Abstract
Tumor-associated carbohydrate antigens (TACAs) are key components of cancer vaccines. A variety of “native” TACA-based vaccines have shown immunogenicity and protection in pre-clinical animal studies, however the weak immunogenicity, in vivo instability and poor bioavailability, have discouraged their further evaluations in clinical studies. We report on a new “improved” vaccine prototype 8 composed by four clustered Tn-antigen mimetics and a T-helper cell peptide epitope that are conjugated to a cyclopeptide carrier. Immunization of mice with vaccine 8 (i) was safe, (ii) induced a strong and long-lasting Tn-specific IgM/IgG antibodies able to recognize “native” carbohydrate antigens; (iii) produced high titres of IgG1, IgG2a and IgG3 antibodies; (iv) raised a significant antibody-dependent regression of tumors and protection. All together, these findings pave the way for a clinical development of vaccine 8 as a safe and effective therapeutic vaccine against Tn-expressing cancers.
Keywords: Tn-antigen mimetic, synthetic vaccine, cancer, therapeutic vaccine, immunity
Cancer cells undergo significant modifications in carbohydrate expression. These alterations, mainly aberrant glycosylation, known as tumor associated carbohydrate antigens (TACAs),[1,2] can be used as diagnostic tumoral markers or therapeutic targets.[3] The most common TACA is the α-Tn antigen, a GalNAc residue which is α-linked to a serine or threonine residue (α-GalNAc-O-Ser/Thr);[4] α-Tn is detected in up to 90% of human breast, ovary and colon carcinomas.[5] The induction of IgG antibodies (Abs) against TACAs is known as a difficult task[3] and it is not surprising because several TACAs are self-antigens and therefore well tolerated by the immune system.[6-10] The shedding of TACAs by growing tumors exacerbates this tolerance.[6,9,11] Conversely, under appropriate conditions, α-Tn can induce tumor-specific IgG in mice and in non-human primates.[12] In addition, the rates of Tn expression is statistically higher in tumor with respect to healthy tissue.[13,14] These observations have raised the hope that TACAs, and Tn in particular, might be used as specific targets for humoral-mediated cancer vaccines. As known, TACAs have to be covalently linked to a T-helper epitope to induce a strong and long-lasting production of high affinity IgG Abs.[15] The cross reaction of these IgG Abs with tumors-expressing TACAs, with or without the cooperation of other immune cells, ultimately leads to a regression of cancer.[3, 16-20] A wide variety of such immunogenic constructs have been synthesized showing promising immunogenicity in pre-clinical animal studies. However till now, none of them entered in clinical trials successfully, as indeed observed with the promising Theratope vaccine for which neither an overall benefit nor an increasing of survival were observed for patients in phase III studies.[21] One reason of these failures is the sensitivity of TACAs to endogenous glycosidases which reduces their in vivo bioavailability.[22-24] As a consequence, structural modifications of native TACAs including C- and S-glycosides,[25-27] deoxyfluoroglycosides[28-30], truncated antigens[31] or thioether-bridged mimetics[32] have been recently proposed to provide structures more stable than those of the parent antigens, without interfering with their B-cell immunogenicity.[33,34]
In this study, we hypothesized that TACA-based vaccines displaying mimetics instead of native Tn antigens could be more resistant to enzymatic degradation. We thus expected that this resistance might translate into an increased in vivo bioavailability and, hence, into a stronger and long-lasting immunogenicity and protective efficacy. To test this hypothesis, we focused on previous vaccine prototypes[35] based on a cyclopeptide carrier (named Regioselectively Addressable Functionalized Template “RAFT”)[35] that was decorated with clusters of GalNAc, the saccaridic epitope of the Tn antigen, and with either T-helper[12] or chimeric T-helper/T-cell peptide epitopes.[16,17,36] Although these constructions were able to promote tumour regression and improved survival rate in mice,[16,17,36] its sensitivity to enzymatic degradation may compromise clinical studies. We thus prepared a new prototype of fully synthetic antitumor vaccine, 8, based on the same model (Figure 1), but containing four residues of a Tn-antigen mimetic (MIM_Tn). This bioactive epitope is a 2-deoxy-2-thio-α-O-galactoside which retains the 4C1 chair conformation of the native antigen.[37] As depicted in Figure 1, MIM_Tn presents a carboxylic residue which can be used for the conjugation, by an amidic linkage, to the four Lys of the RAFT. This cyclopeptide carrier also displays an immunostimulant peptide epitope (OvaPADRE) linked to the Lys residue on the bottom side. The safety, immunogenicity and protective efficacy of the resulting clustered Tn-antigen mimetics-based construct 8 were assessed in mice.
