Abstract
The aim of this study was to compare glial-derived neurotrophic factor (GDNF) treatment with brain-derived neurotrophic factor (BDNF) treatment of retinal transplants on restoration of visual responses in the superior colliculus (SC) of the S334ter line 3 rat model of rapid retinal degeneration (RD). RD rats (age 4–6 weeks) received subretinal transplants of intact sheets of fetal retina expressing the marker human placental alkaline phosphatase (hPAP). Experimental groups included: (1) untreated retinal sheet transplants, (2) GDNF-treated transplants, (3) BDNF-treated transplants, (4) none surgical, age-matched RD rats, (5) sham surgery RD controls, (6) progenitor cortex transplant RD controls, and (7) normal pigmented rat controls. At 2–8 months after transplantation, multi-unit visual responses were recorded from the SC using a 40 ms full-field stimulus (−5.9 to +1 log cd/m2) after overnight dark-adaptation. Responses were analyzed for light thresholds, spike counts, response latencies, and location within the SC. Transplants were grouped into laminated or rosetted (more disorganized) transplants based on histological analysis. Visual stimulation of control RD rats evoked no responses. In RD rats with retinal transplants, a small area of the SC corresponding to the position of the transplant in the host retina, responded to light stimulation between −4.5 and −0.08 log cd/m2, whereas the light threshold of normal rats was at or below −5 log cd/m2 all over the SC. Overall, responses in the SC in rats with laminated transplants had lower response thresholds and were distributed over a wider area than rats with rosetted transplants. BDNF treatment improved responses (spike counts, light thresholds and responsive areas) of rats with laminated transplants whereas GDNF treatment improved responses from rats with both laminated and rosetted (more disorganized) transplants. In conclusion, treatment of retinal transplants with GDNF and BDNF improved the restoration of visual responses in RD rats; and GDNF appears to exert greater overall restoration than BDNF.
Keywords: retinal transplantation, retinal degeneration, superior colliculus electrophysiology, BDNF, GDNF, transgenic rat, retinotectal, spike analysis
1. Introduction
Age-related macular degeneration and retinitis pigmentosa are two types of retinal diseases in which the photoreceptors undergo cell death leading to progressive vision loss and ultimately blindness (Cronin et al., 2007; Djojosubroto and Arsenijevic, 2008). It is estimated that more than 15 million people globally suffer vision loss due to retinal degeneration (Chader, 2002). Yet, there is currently no effective treatment available for either disease type (Kennan et al., 2005; Shintani et al., 2009) even though there are various research results that suggest potential approaches. One of these is subretinal transplantation of intact sheets of fetal retina with its attached retinal pigment epithelium (Aramant and Seiler, 2004; Radtke et al., 2008; Seiler et al., 2008a).
The pigmented transgenic S334ter line 3 rat is a retinal degeneration model that expresses a mutated rhodopsin gene in which a termination codon at residue 334 of the opsin transgene causes a protein lacking the 15 carboxyl-terminal amino acids. This C terminus is involved in the rhodopsin localization to the outer segments. Its absence leads to photoreceptor cell death (Green et al., 2000; Liu et al., 1999). Multiple mutations within the C terminus have been identified in patients with retinitis pigmentosa (Berson et al., 2002). Therefore, using the S334ter line 3 rats allows researchers to investigate an animal model with a disease similar to human retinitis pigmentosa.
Seiler and Aramant have designed a special device and methodology to deliver sheets of fetal retinal neuroblastic progenitor cells into the subretinal space (Aramant and Seiler, 2002, 2004). The advantage of such an approach is that many retinal degeneration patients need not only new retinal pigment epithelium and photoreceptor cells but also other retinal cells. This approach meets this criterion. In addition, the neuroblastic retinal progenitor sheets already have their three-dimensional primordial circuitry established between the cells which leads to a morphological development resembling a normal retina (review: Aramant and Seiler, 2002, 2004). Transplantation of retinal sheets has been found to restore visual responses in the superior colliculus (SC) of different retinal degeneration models long time after transplantation (Sagdullaev et al., 2003; Thomas et al., 2004; Woch et al., 2001). Using trans-synaptic virus tracing, Seiler and colleagues showed that not only the transplant formed synaptic connectivity with host retina (Seiler et al., 2005) but also the visually responsive site in the SC can be traced back to the transplant area in the host retina (Seiler et al., 2008c). In addition, synapses of transplant neurons with the host retina have been demonstrated in the host inner plexiform layer on the ultrastructural level with transplants that restored visual responses in the SC (Seiler et al., 2010b). However, in most transplantation experiments, the level of visual restoration was apparent only in a restricted area of the SC indicating that the area of integration between the transplant and the host retina is limited (Seiler et al., 2008b). Therefore, we sought to improve the functional efficacy of the transplants.
The correct synaptic connections of retinal neurons during development are improved by trophic factors (Hu et al., 2005; Lein and Shatz, 2000; von Bartheld et al., 2001), whereas cells that make inappropriate connections do not receive trophic support and undergo apoptosis (Crespo et al., 1985). For this reason, it is hypothesized that if the removal of neurotrophic factors from the cellular environment can stimulate cell death, then adding exogenous trophic factors may have neuroprotective effects in the retina (Faktorovich et al., 1990) and hence the therapeutic effect of neurotrophins as a general protective strategy to slow the progression of retinal degeneration. Neurotrophins involved in the development and integration of the central nervous system, such as brain-derived neurotrophic factor (BDNF), have a modulatory influence during the development of the visual sensory system (Berardi and Maffei, 2004; von Bartheld et al., 2001). Studies performed in normal and retinal degenerate (RD) rat models demonstrated that BDNF has a neuroprotective role (Chaum, 2003; Gauthier et al., 2005; Ikeda et al., 2003; Keegan et al., 2003; Lawrence et al., 2004; Nakazawa et al., 2002; Paskowitz et al., 2004). A previous study demonstrated that BDNF treatment of retinal transplants improved restoration of visual responses (Seiler et al., 2008b). However, BDNF would be difficult to obtain in clinical grade. Glial-derived neurotrophic factor (GDNF) has been used in clinical trials on Parkinson patients (Nutt et al., 2003; Patel et al., 2005) and was shown to significantly attenuate the degeneration of adult rat retinal ganglion cells (Yan et al., 1999), protect against oxidative damage induced retinal degeneration (Dong et al., 2007), and exert both histological and functional protection of rod photoreceptors in the retinal degeneration (rd/rd) mouse model of retinitis pigmentosa (Frasson et al., 1999). Thus, the present investigation evaluates in the SC of a rat model of rapid retinal degeneration whether treatment of transplants with GDNF is more effective than BDNF to enhance the restoration of visual responses.
2. Materials and methods
2.1. Animals
Normal pigmented rats without any retinal transplant and pigmented transgenic S334ter line 3 RD rats expressing a mutated human rhodopsin protein (Sagdullaev et al., 2003) were used in this study. All rats were group housed (4 animals per Plexiglas cage) and maintained in the vivarium on a 12-h light/dark cycle (lights on from 06:30 to 18:30 h) at an ambient temperature of 21.5 ± 0.8 °C and a relative humidity of 50%. The normal donor rats were bred from transgenic rats positive for human placental alkaline phosphatase (hPAP; Kisseberth et al., 1999) (breeders originally a gift of Dr. Eric Sandgren, Univ. of Wisconsin, Madison, WI) and ACI rats obtained from Harlan Laboratories (Indianapolis, IN, USA); whereas the RD rats were the F1 generation of a cross between albino homozygous S334ter line 3 and pigmented Copenhagen rats (Harlan Laboratories). These RD rats were originally produced by Xenogen Biosciences (formerly Chrysalis DNX Transgenic Sciences, Princeton, NJ), and supplied with the support of the National Eye Institute by Dr. Matthew LaVail, University of California, San Francisco (http://www.ucsfeye.net/mlavailRDrat models.shtml).
