Abstract
Polycomb group (PcG) proteins are key regulators in establishing a transcriptional repressive state. Polycomb Repressive Complex 2 (PRC2), one of the two major PcG protein complexes, is essential for proper differentiation and maintenance of cellular identity. Multiple factors are involved in recruiting PRC2 to its genomic targets. In this review we will discuss the role of DNA sequence, transcription factors, pre-existing histone modifications, and RNA in guiding PRC2 towards specific genomic loci. The DNA sequence itself influences the DNA methylation state, which is an important determinant of PRC2 recruitment. Other histone modifications are also important for PRC2 binding as PRC2 can respond to different cellular states via crosstalk between histone modifications. Additionally, PRC2 might be able to sense the transcriptional status of genes by binding to nascent RNA, which could also guide the complex to chromatin. In this review we will discuss how all these molecular aspects define a local chromatin state which controls accurate, cell-type specific epigenetic silencing by PRC2.
Keywords: Polycomb, epigenetics, chromatin, transcription regulation, crosstalk
1. Introduction: Role of Polycomb in development
The role of polycomb group (PcG) proteins as repressors of early developmental genes was first described in Drosophila melanogaster. PcG proteins were shown to control segmentation during early embryogenesis by maintaining temporal and spatial repression of Hox genes (Lewis 1978; Duncan 1982). In mouse, various knockout studies have demonstrated a similar role for PcG proteins in the maintenance of a repressive transcriptional state (reviewed in: Aloia et al. 2013; Signolet and Hendrich 2015). PcG proteins can form different multi-subunit protein complexes, of which Polycomb Repressive Complex 1 and 2 (PRC1 and PRC2) have been characterized most extensively (see Box 1). Both PRC complexes are histone modifiers. PRC2 catalyses mono-, di-, and trimethylation of histone H3 on lysine K27 (H3K27me1/2/3) by its subunit Ezh2, and PRC1 catalyses monoubiquitylation of histone H2A on lysine 119 (H2AK119ub1) by its subunit Ring1 (Czermin et al. 2002; Kuzmichev et al. 2002; Müller et al. 2002; de Napoles et al. 2004; Pengelly et al. 2013).
BOX 1. Polycomb complex compositions.
PcG proteins contribute to two major protein complexes: Polycomb Repressive Complex (PRC) 1 and PRC2. PRC1 has multiple complex compositions, each with its own properties as reviewed by (reviewed in: Turner & Bracken 2013; Di Croce & Helin 2013). There are two major PRC1 complexes, each containing different core subunits: (i) Cbx, Phc, Ring, and Pcgf, or (ii) Rybp, Ring, and Pcgf. Each of these subunits have different paralogs (Turner and Bracken 2013). The catalytic subunit of PRC1 can be either Ring1a or Ring1b, which monoubiquitylate histone H2A on lysine 119 (H2AK119) (de Napoles et al. 2004), however, their activity depends on the complex composition (Turner and Bracken 2013).
The core components of PRC2 are Enhancer of zeste (Ezh2), Embryonic ectoderm development (Eed), and Suppressor of zeste 12 (Suz12). These subunits exist as monomers in the complex in a 1:1:1 stoichiometry (Smits et al. 2013; Xu et al. 2015), and comprise the minimal composition necessary for catalytic activity of Ezh2, resulting in mono-, di-, or trimethylation of H3K27 (Cao and Zhang 2004; Pasini et al. 2004; Nekrasov et al. 2005). Non-core PRC2 proteins such as RbAp48/46, PCL1/2/3, AEBP2, Jarid2, c17orf96 and C10orf12 can be substoichiometrically present in the complex (Smits et al. 2013), and can increase the catalytic activity (e.g. RbAp46/48 and AEBP2) or the binding and targeting of PCR2 (e.g. Jarid2 and PCL) (reviewed in: Vizán et al. 2015).
Ezh2 is the only PRC2 core subunit known to have a paralog, namely Ezh1. Expression of Ehz2 and Ezh1 is dissimilar, and are found in complexes with distinct composition and function. Ezh2 generally forms a core together with both Eed and Suz12, whereas Ezh1 has been found alone or in a complex together with Suz12 (Xu et al. 2015). Although both molecules show a partial redundancy in catalytic activity and localization, Ezh2 is generally believed to deploy di-and tri-methylation of H3K27 on repressed genomic loci, whereas Ezh1 is more associated with monomethylation of H3K27 on regions with active transcription (Mousavi et al. 2012; Xu et al. 2015). During cell differentiation, the ratio between Ezh1 and Ezh2 containing PRC2 changes, with Ezh2 levels decreasing and Ezh1 levels increasing upon differentiation (Margueron et al. 2008; Mousavi et al. 2012; Xu et al. 2015). To date, most studies on PRC2 focused on the Ezh2 containing variant and its function in transcriptional silencing.