Figure 1.
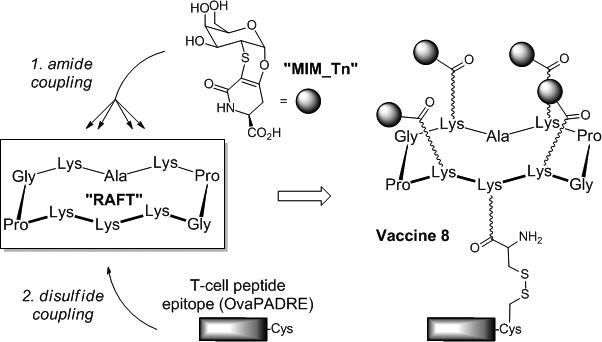
General strategy for the construction of the vaccine 8 displaying the α-Tn antigen mimetic MIM_Tn.
The synthesis of 8 started with the conjugation between 1 and the acetylated mimetic 2 in the presence of PyBOP in DMF (Scheme 1). The conjugate 3 was afforded in 62% yield after precipitation in diethyl ether. Acetyl and Boc groups were removed by treatment with trifluoroacetic acid and sodium methoxide, respectively, providing 4 in 93% yield. An activated cysteine with S-3-nitro-2-pyridinesulfenyl (NPys) group was next coupled to the free Lys of 4 and the resulting compound 6 was reacted with the peptide epitope 7. The complete conversion of 6 was observed by HPLC in 1 h, nonetheless the construct 8 was obtained in a moderate yield (22%) after HPLC purification.
Scheme 1.

Synthetic procedure for the preparation of compound 8. (a) 2, PyBOP, DIPEA, DMF, rt, 1 h, 62%; (b) i: TFA/CH2Cl2 (1/1, v/v), rt, 30 min; ii: MeONa, MeOH, rt, 4 h, 93% for two steps; (c) BocCys(NPys)CO2Su, DIPEA, DMF, rt, 1 h; (d) TFA/CH2Cl2 (1/1, v/v), rt, 30 min, 79% for two steps; (e) 7, iPrOH/AcONa 25mM pH 5 (1/1, v/v, 1 mM), rt, 2 h, 22%.
The safety and immunogenicity in vivo of 8 were firstly evaluated. B10.D1 mice were immunized subcutaneously with 8 in CpG1826 adjuvant, three times at 14-days intervals (Group 1, 10 mice/group). To control the response specificity and evaluate the effect of non-specific immune responses induced by CpG1826, a second group of mice (Group 2) was treated with CpG1826 alone. Ten days after the final immunization, post-immune sera were collected and the titres of IgG/IgM Abs were determined using an enzyme-linked immunosorbent assay (ELISA assay) (see SI, Figure 2SA). It is noteworthy that no adverse effects (e.g. local inflammation, systemic reactions, weight loss or death of treated mice) was observed during and after the course of immunization thus confirming the safety of the construct 8 formulation. Ten days after the third immunization, significant levels of mucin-specific IgG/IgM Abs were induced in immunized mice (Group 1) unlike in Group 2 and Group 3 (mice injected with PBS). The longevity of the IgG/IgM Abs was determined indicating that a significant amount of IgG/IgM Abs was still present in the serum 240 days after the last immunization(see SI, Figure 2SB). More interestingly, a high amount of IgG1, IgG2 and IgG3 Abs subclasses was observed, which suggests that a broad and balanced IgG immune response has been elicited (see SI, Figure 3S). In addition the ratio of IgG2a/IgG1 indicated a higher induction of type 2 than type 1-T helper response.