The study (Table 1) consisted of seven groups of male and female rats (weighing 135–430 g): (1) seven RD rats with untreated retinal progenitor transplants, (2) eighteen RD rats with GDNF microsphere-coated retinal progenitor transplants, (3) eleven RD rats with BDNF microsphere-coated retinal progenitor transplants, (4) thirteen non-transplant controls of age-matched RD rats, (5) eight sham surgery RD rats, (6) six RD rats with progenitor cortex transplants, and (7) ten normal pigmented rats of the ACI-hPAP strain (that was used for obtaining donor tissue). Injections of growth factor microspheres alone were not included as controls because it had been shown in a previous study that treatment of S334ter-3 rats with BDNF microspheres alone did not result in restoration of visual responses in the SC to low light, and had only a transient initial effect at one week post-op (Seiler et al., 2008b). All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as our Institution’s Animal Welfare Committee Guidelines and the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
Table 1.
Experimental groups.
| Normal retina No transplant |
S334ter-3 with degenerate retina (RD) |
||||||
|---|---|---|---|---|---|---|---|
| Transplant |
No transplant |
||||||
| E19 retina +GDNF | E19 retina +BDNF | E19 retina (no factor) | E19 cortex | Sham | No surgery | ||
| N | 10 | 18 | 11 | 7 | 6 | 8 | 13 |
| Age at surgery (d) | n.a. | 32.2 ± 1.1 | 33.3 ± 1.1 | 35.1 ± 3.5 | 24.8 ± 0.2 | 28.1 ± 0.8 | n.a. |
| Age at recording (d) | 94−239 | 92−250 | 87−303 | 121−298 | 86−149 | 90−302 | 37−113 |
| 185 ± 14 | 143 ± 8.7 | 160 ± 25.6 | 224 ± 27 | 111 ± 12 | 222 ± 27 | 67 ± 8.4 | |
| Time post-surgery (d) | n.a. | 55−217 | 54−269 | 88−242 | 61−125 | 62−268 | n.a. |
| 111 ± 8.9 | 126 ± 25 | 189 ± 26 | 86 ± 11 | 194 ± 27 | |||
| Visual threshold (log cd/m2) | −4.9 ± 0.3 | −1.8 ± 0.2 | −1.8 ± 0.3 | −1.7 ± 0.2 | n.a. | n.a. | n.a. |
All variations are expressed as standard error of the mean (S.E.M.).
2.2. Preparation of GDNF/BDNF microspheres
A detailed protocol for the preparation of GDNF or BDNF microspheres (average diameter 1 ± 0.5 μm) was previously reported (Mahoney and Saltzman, 2001; Seiler et al., 2008b). Briefly, 100 μl of 500 μg GDNF (Invitrogen, Baltimore, MD, USA) or BDNF (Abazyme, Needham, MA, USA) was added to s solution of polylactide-co-glycolide (PLGA, 50:50) (Mn = 54,100 Birmingham Polymers), dissolved in methylene chloride which was then processed further by adding aqueous 1% polyvinyl alcohol (4 ml PVA, Mw = 25,000, Polysciences). After further stirring and centrifugation, the microspheres were frozen in liquid nitrogen and lyophilized for 24 h. These microspheres were kept at 4 °C until use.
To induce tissue adherence, a suspension of 5 mg GDNF or BDNF microspheres was incubated shortly before transplantation for 1 h in 10 μg/ml poly-DL-lysine in phosphate-buffered saline (PBS) so that the microspheres develop a positive charge. Microspheres were washed three times with Hibernate E medium (Brain-Bits, Springfield, IL) containing B-27 supplements (Invitrogen, Baltimore, MD, USA) and resuspended in Hibernate E medium at a concentration of 5 mg microspheres/ml.
2.3. Donor tissue
Transgenic rats carrying the hPAP gene were used as the source of donor tissue for these studies. At day 19 of gestation (day of conception = day 0), fetuses were removed by cesarean section. Transgenic fetuses were identified by histochemistry for hPAP (Kisseberth et al., 1999). Embryos were stored on ice in Hibernate E medium with B-27 supplements for up to 6 h until dissection. Retinas were dissected free from surrounding tissue. For GDNF or BDNF microsphere treatment, a drop of a freshly prepared GDNF or BDNF microsphere solution (50 μl, corresponding to 41.5 ng GDNF or BDNF in Hibernate E medium) was added to a retinal flat mount which was then incubated overnight on ice or in the refrigerator to attach the microspheres to the donor tissue. Retinal sheets were cut into rectangular pieces of 1−1.5 × 0.6 mm to fit into the flat nozzle of the previously described custom-made implantation tool (Aramant and Seiler, 2002; Seiler and Aramant, 1998). The orientation of the donor tissue could easily be observed in the dissection microscope. It was not possible to quantify how much GDNF or BDNF adhered to the donor tissue, although the amount of GDNF or BDNF was thought to be less than 8 ng.
2.4. Transplantation procedure
The transplantation procedure was performed according to previously described methodology (Aramant and Seiler, 2002). S334ter-3 rats (P25–56) were intraperitoneally (i.p.) anesthetized with ketamine/xylazine (37.5 mg/kg ketamine and 5 mg/kg xylazine). Their pupils were dilated with 1% Atropine eye drops (Bausch & Lomb, Tampa, FL, USA), and local anesthesia was provided by tetracaine eye drops (0.5%) (Bausch & Lomb). A small incision (~1.0 mm) was made posterior to the pars plana, parallel to the limbus. The implantation instrument was inserted with extreme care to minimize disturbance of the host retinal pigment epithelium. The graft was released into the subretinal space posteriorly at the nasal quadrant near the optic disc. Transplants were placed into the left eye only, leaving the right eye as a control. The incision was closed with two 10–0 sutures and the eyes were treated with gentamycin and artificial tears ointment. The rats were placed in an incubator for recovery. Transplant placement was verified by post-surgical eye exams and ocular coherence tomography. Rats with transplants misplaced into the vitreous or choroid were discarded from the study.
2.5. Electrophysiological recording of visual responses in the SC
Electrophysiological recordings were made in the SC of RD rats 2–9 months (55–269 d) after retinal transplantation (see Table 1). Rats were selected based on positive surgical outcome (i.e., successful implantation in the subretinal space) and Ocular Coherence Tomography imaging (Seiler et al., 2010a). On the day prior to electrophysiological assessment, each rat was dark-adapted in a Plexiglas cage, which was placed inside a plastic container with an oversized black drapery wrapped around it to prevent any light from reaching inside to the animal. On the day of the recording, the experimental room was kept in complete darkness and was lit only by a small red light intermittently between recordings and during preparatory tasks. To minimize the rats’ exposure to even this red light, their eyes were covered with a custom-made eye cap that was removed only during photic stimulation and electrophysiological recordings. All recordings were conducted in complete darkness, except for the flashes from the photic stimulation.
Rats were initially anesthetized with an injection of ketamine/xylazine (37.5 mg/kg ketamine and 5 mg/kg xylazine, i.p.) and placed into a stereotaxic apparatus where they received Isoflurane anesthesia (1–2% mixed with 40% oxygen) through a gas anesthesia mask (Stoelting, Wood Dale, IL, USA). For the craniotomy, an incision of 2.0–2.5 cm was made to expose the skull. A hole of ~0.5 cm in diameter was drilled through the skull above the SC. A glass, disposable Pasteur pipet with custom-pulled tip was used to aspirate part of the cortex to expose the SC surface for recording. Multi-unit responses were recorded from the superficial laminae of the SC using tungsten microelectrodes (0.5 megaohms (MΩ) impedance; MicroProbe, Inc., Gaithersburg, MD). Using the coordinate system on the stereotaxic apparatus, microelectrodes were systemically placed on the recording sites (30–80 μm apart) throughout the SC surface (max. 0.3 mm depth). At each recording location, ten sweeps of a full-field stimulus were delivered by a Sigma SA-7 camera with a shutter mechanism that controlled the duration of light exposure (40 ms) onto a round screen (190 cm diameter) that was placed 10 cm in front of the rat’s eye. The light originated from a 150 W fiber optic power supply (Dyna Lite 150) (as described in Thomas et al., 2005). To regulate the light intensity, neutral density filters were placed in front of the screen, resulting in a range of +1.0 to −5.9 log cd/m2 of luminance that was actually delivered to the rat. All electrophysiological responses were recorded using a digital data acquisition system (Powerlab; ADI Instruments, Mountain View, CA). Stimuli were repeated 10 times at approximately 10 s intervals. All spikes occurring 100 ms before to 300 ms after the onset of the photic stimulus were counted. The sum was averaged across 10 stimulus presentations. To assess baseline spike activity, each set of 10 stimuli was preceded by a control “stimulus” in which the shutter was blocked by a tight-fitting lens cover.