Post-translational modifications can regulate transcription, because they can function as a docking site or modulate the affinity of nuclear proteins (Musselman et al. 2012b). In this way PcG proteins can limit the accessibility of DNA for the transcription machinery by compacting chromatin (reviewed in: Di Croce & Helin, 2013; Schwartz & Pirrotta, 2013). Besides altering the accessibility of chromatin PcG proteins can as well mediate epigenetic repression by counteracting activating histone modifications (figure 1A, B). In contrast to PcG proteins some of the Trithorax Group (TrxG) proteins catalyse trimethylation of histone H3 on lysine K4 (H3K4me3) and lysine K36 (H3K36me3) at genes that are transcriptionally active. Various studies have highlighted that PcG proteins antagonize transcriptional activation by TrxG proteins (reviewed in: Steffen & Ringrose 2014). PcG proteins also counteract activating histone modifications at regulatory elements across the genome. Methylation of H3K27 prevents acetylation of this lysine (H3K27ac), a modification which is enriched at active enhancer regions (Ferrari et al. 2014).
Figure 1. Roles of PRC2.
The activity of PRC2 is different at functionally distinct genomic regions. A) PRC2 inhibits gene activation by trimethylation of H3K27 at transcription start sites (TSSs), which prevents Mll or Set1-mediated trimethylation of H3K4 at the TSS. B) Methylation of H3K27 by PRC2 on enhancers prevents activation by antagonizing acetylation of this substrate by p300. C) Upon transcription, monomethylation of H3K27 by PRC2 co-occurs with H3K36me3 deposition by Setd2.
These biochemical mechanisms via which PcG proteins mediate transcription silencing have been extensively studied. At the same time, how PRC complexes are directed to their genomic targets remains an important question. This review is focussed on the several aspects that affect the recruitment of PRC2 to its genomic targets: DNA sequence, transcription factors, pre-existing histone modifications, and RNA. First we will briefly summarize recent findings on polycomb mediated transcriptional regulation. After that we will discuss in more detail the recent findings on PRC2 recruitment.
2. Sequential polycomb action: a paradigm under pressure
Trimethylated H3K27 can serve as a docking site for PRC1 component PC (Cbx in mammals) (Cao et al. 2002). In the absence of enzymatically active PRC2, H3K27 cannot be trimethylated and PRC1 binding is lost (Cao et al. 2002; Wang et al. 2004; Boyer et al. 2006). These observations gave rise to the sequential or hierarchical model, which postulates that once PRC2 is recruited and trimethylates H3K27, PRC1 is recruited by virtue of the affinity of its Cbx subunit for this methylated residue. However, not all recent findings fit the classical sequential model, suggesting alternative mechanisms for the establishment of polycomb-mediated regulation of transcription.
The classical model predicts co-occurrence of PRC1 and PRC2 subunits on genomic loci, however, genome-wide profiling studies in embryonic stem cells (ESCs) showed that PRC1 and PRC2 proteins share only a subset of binding sites (Boyer et al. 2006; Ku et al. 2008; Blackledge et al. 2014). Early ChIP-on-chip assays in mouse ESCs indicated that merely 25% of all PcG enriched transcription start sites (TSS) were occupied by all four proteins that were profiled: PRC1 components Phc1 and Rnf2, and PRC2 components Eed and Suz12 (Boyer et al. 2006). More recently, ChIP-sequencing assays on Ring1b and Ezh2 binding showed that almost 90% of the Ring1b binding sites were also occupied by Ezh2, whereas only 50% of the Ezh2 binding sites bound Ring1b as well (Ku et al. 2008). A stronger, but still not perfect overlap for Ezh2 at Ring1b targets was found by Blackledge and colleagues. In their study Ring1b and Ezh2 shared about 80% of their targets (Blackledge et al. 2014). These findings show that PRC1 and PRC2 do not always bind the same regions, contrary to what may be expected on basis of the classical model of PRC2 and PRC1 action.