Flow cytometry was next used to analyse the binding of the immune serum Abs to mouse (NT2 and TA3HA) and human (MCF7) cancer cell lines which express native TACAs. When NT2, TA3HA and MCF7 cells were treated with sera from immunized mice, we observed a significant enhancement of the fluorescence intensity whereas sera from the control groups did not exhibit any interaction (see SI, Figure 4S). These results demonstrate that Abs generated by the Tn-mimetic based vaccine 8 recognize tumor cells expressing the native antigen at their surface, which clearly confirms the tumoral specificity of the Ab response.
The immunotherapeutic efficacy of 8 was also determined by assessing tumor growth and mice survival rate. To develop tumor, female B10.D1 mice were implanted subcutaneously with NT2 cells then treated with: (i) 8 in CpG1826 adjuvant (Group 1, 10 mice/group); (ii) CpG1826 adjuvant alone (Group 2); (iii) with PBS alone (mock, Group 3). Tumor volume and mice survival were recorded up to 60 days after the final immunization. As shown in Figure 2A, the tumour diameter was significantly reduced in mice from Group 1 compared to mice from Group 2 (p< 0.005) and Group 3 (p< 0.002).
Figure 2.

Immunotherapeutic efficacy of 8. A Tumor progression; B Survival (see SI for details).
The strong immunotherapeutic effect of 8 was also evident from Figure 2B. Of 10 mice vaccinated with 8, 7 were alive after 8 weeks after tumor inoculation, whereas 1 and 0 of the 10 survived in Groups 2 and 3, respectively. To verify the involvement of B-cells in the observed protection, in vivo depletion of B, CD4+ or CD8+ T-cells was performed in immunized mice using specific mAbs. Interestingly, only the depletion of B cells significantly abrogated the protection induced by 8 against tumour progression (Figure 3A) and death (Figure 3B), suggesting that protection is mainly due to B cells.
Figure 3.

Effect of in vivo depletion of B-cells, CD4+ and CD8+ T cells in the tumor progression (A) and survival (B) induced by 8. Vaccinated mice were i.p. injected with six doses of 100 μL of PBS containing mAb GK1.5 (anti-CD4+), a mAb 2.43 (anti-CD8+), mAb CD20-1 (anti–B cell) or hamster immunoglobulin treated control on day − 7, −1, 0, 2, and 5 post-tumor transplant. Depletion of B and T cells was assessed by flow cytometry analysis of splenocytes at the end of the experiment (days 12-13 post-inoculation).
Concluding, though the discovery of a potent carbohydrate-based cancer vaccine remains a chimerical and long-standing goal, “tailored” synthetic immunogenic constructs offer safety, reliability and cost advantages over traditional methods (e.g. live vectors, tumor cells-APC fusion, genetic immunization).[3,20,36]
In this communication, we reported on the first use of a simple and structurally stable mimetic of the mucin antigen α-Tn for the synthesis of an unprecedented fully synthetic vaccine. We demonstrated that this vaccine prototype elicits a robust and long-lasting IgG/IgM Abs response and induces a protection in mice through a B cell-mediated mechanism. Interestingly, these Abs were shown to bind to MCF-7 human breast cancer cell lines expressing the native carbohydrate antigens on their surface, suggesting that biologically relevant Ab specificities were induced.[41] Though the in vivo mechanism by which 8 raises a carbohydrate-specific response is still unclear, our findings represent a step forward the development of “armed” synthetic therapeutic vaccines against cancers. This fully synthetic approach indeed addresses the problems associated to the use of large carrier proteins to deliver weakly antigenic carbohydrate molecules and override the necessity of “booster” injections to convert the initial, transient IgM response into a strong, durable IgG one. This new vaccine would, not only break the self-tolerance (a central problem in cancer immunotherapy), but also boost carbohydrate B-cell response which are both essential to enhance the protective adaptive immunity against cancers.