Spikes were defined as upwards or downwards deflections that were at least twice the baseline amplitude. Baseline activity occurring during both the 100 ms period preceding each light stimulus and the blank trial in which no stimulus was presented served as controls for the occurrence of a response. A response was defined across the set of 10 photic stimuli as >10% increase of spike counts over baseline or 10 spikes in the case of zero baseline activity.
Visual thresholds were defined as the lowest light level at which a response could be obtained. Visual thresholds were usually only determined for one responsive site within the SC, and responsive areas were determined at a higher light intensity of −1.33 or 0.08 log cd/m2.
The response latency was defined as the period of time (ms) between stimulus onset and the first evoked spike. Because time zero for stimulus onset was the electrical signal to the camera shutter, relatively long latency times resulted from the time required for the (mechanical) shutter on the camera to open to allow the light out. Since stimulus onset was determined by the mechanical operation of the shutter, latency should be considered in relative rather than absolute terms.
The following characteristics of visual responses were analyzed: (1) visual thresholds, (2) spike counts, (3) response latencies, and (4) location within the SC.
2.6. Histology
At the end of the recording session, animals were perfused through the ascending aorta with 4% paraformaldehyde in 0.1 M sodium (Na) phosphate buffer (sometimes with 0.1–0.4% glutaraldehyde added) or the eyes were immersion fixed in 4% paraformaldehyde. Eye cups were dissected and immersed in 4% paraformaldehyde in 0.1 M Na-phosphate buffer for 2–4 h and washed three times with 0.1 M Na-phosphate buffer. Dissected eye cups, oriented along the dorso-ventral axis, were infiltrated with 30% sucrose overnight, embedded in OCT compound (International Medical Equipment, Inc., San Marcos, CA, USA), and frozen in isopentane on dry ice. Transverse sections of the retina were cut at 10 μm thickness on a cryostat and mounted onto slides. Every fifth slide was stained by histochemistry for hPAP (Kisseberth et al., 1999; Mujtaba et al., 2002).
Alternatively, eye cups were embedded in 4% agarose, and 80–100 μm slices were cut on a vibratome.
A series of sections through the full extent of selected transplants were evaluated at the light microscopic level by immunohistochemistry for various markers.
Frozen sections were washed with PBS and blocked for at least 30 min in 20% goat serum. The following primary antibodies were used: a rabbit monoclonal antibody against hPAP (clone SP15) (Epitomics, Burlingame, CA; dilution 1:50) which required antigen retrieval of dried frozen sections for 20 min at 70 °C using HistoVT One (Nacalai USA Inc., San Diego CA); and rabbit antisera against red-green opsin and blue opsin (1:2000) (Chemicon, Temecula, CA, dilution of 1:2000); in combination with mouse monoclonal antibodies: against recoverin (gift of Dr. McGinnis, Univ. of Oklahoma (McGinnis et al., 1997)); anti-syntaxin 1 (HPC-1), 1:500 (Barnstable et al., 1985) (gift of Dr. Barnstable, now at Penn State College of Medicine, Hershey, CA), protein kinase C (PKC) (Biodesign, Saco, ME; dilution 1:100), or rhodopsin (rho1D4, dilution 1:50; gift of Dr. Molday, Univ. of British Columbia (Molday and MacKenzie, 1983)).
Sections were incubated in primary antibodies overnight at 4 °C, followed by five PBS washes. The binding of the primary antibody was detected using a 1:200 dilution of a combination of AF488 or rhodamine-X-anti-rabbit IgG and Rhodamine-X of AF488 anti-mouse-IgG, respectively (Molecular Probes, Eugene, OR), and coverslipped with DAPI (4′,6′-diamidino-2-phenylinode hydrochloride) containing Vectashield mounting medium (Vector Labs). Sections were imaged using a Zeiss LSM510 and LSM710 confocal microscope. Five to 12 confocal slices were combined in a projection image.
2.7. Data analysis
Multi-unit responses to light intensities and baseline activities were evaluated off-line using the Scope Application program (ADI Instruments, Mountain View, CA). Responses within 300 ms after the photic stimulus onset were analyzed for visual thresholds, average spike counts, response latencies, and location within the SC. Statistical comparisons were made using a one-way analysis of variance (ANOVA) with subsequent post hoc tests from a statistics package of GraphPad Software, Inc. (San Diego, CA). The varying N of different experimental groups was taken in account. A significance level of 0.05 was applied throughout all analyses. Graphs were prepared using Sigmaplot version 11 (Systat Software, Inc., San Jose, CA).
3. Results
3.1. Representative examples of groups
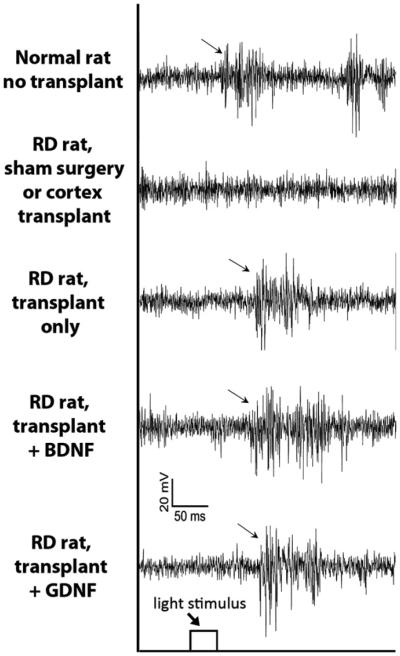
Fig. 1 shows representative responses from the different experimental groups after a 40 ms light stimulus of the same light level. At this stimulus level, strong multi-unit responses were evoked in normal control rats and in RD rats receiving non-treated, GDNF-treated or BDNF-treated retinal transplants. It is evident that responses evoked from the SC in each group were quite similar in magnitude and duration, although responses from transplanted RD showed a longer response latency. No responses were evoked in control RD rats.
Fig. 1.
Examples of recording traces from different experimental groups to a 40 ms light stimulus (stimulus level – 0.08 log cd/m2). Examples of multi-unit spike activities could be recorded in the SC of normal and rats with transplants but not in different control RD rats. Note that the response latency is longer in the transplanted rats than in the normal rat.
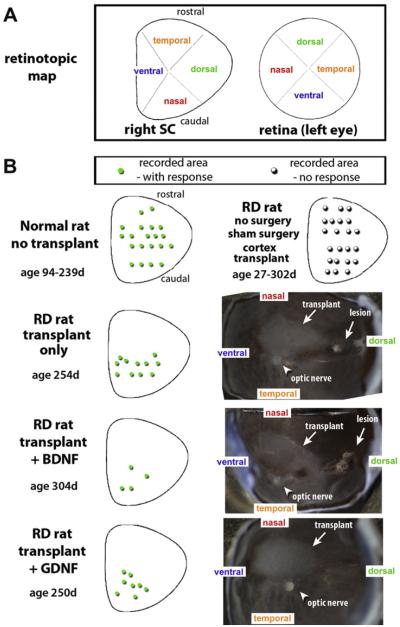
Fig. 2A illustrates how the retina is divided into four quadrants (i.e., ventral, nasal, dorsal, and temporal) that correspond topographically to those in the SC. Fig. 2B shows examples of the spatial distribution of responses of laminated transplants of the three different retinal transplant groups (no treatment; BDNF; GDNF), together with the normal control and sham surgery RD control. The position of the transplant in the eye cup was determined after dissection of the eye cup (Fig. 2B), and compared with SC recording results (Figs. 1–3) and histological results (Figs. 4–7). The location of responses in the SC to photic stimulation corresponded to the position of the transplant in the retina of each rat (Fig. 2B). The SC in sham surgery rats (and cortex transplanted rats, Fig. 2B) failed to respond to any photic stimulation.
Fig. 2.
Retinotopic distribution of responses (individual experiments). (A) Diagram of retinotopic map of the right SC and the left retina. (B) Each rat was recorded over the whole extent of the SC. Normal rats: responses all over the SC (green dots); control degenerate rats: no responses (hollow black dots). In the transplanted rats (histology shown in Figs. 6A–F and 7), the location of responses in the SC to photic stimulation (green dots in diagrams in left column) corresponds to the position of the transplant in the retina (dissected eye cups, right column). For sake of clarity, transplant diagrams show only areas with responses. All eye cups were oriented along the dorso-ventral axis. Transplants appear whitish.