Independent functions and recruitment mechanisms for PRC1 and PRC2 have been identified. Genomic and proteomic analysis of PRC1 complexes identified six major groups, containing distinct subunits and differing in genomic binding, of which only a small subset co-localized with H3K27me3 (Gao et al. 2012). Furthermore, it is demonstrated that PRC1 recruitment is not solely dependent on H3K27me3, as it can still deposit H2AK119ub and repress gene transcription in PRC2-deficient mouse ESCs (Tavares et al. 2012). Although PRC2 can still be involved in recruiting PRC1 to shared binding sites, recent studies showed that PRC1 can also be involved in the recruitment of PRC2 (Blackledge et al. 2014; Cooper et al. 2014; Kalb et al. 2014). Knockdown of PRC1 not only resulted in a loss of H2AK119ub, but also in reduced PRC2 binding (Blackledge et al. 2014). The role of H2AK119ub in PRC2 recruitment will be further discussed in sections 3. and 5.2. of this review. These findings suggest that the order of events can be bidirectional rather than unidirectional as described in the classical model.
Another caveat in the classical model is that it only focusses on the H3K27 trimethylation by PRC2, even though PRC2 also catalyses mono- and dimethylation of H3K27 (Ferrari et al. 2014). In the past, genome-wide studies in murine ESCs identified PcG proteins and H3K27me3 in the vicinity of the transcription start site (TSS, figure 1A) of genes, many of which encode transcription factors with important functions in development (Bernstein et al. 2006; Boyer et al. 2006). More recently, Ferrari and colleagues characterized the distribution of H3K27me1 and H3K27me2 in mouse ESCs, and found them to be located at functionally distinct genomic regions. H3K27me1 is mainly enriched in the bodies of actively transcribed genes (figure 1C), whereas H3K27me2 was broadly distributed throughout the genome, covering approximately 70% of all histones. Genes and enhancers covered with H3K27me2 were deprived of marks associated with genomic activation, and associated with low expression levels (Ferrari et al. 2014).
However, H3K27me2 is not highly abundant throughout Xenopus development. Mass spectrometry (MS) based analysis showed that H3K27me2 levels rose from 3% in blastula stage to 15% in tadpoles (Schneider et al. 2011). Furthermore, culture conditions might influence dimethylation levels. When ESCs are cultured in 2i medium instead of serum, trimethylation levels of H3K27 reduce dramatically (Marks et al. 2012). However, even if H3K27me2 is not generally distributed throughout the whole genome PRC2 can also counteract acetylation of H3K27 at enhancers by trimethylation (Pinello et al. 2014; Abou El Hassan et al. 2015).
The picture that now emerges constitutes complementing biochemical PRC1 and PRC2 activities, but also shows previously unknown roles in the regulation of transcription. In the next paragraphs we will discuss the molecular determinants involved in recruiting PRC2 to its genomic targets.
3. Sequence context of PRC2 action: Genetic prerogative or epigenetic consequence?
CpG dinucleotide density and its methylation status are good predictors of mammalian PRC2 recruitment. Analysing the DNA underlying PRC2-bound loci for sequence features in mammals revealed an enriched representation of CpG dense regions (Lee et al. 2006). CpG richness is a feature that is also found at the TSS of genes marked by H3K4me3 (Bernstein et al. 2006). Indeed, insertion of CpG-rich elements was sufficient for the recruitment of PRC2 and deposition of H3K27me3, as well as H3K4me3, to exogenous loci in mouse ESCs (Mendenhall et al. 2010). Vice versa, a comparative study of mouse and human ESCs showed that loss of CpG-rich elements resulted in loss of H3K27me3 deposition at these regions (Lynch et al. 2012).
CpG dinucleotides can be subjected to methylation, which prevents them from binding PRC2 (Bartke et al. 2010). Mass spectrometry (MS) based analysis showed that incorporation of methylated CpG DNA in nucleosomes antagonized the binding of PRC2 subunit Eed (Bartke et al. 2010). Indeed, mutual exclusion of CpG-island (CGI) methylation and H3K27me3 deposition was demonstrated in vertebrate genomes (Bogdanovic et al. 2011; Lynch et al. 2012). At loci with low CpG dinucleotide density, however, DNA methylation and H3K27me3 were found to co-occur (Brinkman et al. 2012). Not only CpG density, but also G+C richness is a property of methylation-free regions. Deposition of either H3K4me3 or H3K27me3 is the default chromatin state at these loci, as was shown by integration of artificial CGI-like DNA sequences into the genome of ESCs (Wachter et al. 2014). CpG-richness at promoters is particularly prevalent in mammals. In non-mammalian vertebrates relatively few CpG dinucleotides overlap with gene promoters. Even so, promoters in non-mammalian vertebrates contain non-methylated clusters of CpGs, called non-methylated islands (NMI), which are highly conserved across species (Long et al. 2013b). In Xenopus embryos, trimethylation of either H3K27 or H3K4 is closely associated with the presence of NMIs (van Heeringen et al. 2014). During gastrulation H3K27 trimethylation is acquired in pre-existing hypomethylated regions in Xenopus. These studies show conserved PRC2 recruitment to hypomethylated regions in vertebrates.
