 31.Keller A, Bader SL, Shteynberg D, Hood L, Moritz RL. Automated Validation of Results and Removal of Fragment Ion Interferences in Targeted Analysis of Data Independent Acquisition MS using SWATHProphet. Mol. Cell Proteomics. 2015 doi: 10.1074/mcp.O114.044917. mcp.O114.044917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudomin EL, Carr SA, Jaffe JD. Directed sample interrogation utilizing an accurate mass exclusion-based data-dependent acquisition strategy (AMEx) J. Proteome Res. 2009;8:3154–3160. doi: 10.1021/pr801017a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsou C-C, Avtonomov D, Larsen B, Tucholska M, Choi H, Gingras A-C, Nesvizhskii AI. DIA-Umpire: comprehensive computational framework for data-independent acquisition proteomics. Nat Chem Biol. 2015;12:258–264. doi: 10.1038/nmeth.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 35.Xu G, Wang J, Wu Z, Qian L, Dai L, Wan X, Tan M, Zhao Y, Wu Y. SAHA Regulates Histone Acetylation, Butyrylation, and Protein Expression in Neuroblastoma. J. Proteome Res. 2014;13:4211–4219. doi: 10.1021/pr500497e. [DOI] [PubMed] [Google Scholar]
- 36.Wu Q, Xu W, Cao L, Li X, He T, Wu Z, Li W. SAHA treatment reveals the link between histone lysine acetylation and proteome in nonsmall cell lung cancer A549 Cells. J. Proteome Res. 2013;12:4064–4073. doi: 10.1021/pr4004079. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Guerin-Peyrou TG, Sharma GG, Park C, Agarwal M, Ganju RK, Pandita S, Choi K, Sukumar S, Pandita RK, et al. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Molecular and Cellular Biology. 2008;28:397–409. doi: 10.1128/MCB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.