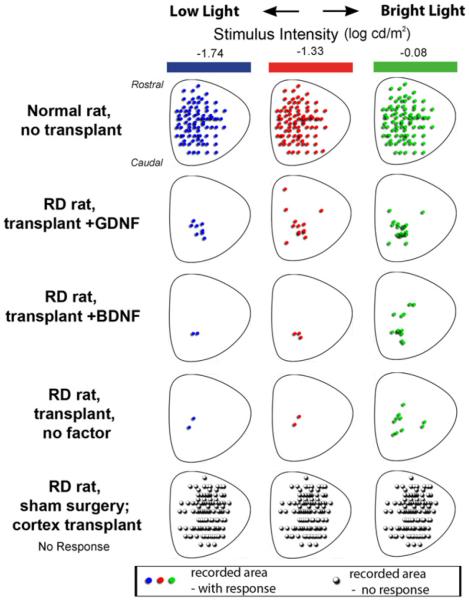
Fig. 3.
Spatial distribution of responses in the SC of normal rats (N = 10); RD rats with GDNF-treated transplants (N = 17), BDNF-treated transplants (N = 10); untreated transplants (N = 7); and sham surgery (N = 8) and cortex RD transplanted rats (N = 6) corresponding to photic stimulus intensities of −1.74 (blue dots), −1.33 (red dots) and −0.08 (green dots) log cd/m2. For sake of clarity, transplant diagrams show only areas with responses in at least one animal. For rat ages and post-surgical times see Table 1.
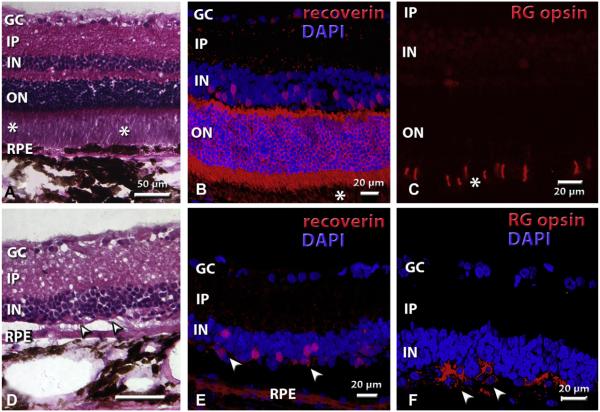
Fig. 4.
Histology of normal retina (A–C) and degenerate (RD) retina (D–F). Asterisks indicate photoreceptor outer segments. In (D–F), arrow heads indicate remaining cones in the RD retina. For explanation of label abbreviations, see abbreviation list. (A) Normal retina, hematoxylin–eosin (H–E) stain. There are 10–11 rows of nuclei in the outer nuclear layer. (B) Staining for recoverin (specific for rod and cone photoreceptors and cone bipolar cells). Nuclei are stained blue with DAPI. There is strong staining of photoreceptor somas in the outer nuclear layer, inner segments, and the photoreceptor terminal layer in the outer plexiform layer. (C) Staining for red-green opsin (RG opsin) in cone outer segments in normal retina. (D) RD retina, sham surgery, age 289 d (H–E staining). (E) Recoverin staining of RD host retina outside transplant area (retina + GDNF transplant, age 250 d). Cone bipolar cells in the inner nuclear layer are more intensely stained than residual cones. (F) RG opsin staining of residual cones in RD host retina outside transplant area (retina only transplant, age 254 d). Magnification bars: 50 μm (A, D); 20 μm (B, C, E, F). All images are oriented with the ganglion cell layer up and the RPE layer down.
Fig. 7.
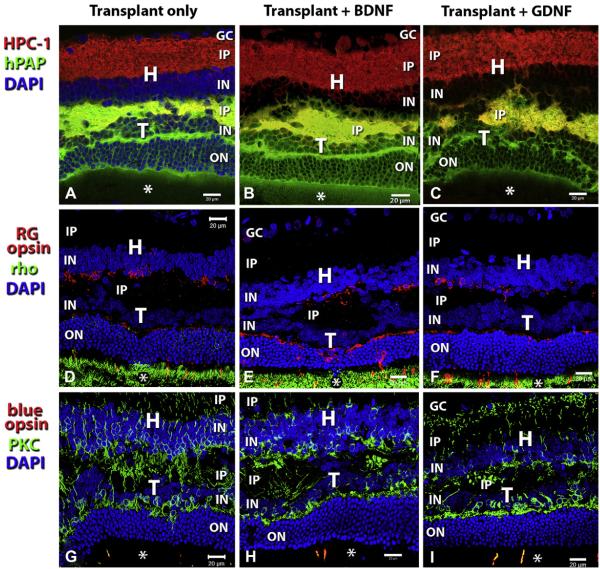
Analysis of 3 different laminated transplants (same transplants as Fig. 6A–F). Each column shows images of the same transplant, stained for antibodies indicated on the left side. All images are projection stacks of confocal images taken at several different focus levels. In (A), (D)–(I), nuclei are counterstained with DAPI (blue). Photoreceptor outer segments are indicated by asterisks. (A)–(C) Rabbit anti hPAP (green) in combination with mouse HPC-1 (anti-Syntaxin-1, red). HPC-1 stains synaptic layers and, more faintly, the cytoplasm of amacrine cells in the inner nuclear layer. The DAPI stain has been omitted from (B) and (C) so that the amacrine cell staining can be seen. (D)–(F) rabbit anti red-green opsin (red) in combination with mouse anti-rhodopsin (green). Strong staining of transplant outer segments for rhodopsin. Note that there is no rhodopsin staining in the host retina although there are scattered cell bodies of host cones at the transplant-host interface. Cone outer segments can only be seen in the transplant. Cone opsin also stains cone terminals in the outer plexiform layer of the transplant and processes of host cones in the host outer plexiform layer. (G)–(I) Combination of rabbit anti blue opsin (red) with mouse anti PKC (green) which stains rod bipolar cells and blue cones. Outer segments of blue cones (very few) can only be seen in the transplant. PKC staining of bipolar cells is stronger in the transplant than in the host retina (best seen in I). Scale bars = 20 μm.
3.2. Spatial distribution of responses in SC (group data)
Responses to stimulus levels of −1.74 to −0.08 log cd/m2 are broadly distributed in the SC of normal rats (Fig. 3). However, the spatial distribution of responses in the SC of RD rats with GDNF-treated transplants (N = 17), BDNF-treated transplants (N = 10) and untreated transplants (N = 7) to these stimuli depended on stimulus level, i.e., the spatial distribution of responses varied with stimulus intensity. At low light (−1.74 log cd/m2), the area of responses was around the nasal quadrant in the SC. This area of responses widened as the stimulus intensity increased to −0.08 log cd/m2. The RD rats with GDNF-treated transplants showed the most response areas compared to RD rats with BDNF-treated and untreated transplants. No responses were found in age-matched non-surgery (N = 13), sham surgery (N = 8) or cortex transplanted RD rats (N = 6).