DNA binding proteins that direct PRC2 toward NMIs might operate via PRC1 (Farcas et al. 2012). Unmethylated CxxC domains can be recognized by Zinc finger (ZF)-CxxC domain proteins, such as KDM2B (Long et al. 2013a). Affinity purification of KDM2B from ESCs followed by MS revealed that it forms a complex with the PCR1 subunit Ring1b. Recruitment of KDM2B to promoters leads to H2AK119ub deposition, followed by PRC2 binding and H3K27me3-mediated silencing (Farcas et al. 2012). Removal of the ZF-CxxC domain of KDM2B resulted in loss of Ring1b binding at roughly half of the Ring1b binding sites in mouse ESCs. In addition, KDM2B binding sites showed reduced levels of ubiquitinated H2AK119 and Suz12 recruitment in KDM2B deficient cells. Targeted KDM2B binding induced local enrichment of Ring1b, H2AK119ub, Ezh2, and H3K27me3, independent of its demethylase activity. Hence, KDM2B mediates PCR1 recruitment to NMIs, and is required for PRC2-catalyzed trimethylation of H3K27 at these loci (Blackledge et al. 2014). PRC1-independent recruitment of PRC2 to unmethylated DNA might also occur via PRC2-accessory proteins with DNA binding capacity, such as Jarid2. Jarid2 was shown to co-occur with PRC2 genome-wide, and motif analysis in ESCs showed that Jarid2-PRC2 bound loci were enriched for both CCG-repeats and GA-rich regions (Peng et al. 2009).
Computational analyses to identify sequences that recruit PRC2 suggest a central role for NMIs (figure 2). A Support Vector Machine trained on a subset of sequences underlying H3K27me3 domains, accurately predicted H3K27me3 status of unknown sequences in a cross-species analysis in frog, zebrafish, and human, CpG-density differences between mammals and other vertebrates notwithstanding (van Heeringen et al. 2014). This pan-vertebrate sequence conservation within NMIs suggests that additional genetic factors determine when and where NMIs become marked by H3K27me3 or by H3K4me3. The next section will further discuss the role of specific sequence properties and transcription factor (TF) binding sites in PRC2 recruitment.
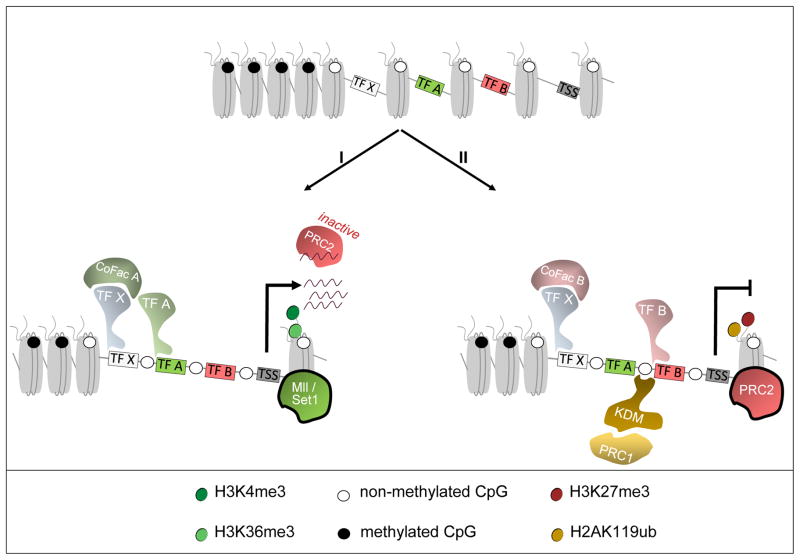
Figure 2. Sequence context of PRC2 action.
Non methylated Islands (NMIs) are susceptible for gene activation by TrxG proteins (for example Mll, Set1) or repression by PcG proteins. (I) Mll catalyses H3K4me3 in the presence of transcription factors (TF) that facilitate binding of Mll, creating a permissive state for transcription. PRC2 might recognize these actively transcribed regions by binding nascent RNA, but is antagonized by Mll. (II) In the absence of transcription activating factors, PRC2 can bind at NMIs via positioning by TFs or their cofactors (CoFac). Zinc Finger-CxxC domain proteins (KDM) that bind PRC1, can also stimulate PRC2 recruitment by providing a docking site, H2AK119ub.