Supplementary Material
Acknowledgements
This work was supported by Labex Arcane (ANR-11-LABX-003), the ANR-12-JS07-0001-01 “VacSyn”; by Ente Cassa di Risparmio di Firenze and AIRC, IG Grant 2012 to CN, the COST action CM1102 to OR and CN, by the PHC Galileo Program 2013 to OR and CN, and by Public Health Service Research NIH grants EY14900 and EY019896 to LBM.
References
- 1.Hakomori S. Adv. Exp. Med. Biol. 2001;491:369. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 2.Syrigos KN, Karayiannakis AJ, Zbar A. Anticancer Res. 1999;19:5239. [PubMed] [Google Scholar]
- 3.Chentoufi AA, Nesburn AB, BenMohamed L. Arch. Immunol. Ther. Exp. 2009;57:409. doi: 10.1007/s00005-009-0049-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L, Virol J. 2005;79:15289. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer GF. J. Mol. Med. 1997;75:594. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 6.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Nat. Chem. Biol. 2007;10:663. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarp MA, Clausen H. Biochim. Biophys. Acta. 2008;1780:546. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Sørensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, Burchell J, Clausen H. Glycobiology. 2006;16:96. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, Spitaleri G, Pietri E, Rescigno M, de Braud F, Cardillo A, Munzone E, Rocca A, Bonizzi G, Brichard V, Orlando L, Goldhirsch A. Ann. Oncol. 2006;17:750. doi: 10.1093/annonc/mdj083. [DOI] [PubMed] [Google Scholar]
- 10.Tarp MA, Sørensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, Clausen H. Glycobiology. 2007;17:197. doi: 10.1093/glycob/cwl061. [DOI] [PubMed] [Google Scholar]
- 11.Slovin SF, Keding SJ, Ragupathi G. Immunol. Cell. Biol. 2005;83:418. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 12.Grigalevicius S, Chierici S, Renaudet O, Lo-Man R, Deriaud E, Leclerc C, Dumy P. Bioconjug. Chem. 2005;16:1149. doi: 10.1021/bc050010v. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez-Martin C, Cuevas E, Gil-Martin E, Fernandez-Briera A. Oncology. 2004;67:159–65. doi: 10.1159/000081003. [DOI] [PubMed] [Google Scholar]
- 14.Manimala JC, Li Z, Jain A, VedBrat S, Gildersleeve JC. ChemBioChem. 2005;6:2229–41. doi: 10.1002/cbic.200500165. [DOI] [PubMed] [Google Scholar]
- 15.Berti F, Adamo R. ACS Chem. Biol. 2013;8:1653. doi: 10.1021/cb400423g. [DOI] [PubMed] [Google Scholar]
- 16.Renaudet O, Dasgupta G, Bettahi I, Shi A, Nesburn AB, Dumy P, BenMohamed L. PLoS One. 2010;5:e11216. doi: 10.1371/journal.pone.0011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bettahi I, Dasgupta G, Renaudet O, Chentoufi AA, Zhang X, Carpenter D, Yoon S, Dumy P, BenMohamed L. Cancer Immunol. Immunother. 2009;58:187. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Eur. J. Immunol. 2004;34:3102. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 19.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingstone PO, Bay S, Leclerc C. Cancer Res. 2004;64:4987. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 20.Vichier-Guerre S, Lo-Man R, BenMohamed L, Deriaud E, Kovats S, Leclerc C, Bay S. J. Pept. Res. 2003;62:117. doi: 10.1034/j.1399-3011.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 21.Miles D, Roché H, Martin M, Perren TJ, Cameron DA, Glaspy J, Dodwell D, Parker J, Mayordomo J, Tres A, Murray JL, Ibrahim NK, the Theratope® Study Group Oncologist. 2011;16:1092. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohyama C. Int. J. Clin. Oncol. 2008;13:308. doi: 10.1007/s10147-008-0809-8. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Jiao L, Guo Z, Li X, Ba C, Zhang J. Carbohydr. Res. 2008;343:3015. doi: 10.1016/j.carres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Paulsen H, Brockhausen I. Glycoconj. J. 2001;18:867. doi: 10.1023/a:1022244324787. [DOI] [PubMed] [Google Scholar]