3.3. Histology
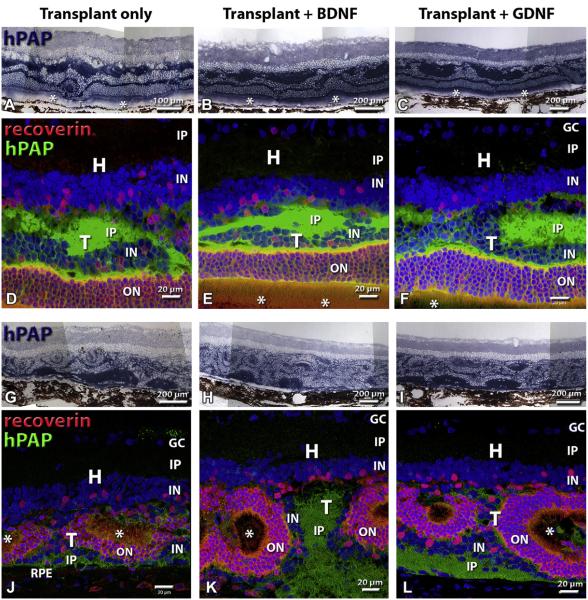
Fig. 4 compares the histology of normal retina (Fig. 4A–C) to degenerate S334ter-3 retina (Fig. 4–F) by hematoxylin–eosin staining and immunohistochemistry for recoverin and red-green cone opsin. Normal rat retina contains about 10 rows of nuclei in the outer nuclear layer (Fig. 4A) which stain strongly for recoverin (Fig. 4B). Cone opsin staining is only found in the outer segments (Fig. 4C). S334ter rats without surgery, with sham surgery, or in transplanted eyes outside the transplant area had lost their outer nuclear layer and had only few cones left (Fig. 4D–F). Recoverin staining showed stronger stain of cone bipolar cells in the inner nuclear layer than of the remaining cones which had lost their outer segments (Fig. 4E). Cone opsin staining revealed staining of abnormal cone processes, and absence of outer segments (Fig. 4F). All fetal cortex transplants survived and developed large masses in the subretinal space (Fig. 5A, B), containing neurons and glia (not shown). Cortex-transplant-derived oligodendrocytes apparently had migrated to myelinate axons of host retinal ganglion cells (Fig. 5B). Transplants could be identified by blue-purple hPAP staining (Figs. 5B; 6A–C, G–I) and immunohistochemistry for hPAP (Figs. 6D–F, J–L; 7A–C). Retinal transplants could be divided up based on their organization (Table 2, Fig. 6). About half of the transplants contained large areas with appropriate lamination, including photoreceptor outer segments in contact with the host RPE (Figs. 6A–F; 7). However, most laminated transplants also contained rosetted areas (defined as spheres of photoreceptors, surrounded by other retinal layers). The remaining transplants contained rosettes only (examples in Fig. 6G–L). The difference in laminar organization could be well observed using recoverin staining showing recoverin-immunoreactive photoreceptor layers arranged in balls with outer segments in the center and the transplant inner plexiform layer facing the RPE (Fig. 6J–L). Fig. 7 shows examples of different cell markers in combination with immunohistochemistry for donor cell markers in laminated transplants which had developed cellular morphologies similar to normal retina. Transplants appeared to extend processes into the host retina (Fig. 7A–C). Staining for red-green opsin (Fig. 7D–F) revealed cones with outer segments in the transplant and abnormal cones without outer segments in the host. Rhodopsin staining (Fig. 7D–F) and blue opsin staining (Fig. 7G–I) was only seen in the transplant, not in the host retina. PKC-immunoreactive rod bipolar cells showed stronger immunoreactivity in the transplants than in the host retina, and processes appeared to cross the transplant–host interface in some places (Fig. 7G–I).
Fig. 5.
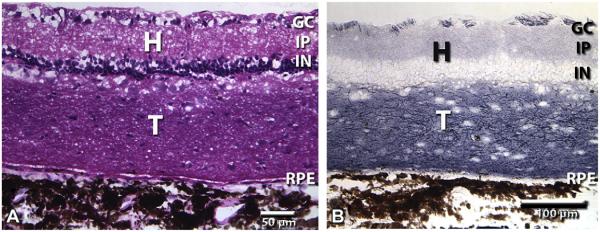
Cortex transplant, 81 d after surgery, age 106 d. No response. (A) H–E staining. (B) hPAP staining (blue-purple). Host ganglion cell axons at the vitreal surface of the retina apparently have been myelinated by transplant-derived oligodendrocytes. Magnification bars: 50 μm (A), 100 μm (B).
Fig. 6.
Comparison of laminated and rosetted transplants. Asterisks (*) indicate photoreceptor outer segments. A, B, C, G, H, I: overview, hPAP staining of transplant (blue-purple). D, E, F, J, K, L: Enlargements; green staining for rabbit anti hPAP (marker for donor cells) in combination with mouse anti recoverin (red). Recoverin stains all photoreceptors and cone bipolar cells. Note that the host retina contains few cone photoreceptors on all images. Nuclei are counterstained blue with DAPI. (A)-(F) Examples of laminated transplants. (A), (D) Non-treated retinal transplant, age 254 d. This rat had responses in 11 areas at bright light; the threshold for visual responses in the SC was at −2.2 log cd/m2. (B), (E) BDNF-treated transplant, age 304 d; threshold −3.6 log cd/m2; responses in 5 areas in bright light. (C), (F) GDNF-treated transplant, age 250 d; threshold −1.7 log cd/m2; responses in 8 areas in bright light. (G)–(L) Examples of rosetted transplants. (G), (J) Non-treated retinal-transplant, age 260 d. This rat had only faint responses in bright light (−0.91 log cd/m2). (H), (K) BDNF-treated transplant, age 93 d; threshold −1.1 log cd/m2, response in 4 areas in bright light. (I), (L) GDNF-treated transplant; age 131 d; threshold −2.4 log cd/m2, responses in 9 areas in bright light. Magnification bars: 100 μm (A); 200 μm (B, C, G–I); 20 μm (D–F, J–L). All images are oriented with the ganglion cell layer up and the RPE layer down.
Table 2.
Overview of transplant results.
| Visual threshold (log cd/m2) |
Responsive areas(%) at −0.08 log cd/m2 |
|||
|---|---|---|---|---|
| Laminated transplants |
Rosetted transplants |
Laminated transplants |
Rosetted transplants |
|
| E19 retina + GDNF | −2.0 ± 0.1 N = 8 |
−1.7 ± 0.2 N = 10 |
34.2 ± 2.2* N = 8 (*P < 0.05) |
20.2 ± 3.8 N = 10 |
| E19 retina + BDNF | −2.4 ± 0.5 N = 4 |
−1.5 ± 0.4 N = 7 |
25.8 ± 5.9 N = 4 |
19.8 ± 2.4 N = 7 |
| E19 retina (no factor) | −2.1 ± 0.2 N = 4 |
−1.2 ± 0.2 N = 3 |
28.0 ± 5.0 N = 4 |
20.2 ± 7.1 N = 3 |
| Total | −2.15 ± 0.1* N = 16 (*P < 0.05) |
−1.5 ± 0.2 N = 20 |
30.5 ± 2.2** N = 16 (**P < 0.01) |
20.1 ± 2.2 N = 20 |
All variations are expressed as standard error of the mean (S.E.M.).
H = host; T = transplant; RD = retinal degenerate (S34ter-3 transgenic rat); SC = superior colliculus; HE = hematoxylin–eosin; hPAP = human placental alkaline phosphatase; DAPI = 4’,6-diamidino-2-phenylindole dihydrochloride; GC = ganglion cell layer; IP = inner plexiform layer; IN = inner nuclear layer; ON = outer nuclear layer; RPE = retinal pigment epithelium; HPC-1 = syntaxin 1; rho = rhodopsin; PKC = protein kinase C; RG opsin = red-green opsin.
3.4. Patterns of response
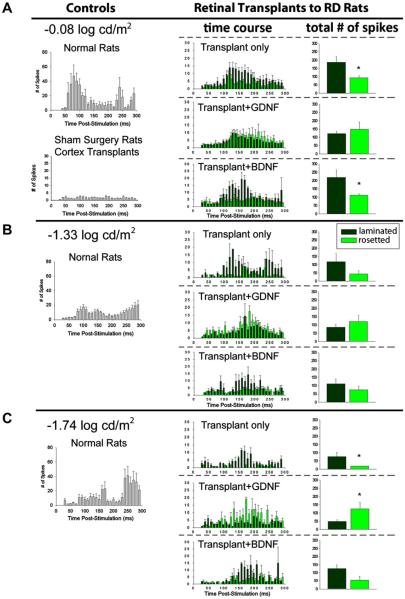
Fig. 8 compares the patterns of response in the SC of various groups of RD rats with transplants to normal, sham surgery and cortex transplanted rats at −0.08 log cd/m2 (Fig. 8A), −1.33 log cd/m2 (Fig. 8B) and −1.74 log cd/m2 (Fig. 8C) photic stimulation. In normal rats without any transplant, the average number of spikes reached 47 spikes 80 ms after photic stimulation at −0.08 log cd/m2. This peak decreased in amplitude and shifted to longer latencies as the stimulus intensity was reduced.
Fig. 8.
Difference of response patterns in SC between experimental groups at 3 light intensities, showing a difference in mechanism of action between BDNF and GDNF (analysis of spike counts within 300 ms after light stimulus). RD rats with retinal transplants (middle and right column) were compared to normal and control RD rats (left column) at −0.08, −1.33 and −1.74 log cd/m2 photic stimulation. Results of laminated and rosetted transplants are shown separately. The left and middle columns show spike counts for every 20 ms within 300 ms after light stimulus. The right column shows the total number of spikes within 300 ms. Laminated transplants without treatment and with BDNF treatment show higher spike counts than rosetted transplants within the same treatment group. In the GDNF-treated group, there is a trend towards higher spike counts in the rosetted transplant group at luminances of −0.08 and −1.33 log cd/m2; and a significantly higher spike count in the rosetted transplant group at a luminance of −1.74 log cd/m2. * indicates a significant difference, p < 0.05.
Spike counts from the RD rats with transplants were subgrouped according to the morphological organization of the transplants (predominantly laminated vs. rosettes only) (see histological examples in Fig. 6 and Table 2). At −0.08 log cd/m2 stimulation, the RD rats with untreated transplants (Fx,y = 25.9; p < 0.05) and BDNF-treated transplants (F1,11 = 9.663; p < 0.05) had larger responses from laminated transplants than rosetted transplants; whereas RD rats with GDNF-treated transplants showed increased spike counts in the SC from rosetted transplants more than from laminated transplants (Fig. 8A). Similar trends were found at −1.33 and −1.74 log cd/m2 stimulation in which the RD rats with GDNF-treated transplants evoked significantly more responses from rosetted (Fx,y = 8.31; p < 0.05) than laminated transplants (Fig. 8B and C). Furthermore, unlike the peak amplitude of normal rats, the peak amplitude for all three transplanted groups was at least 120 ms after stimulation at −0.08, −1.33 and −1.74 log cd/m2. There was no response elicited from sham surgery and cortex transplanted rats at all three stimulus intensities.
3.5. Lamination vs. rosettes
Data from all three transplanted groups (GDNF-treated, BDNF-treated and untreated transplants) were separated into laminated and rosettes, based on the morphological organization of the transplants. Table 2 compares the response thresholds and the percentage of responsive areas in the SC of RD rats with laminated transplants and RD rats with rosetted transplants in the different experimental groups. Within groups, the differences were not statistically significant due to the small sample size, except that the percentage of responsive areas in the GDNF-treated group was larger in laminated transplants. The RD rats with laminated transplants showed significantly 10% larger responsive areas in the SC than the rosetted transplants (t34 = 3.273; p < 0.01). The response threshold in the SC of RD rats with laminated transplants was at −2.15 log cd/m2 whereas the RD rats with rosetted transplants had a significantly higher response threshold of −1.5 log cd/m2 (t34 = 2.629; p < 0.1).
3.6. Response threshold
The response magnitude (spike counts) depended on the intensity of the light stimulus (Fig. 9). The best threshold of normal rats was at −6.1 log cd/m2 (average −4.6) whereas the best threshold of RD rats with GDNF-treated, BDNF-treated and untreated transplants was at −2.7 log cd/m2, −3.6 log cd/m2 and −2.7 log cd/m2, respectively (for averages see Table 2). Above their respective thresholds, the response functions of the implanted animals were significantly higher than control S334ter animals and below the normal controls. Interestingly, they increased monotonically beyond the stimulus level at which the function from normal controls saturated.
Fig. 9.
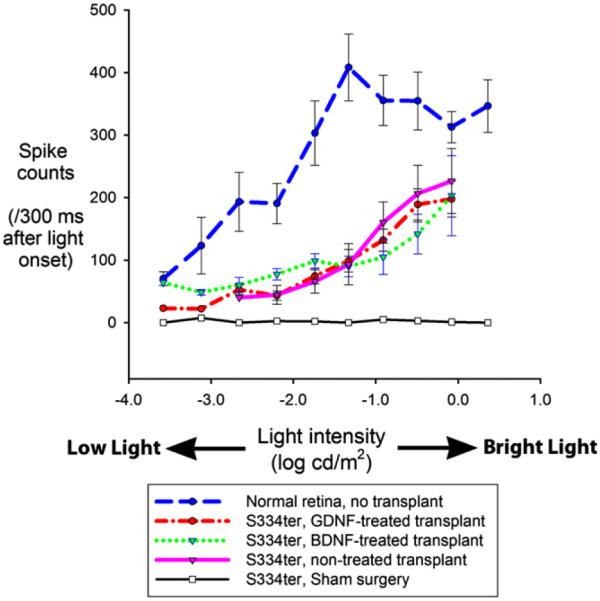
Response functions (total spike counts within 300 ms after stimulus) of normal rats (blue), RD rats with GDNF-treated (red), BDNF-treated (green) and untreated transplants (purple), and sham surgery RD rats (black).
3.7. Response onset latency
At low light intensity (−2.2 log cd/m2 luminance), the response onset latency of normal rats was 94 ± 12.8 ms while that of RD rats with untreated transplants was 157 ± 2.5 ms (p < 0.05; Fig. 10). At this same level of luminance, the response onset latencies were shorter in the RD rats with BDNF-treated transplants (145 ± 26 ms; p > 0.05) and GDNF-treated transplants (128 ± 14 ms; p < 0.05) compared to rats with untreated transplants. As the luminance increased, latencies shortened and differences in the onset latencies among groups of normal rats and RD rats became non-significant.
Fig. 10.
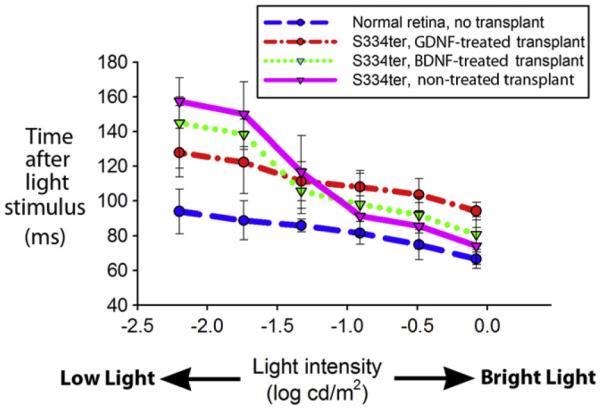
The response onset latency of normal rats (blue), rats with GDNF-treated transplants (red), rats with BDNF-treated transplants (green), and rats with untreated transplants (purple). At the lowest luminances, GDNF and BDNF-treated transplants have shorter latencies than untreated transplants. Time zero on the Y-axis is the electrical signal to the camera shutter.
4. Discussion
Our data show that treatment of intact sheets of fetal retina with neurotrophic factors before subretinal transplantation into recipient rats with retinal degeneration enhanced the recipients’ response to photic stimulation. BDNF microspheres improved responses of laminated transplants whereas treatment with GDNF microspheres appears to have an effect on both laminated and rosetted transplants as indicated by the average number of spikes recorded from the SC following low light stimulation.
Results from the present study are in accordance with previous investigations which demonstrated that fetal retinal sheet transplantation exerted a long-term rescue and restorative effect on visual function in an animal model of retinal degeneration (Seiler et al., 2008b; Thomas et al., 2004). The area of visual restoration in the SC corresponded to the location of the graft under the host retina (Figs. 2 and 3). In the transplanted rats, the responsive area responsive areas was very small in low light and increased with stronger light intensity. Seiler et al. (2008b) showed that 80% of transplanted rats treated with BDNF microspheres compared to 57% of transplanted rats not treated with BDNF microspheres responded to a low light intensity of 1 cd/m2 in a confined area of the SC, indicating that BDNF coating improved the functional efficacy of retinal progenitor cell grafts. Similarly, the present study found that transplanted RD rats treated with BDNF microspheres responded better on response threshold and response onset latency measures than transplanted RD rats not treated with BDNF microspheres, despite differences in methodologies to the previous study (Seiler et al., 2008b) such as distance of the photic stimulation to the rat’s eye, interstimulus interval, level of luminance, and duration of the light stimulus.