- 25.Kuberan B, Sikkander SA, Tomiyama H, Linhardt RJ. Angew. Chem. Int. Ed. 2003;42:2073–5. doi: 10.1002/anie.200351099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bundle DR, Rich JR, Jacques S, Yu HN, Nitz M, Ling CC. Angew. Chem. Int. Ed. 2005;44:7725. doi: 10.1002/anie.200502179. [DOI] [PubMed] [Google Scholar]
- 27.Awad L, Madani R, Gillig A, Kolympadi M, Philgren M, Muhs A, Gerard C, Vogel P. Chem. Eur. J. 2012;18:8578. doi: 10.1002/chem.201200364. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann-Roder A, Kaiser A, Wagner S, Gaidzik N, Kowalczyk D, Westerlind U, Gerlitzki B, Schmitt E, Kunz H. Angew. Chem. Int. Ed. 2010;49:8498. doi: 10.1002/anie.201003810. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, Zheng XJ, Huo CX, Wang Y, Zhang Y, Ye XS. ACS Chem. Biol. 2011;6:252. doi: 10.1021/cb100287q. [DOI] [PubMed] [Google Scholar]
- 30.Oberbillig T, Mersch C, Wagner S, Hoffmann-Roder A. Chem Commun. 2012;48:1487. doi: 10.1039/c1cc15139h. [DOI] [PubMed] [Google Scholar]
- 31.Guttormsen HK, Paoletti LC, Mansfield KG, Jachymek W, Jennings HJ, Kasper DL. Proc. Natl. Acad. Sci. USA. 2008;105:5903. doi: 10.1073/pnas.0710799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.a Arcangeli A, Toma L, Contiero L, Crociani O, Legnani L, Lunghi C, Nesti E, Moneti G, Richichi B, Nativi C. Bioconjugate Chem. 2010;21:1432. doi: 10.1021/bc900557v. [DOI] [PubMed] [Google Scholar]; b Toma L, Di Cola E, Ienco A, Legnani L, Lunghi C, Moneti G, Richichi B, Ristori S, Dell'Atti D, Nativi C. ChemBioChem. 2007;8:1646. doi: 10.1002/cbic.200700208. [DOI] [PubMed] [Google Scholar]
- 33.Lazoura E, Apostolopoulos V. Curr . Med. Chem. 2005;12:1481. doi: 10.2174/0929867054039017. [DOI] [PubMed] [Google Scholar]
- 34.Lazoura E, Apostolopoulos V. Curr . Med. Chem. 2005;12:629. doi: 10.2174/0929867053202188. [DOI] [PubMed] [Google Scholar]
- 35.Galan MC, Dumy P, Renaudet O. Chem. Soc. Rev. 2013;42:4599. doi: 10.1039/c2cs35413f. [DOI] [PubMed] [Google Scholar]
- 36.Renaudet O, BenMohamed L, Dasgupta G, Bettahi I, Dumy P. ChemMedChem. 2008;3:737. doi: 10.1002/cmdc.200700315. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Barbero J, Dragoni E, Venturi C, Nannucci F, Arda A, Fontanella M, Andre S, Canada FJ, Gabius H-J, Nativi C. Chem. Eur. J. 2009;15:10423. doi: 10.1002/chem.200901077. [DOI] [PubMed] [Google Scholar]
- 38.Slovin SF, Keding SJ, Ragupathi G. Immunol. Cell. Biol. 2005;83:418. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 39.Franco A. Anticancer Agents Med. Chem. 2008;8:86. doi: 10.2174/187152008783330888. [DOI] [PubMed] [Google Scholar]
- 40.Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Expert Rev Vaccines. 2005;4:677. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- 41.Kudryashov V, Glunz PW, Williams LJ, Hintermann S, Danishefsky SJ, Lloyd KO. Proc. Natl. Acad. Sci. USA. 2001;98:3264. doi: 10.1073/pnas.051623598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