In contrast to a previous publication (Seiler et al., 2008b), the present study also analyzed the pattern of response, percentage of and response threshold of RD rats in terms of laminated or rosetted transplants. A rosette has photoreceptors and cells of other retinal layers around a central lumen enclosed by an outer limiting membrane (Seiler and Aramant, 1998). The cause of rosette formation in retinal transplants is not well known. It is believed to be a response to the damage of retinal pigment epithelium, Bruch’s membrane or the donor tissue that prevented the maintenance of outer segments (Aramant and Seiler, 2004). When sheets of minimally disturbed fetal retinas can be transplanted, more laminated and less rosetted formation is observed in transplants (Ghosh and Ehinger, 2000). In the current study, it was found that BDNF microspheres improved responses of laminated transplants while treatment with GDNF microspheres appears to have an effect on both laminated and rosetted transplants. Alternatively, GDNF could enhance responsiveness of rosettes that occur in both rosetted and laminated transplants whereas BDNF enhances only purely laminated transplants. It was also observed that response activity in the SC of retinal degenerate rats with GDNF-treated transplants had a shorter response onset latency and larger responsive areas (laminated transplants) compared to rats with BDNF-treated or untreated transplants, suggesting that GDNF also exerted protective effects on transplanted photoreceptors. These findings are in accordance with another study which demonstrated that GDNF vector treatment delayed photoreceptor degeneration by increasing rod photoreceptor survival as indicated by morphometric analysis of the outer nuclear layer thickness in the transgenic S334ter-4 rats (McGee Sanftner et al., 2001). It was also reported that GDNF was more effective in protecting nigrostriatal neurons than BDNF (Sun et al., 2005).
Photoreceptors of the retina have high oxygen and nutrient demands and must maintain a complex equilibrium of extracellular and intracellular ions for phototransduction. This makes the rods and cones especially susceptible to genetic, structural and biochemical insults (McGee Sanftner et al., 2001; Stone et al., 1999; Travis, 1998). Hence, any disturbance in the visual cycle could trigger apoptotic cell death in photoreceptors (McGee Sanftner et al., 2001). Several studies showed that intraocular administration of GDNF significantly slows down photoreceptor degeneration and even partially preserves visual function in the rd 1 mouse (Frasson et al., 1999), the retinal detachment-induced photoreceptor degeneration model (Wu et al., 2002), and Royal College of Surgeons rat (Lawrence et al., 2004). Thus, neurotrophic factors, such as GDNF and BDNF, have the ability to modulate neuronal growth during development to maintain existing cells and to allow recovery of injured neuronal populations. This conclusion is further supported by observations of retinal neurons during development which indicate that proper synaptic connections are reinforced by neurotrophic factors, while cells that make inappropriate connections and do not receive neurotrophic support experience apoptosis (McGee Sanftner et al., 2001).
The current study used several age-matched RD controls that all showed no visual responses: age-matched non-surgery RD rats, transplants of non-visual tissue (cortex), and sham surgeries. All rats were recorded at least 2 months after surgery, often much later (see Table 1). A control of injecting growth factor containing microspheres without transplants was not used. This was done in a previous study (Seiler et al., 2008b) which showed that RD rats treated only with BDNF microspheres (not with transplants) had no responses in the SC to low light. BDNF microspheres had a shortterm effect on optokinetic responses at 1 week post-surgery, but this effect was lost at 3 and 6 weeks post-op whereas rats with BDNF-treated transplants showed significantly better head tracking responses throughout the testing time frame. In other words, BDNF microspheres alone had no long-term effect on the host, except in combination with the developing transplant.
The growth factor release from microspheres follows an exponential decay, with most of the release occurring within the first 4 weeks (Seiler et al., 2008b). This previous study also showed that there is a 100-fold decrease of growth factor release from the microspheres between days 2 and 27. Thus, in the current study, most of the growth factor would have been depleted at the time of recording (>2 months).
Glial cell derived neurotrophic factor and its receptors are synthesized in the retina (Jing et al., 1996; Nosrat et al., 1996; Pachnis et al., 1993), suggesting that GDNF may have an innate neurotrophic role in the retina. GDNF protein has been found to enhance histological and functional protection of photoreceptors in the rd 1 mouse (Frasson et al., 1999), to delay photoreceptor outer segment collapse in vitro (Carwile et al., 1998), and to maintain populations of mouse photoreceptors in vitro (Jing et al., 1996). Yet, it is unknown whether GDNF acts directly or indirectly on photoreceptors in vivo. Harada and colleagues (Harada et al., 2003) showed that GDNF modulates trophic factors release by Muller glial cells in vitro and therefore proposed that GDNF could exert its neuron-protective effect through both direct and indirect pathways. Another study (Delyfer et al., 2005) found that injection of recombinant GDNF enhances the expression of glial L-glutamate /L-aspartate transporter (GLAST) around degenerating photoreceptors, implying that in the rd 1 mouse retina GDNF neuroprotective effect on photoreceptors can be mediated indirectly through activation of Muller glial cells.
In summary, RD rats with GDNF-treated transplants exhibited responses at a shorter response onset latency and over a larger area of the SC (laminated transplants). GDNF treatment of retinal transplants increased spike counts in the SC from rosetted transplants whereas BDNF specifically improved responses from laminated transplants, suggesting that the mechanism of restoration of visual responses by GDNF, as well as its neuroprotective role in the retina, may be different from that of BDNF. Further investigations on BDNF and GDNF are needed, such as behavioral data to examine the functional differences between laminated and rosetted transplants and correlate this to the anatomical findings.
Acknowledgment
The authors wish to thank the Lincy Foundation and private donations for their generous support of this research project. This work was made possible, in part, through access to the Optical Biology Core facility of the Developmental Biology Center, a Shared Resource supported in part by the Cancer Center Support Grant (CA-62203) and Center for Complex Biological Systems Support Grant (GM-076516) at the University of California, Irvine. MJS and RBA have proprietary interests in the transplantation instrument and method. The authors thank Lakshmi Patil, Eric Kitayama, Melissa Jones, Saba Motakef and David Ferguson for their technical assistance, and Dr. Biju Thomas, Doheny Eye Institute, Los Angeles CA, for his help with training and setup.
References
- Aramant RB, Seiler MJ. Retinal transplantation – advantages of intact fetal sheets. Prog. Retin. Eye Res. 2002;21:57–73. doi: 10.1016/s1350-9462(01)00020-9. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog. Retin. Eye Res. 2004;23:475–494. doi: 10.1016/j.preteyeres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Berardi N, Maffei L. The visual neurosciences. In: Chalupa LM, Werner JS, editors. Neurotrophins. Electrical Activity and the Development of Visual Function. Vol. 1. MIT Press; 2004. [Google Scholar]
- Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest. Ophthalmol. Vis. Sci. 2002;43:3027–3036. [PubMed] [Google Scholar]
- Carwile ME, Culbert RB, Sturdivant RL, Kraft TW. Rod outer segment maintenance is enhanced in the presence of bFGF, CNTF and GDNF. Exp. Eye Res. 1998;66:791–805. doi: 10.1006/exer.1998.0488. [DOI] [PubMed] [Google Scholar]
- Chader GJ. Animal models in research on retinal degenerations: past progress and future hope. Vision Res. 2002;42:393–399. doi: 10.1016/s0042-6989(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Chaum E. Retinal neuroprotection by growth factors: a mechanistic perspective. J. Cell Biochem. 2003;88:57–75. doi: 10.1002/jcb.10354. [DOI] [PubMed] [Google Scholar]
- Crespo D, O’Leary DD, Cowan WM. Changes in the numbers of optic nerve fibers during late prenatal and postnatal development in the albino rat. Brain Res. 1985;351:129–134. doi: 10.1016/0165-3806(85)90238-x. [DOI] [PubMed] [Google Scholar]
- Cronin T, Leveillard T, Sahel JA. Retinal degenerations: from cell signaling to cell therapy; pre-clinical and clinical issues. Curr. Gene Ther. 2007;7:121–129. doi: 10.2174/156652307780363143. [DOI] [PubMed] [Google Scholar]
- Delyfer MN, Simonutti M, Neveux N, Leveillard T, Sahel JA. Does GDNF exert its neuroprotective effects on photoreceptors in the rd1 retina through the glial glutamate transporter GLAST? Mol Vis. 2005;11:677–687. [PubMed] [Google Scholar]
- Djojosubroto MW, Arsenijevic Y. Retinal stem cells: promising candidates for retina transplantation. Cell Tissue Res. 2008;331:347–357. doi: 10.1007/s00441-007-0501-8. [DOI] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Hackett SF, Campochiaro PA. Increased expression of glial cell line-derived neurotrophic factor protects against oxidative damage-induced retinal degeneration. J. Neurochem. 2007;103:1041–1052. doi: 10.1111/j.1471-4159.2007.04839.x. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- Frasson M, Picaud S, Leveillard T, Simonutti M, Mohand-Said S, Dreyfus H, Hicks D, Sabel J. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest. Ophthalmol. Vis. Sci. 1999;40:2724–2734. [PubMed] [Google Scholar]
- Gauthier R, Joly S, Pernet V, Lachapelle P, Di Polo A. Brain-derived neurotrophic factor gene delivery to muller glia preserves structure and function of light-damaged photoreceptors. Invest. Ophthalmol. Vis. Sci. 2005;46:3383–3392. doi: 10.1167/iovs.05-0362. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Ehinger B. Full-thickness retinal transplants: a review. Ophthalmologica. 2000;214:54–69. doi: 10.1159/000027472. [DOI] [PubMed] [Google Scholar]
- Green ES, Menz MD, LaVail MM, Flannery JG. Characterization of rhodopsin mis-sorting and constitutive activation in a transgenic rat model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2000;41:1546–1553. [PubMed] [Google Scholar]
- Harada C, Harada T, Quah HM, Maekawa F, Yoshida K, Ohno S, Wada K, Parada LF, Tanaka K. Potential role of glial cell line-derived neurotrophic factor receptors in Muller glial cells during light-induced retinal degeneration. Neuroscience. 2003;122:229–235. doi: 10.1016/s0306-4522(03)00599-2. [DOI] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Tanihara H, Tatsuno T, Noguchi H, Nakayama C. Brain-derived neurotrophic factor shows a protective effect and improves recovery of the ERG b-wave response in light-damage. J. Neurochem. 2003;87:290–296. doi: 10.1046/j.1471-4159.2003.01994.x. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Keegan DJ, Kenna P, Humphries MM, Humphries P, Flitcroft DI, Coffey PJ, Lund RD, Lawrence JM. Transplantation of syngeneic Schwann cells to the retina of the rhodopsin knockout (rho(–/–)) mouse. Invest. Ophthalmol. Vis. Sci. 2003;44:3526–3532. doi: 10.1167/iovs.02-0097. [DOI] [PubMed] [Google Scholar]
- Kennan A, Aherne A, Humphries P. Light in retinitis pigmentosa. Trends Genet. 2005;21:103–110. doi: 10.1016/j.tig.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Keegan DJ, Muir EM, Coffey PJ, Rogers JH, Wilby MJ, Fawcett JW, Lund RD. Transplantation of Schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons rats. Invest. Ophthalmol. Vis. Sci. 2004;45:267–274. doi: 10.1167/iovs.03-0093. [DOI] [PubMed] [Google Scholar]
- Lein ES, Shatz CJ. Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation. J. Neurosci. 2000;20:1470–1483. doi: 10.1523/JNEUROSCI.20-04-01470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Peng M, Laties AM, Wen R. Activation of caspase-3 in the retina of transgenic rats with the rhodopsin mutation s334ter during photoreceptor degeneration. J. Neurosci. 1999;19:4778–4785. doi: 10.1523/JNEUROSCI.19-12-04778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MJ, Saltzman WM. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nat. Biotechnol. 2001;19:934–939. doi: 10.1038/nbt1001-934. [DOI] [PubMed] [Google Scholar]
- McGee Sanftner LH, Abel H, Hauswirth WW, Flannery JG. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol. Ther. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- McGinnis JF, Stepanik PL, Jariangprasert S, Lerious V. Functional significance of recoverin localization in multiple retina cell types. J. Neurosci. Res. 1997;50:487–495. doi: 10.1002/(SICI)1097-4547(19971101)50:3<487::AID-JNR15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Han SS, Fischer I, Sandgren EP, Rao MS. Stable expression of the alkaline phosphatase marker gene by neural cells in culture and after transplantation into the CNS using cells derived from a transgenic rat. Exp. Neurol. 2002;174:48–57. doi: 10.1006/exnr.2001.7847. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest. Ophthalmol. Vis. Sci. 2002;43:3319–3326. [PubMed] [Google Scholar]
- Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, Ebendal T, Hoffer BJ, Olson L. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996;286:191–207. doi: 10.1007/s004410050688. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr., Lozano AM, Penn RD, Simpson RK, Jr., Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Paskowitz DM, Nune G, Yasumura D, Yang H, Bhisitkul RB, Sharma S, Matthes MT, Zarbin MA, Lavail MM, Duncan JL. BDNF reduces the retinal toxicity of verteporfin photodynamic therapy. Invest. Ophthalmol. Vis. Sci. 2004;45:4190–4196. doi: 10.1167/iovs.04-0676. [DOI] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann. Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am. J. Ophthalmol. 2008;146:172–182. doi: 10.1016/j.ajo.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, Aramant RB, Seiler MJ, Woch G, McCall MA. Retinal transplantation-induced recovery of retinotectal visual function in a rodent model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2003;44:1686–1695. doi: 10.1167/iovs.02-0615. [DOI] [PubMed] [Google Scholar]
- Seiler M, Rao B, Aramant R, Yu L, Wang Q, Kitayama E, Pham S, Yan F, Chen Z, Keirstead H. Three-dimensional optical coherence tomography imaging of retinal sheet implants in live rats. J. Neurosci. Methods. 2010a;188:250–257. doi: 10.1016/j.jneumeth.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest. Ophthalmol. Vis. Sci. 1998;39:2121–2131. [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, Keirstead HS. Retinal transplants: hope to preserve and restore vision. Opt. Photon. News. 2008a;19:37–47. [Google Scholar]
- Seiler MJ, Aramant RB, Thomas BB, Peng Q, Sadda SR, Keirstead HS. Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets. Eur. J. Neurosci. 2010b;31:508–520. doi: 10.1111/j.1460-9568.2010.07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Sagdullaev BT, Woch G, Thomas BB, Aramant RB. Transsynaptic virus tracing from host brain to subretinal transplants. Eur. J. Neurosci. 2005;21:161–172. doi: 10.1111/j.1460-9568.2004.03851.x. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Thomas BB, Chen Z, Arai S, Chadalavada S, Mahoney MJ, Sadda SR, Aramant RB. BDNF-treated retinal progenitor sheets transplanted to degenerate rats: improved restoration of visual function. Exp. Eye Res. 2008b;86:92–104. doi: 10.1016/j.exer.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Thomas BB, Chen Z, Wu R, Sadda SR, Aramant RB. Retinal transplants restore visual responses: trans-synaptic tracing from visually responsive sites labels transplant neurons. Eur. J. Neurosci. 2008c;28:208–220. doi: 10.1111/j.1460-9568.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- Shintani K, Shechtman DL, Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009;80:384–401. doi: 10.1016/j.optm.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Peer J. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Retin. Eye Res. 1999;18:689–735. doi: 10.1016/s1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson’s disease. Brain Res. 2005;1052:119–129. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BB, Aramant RB, Sadda SR, Seiler MJ. Light response differences in the superior colliculus of albino and pigmented rats. Neurosci. Lett. 2005;385:143–147. doi: 10.1016/j.neulet.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Seiler MJ, Sadda SR, Aramant RB. Superior colliculus responses to light – preserved by transplantation in a slow degeneration rat model. Exp. Eye Res. 2004;79:29–39. doi: 10.1016/j.exer.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Travis GH. Mechanisms of cell death in the inherited retinal degenerations. Am. J. Hum. Genet. 1998;62:503–508. doi: 10.1086/301772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Wang X, Butowt R. Anterograde axonal transport, transcytosis, and recycling of neurotrophic factors: the concept of trophic currencies in neural networks. Mol. Neurobiol. 2001;24:1–28. doi: 10.1385/MN:24:1-3:001. [DOI] [PubMed] [Google Scholar]
- Woch G, Aramant RB, Seiler MJ, Sagdullaev BT, McCall MA. Retinal transplants restore visually evoked responses in rats with photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 2001;42:1669–1676. [PubMed] [Google Scholar]
- Wu WC, Lai CC, Chen SL, Xiao X, Chen TL, Tsai RJ, Kuo SW, Tsao YP. Gene therapy for detached retina by adeno-associated virus vector expressing glial cell line-derived neurotrophic factor. Invest. Ophthalmol. Vis. Sci. 2002;43:3480–3488. [PubMed] [Google Scholar]
- Yan Q, Wang J, Matheson CR, Urich JL. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF) J. Neurobiol. 1999;38:382–390. doi: 10.1002/(sici)1097-4695(19990215)38:3<382::aid-neu7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]