4. Interplay of transcription factor binding and PRC2 recruitment
4.1. PcG response elements in Drosophila
The first evidence for motif-specific PRC2 recruitment was found in Drosophila. Within the Bithorax complex, a cluster of three homeotic genes which are important in segmental development, specific DNA regulatory elements to which PcG proteins are recruited were identified (Simon et al. 1993). Insertion of these PcG response elements (PREs) in a reporter plasmid resulted in repression of transcription in a PcG-dependent manner (Simon et al. 1993). The first sequence-specific DNA-binding protein that was shown to mediate PcG recruitment to PREs was Pleiohomeotic (Pho). Pho was shown to bind a 17 bp sequence located within a 176 bp fragment located upstream of the engrailed locus, which was previously linked to PcG mediated silencing in transgenic flies. This 17 bp PRE was highly conserved and essential, but not sufficient for the PcG mediated silencing (Brown et al. 1998). Following this discovery multiple more PREs were found in Drosophila and these PREs contained binding motifs for various TFs (like Gaga, Pho, and Zeste binding motifs) (reviewed in: Kassis and Brown 2013).
Locations of PREs throughout the genome were computationally predicted based on diverse TF binding motifs that were enriched in experimentally confirmed PREs (Ringrose et al. 2003). However, two independent genome-wide assays proved that PRC2 and PRC1 bind to some, but not the majority of these predicted PREs in Drosophila (Schwartz et al. 2006; Tolhuis et al. 2006). Genome-wide studies that characterized the binding sites of various sequence-specific DNA-binding proteins have shown co-occupancy of multiple TFs, suggesting a cooperative recruitment of PcG components in Drosophila. However, many of the putative PcG recruiters (TFs like Pho and Gaga) were not solely enriched at PcG binding sites, but also at the H3K4me3-associated TrxG binding sites (Schuettengruber et al. 2009). These results imply that different factors work together to recruit PcG proteins or that these TFs have another function besides PcG repression.
Recently, a study on the function and evolution of PREs shed new light on the functionality, specificity, and cooperativity of PcG recruiters (Schuettengruber et al. 2014). Comparing H3K27 methylation, PH (PRC1) binding, and DNA sequence in five different Drosophila species showed that, despite variations in the underlying sequence, PcG domains were highly conserved in syntenic regions. Unexpectedly, not the DNA sequence, but the TF binding itself was highly conserved, with both Pho and Dorsal Switch Protein (Dsp1) binding to low specificity sites at the PcG domains. Cooperative binding sites for Pho and Dsp1 showed the highest overlap with PcG domains, and prediction of Pho binding was more accurate as a function of PH binding and Pho motifs, compared to TF motifs alone. This suggests a bidirectional interaction between PcG proteins and other proteins, stabilizing the PcG domains (Schuettengruber et al. 2014).
4.2. PcG and transcription factor motifs in vertebrates
PRE-like mechanisms of PRC2 recruitment have been elusive in vertebrates as no clear ortholog to any of the Drosophila PRC2-recruiting factors has been found. However, a variety of TFs influence PRC2 recruitment in vertebrates. The first H3K27me3 and PcG profiling studies in ESCs already suggested a possible relation between PcG proteins and TFs, based on the co-localization of PcG components with pluripotency factors Oct4, Sox2 and Nanog (Bernstein et al. 2006; Boyer et al. 2006; Lee et al. 2006). More recent studies suggest that the correlation between DNA sequence and histone modifications might be the result of TF-mediated recruitment of histone modifiers (figure 2) (Benveniste et al. 2014). Analyses of TF binding from genome-wide profiling studies in H1 cells, K562 cells, and GM12878 cells demonstrated that TF binding more accurately predicted the presence of H3K4me1, H3K4me3, H3K9ac, H3K27ac or H3K27me3 at promoters and enhancers, compared to the DNA sequence itself. This indicates that TFs might form a link between specific DNA sequences and the histone modifiers (Benveniste et al. 2014).
Conversely, deletion of motifs for transcription activators from NMIs was found to be sufficient for PRC2 recruitment and H3K27me3 deposition in ESCs (Mendenhall et al. 2010). Minimal DNA sequence elements capable of autonomously recruiting PRC2 were recently defined by using iterative genome editing in mouse ESCs. This demonstrated the influence of surrounding sequences on PRC2 recruitment, as an active enhancer-promoter sequence surrounding CG-rich sequences was shown to prevent PRC2 recruitment and trimethylation of H3K27 at these loci (Jermann et al. 2014). Jermann and colleagues proposed that CGIs bind PRC2 by default, provided that they are devoid of DNA methylation and are not transcriptionally active. Inhibition of RNA polymerase II was indeed sufficient to obtain Suz12 binding and trimethylation of H3K27me3 in mouse ESCs (Riising et al. 2014). Sites with increased H3K27me3 upon transcriptional inhibition were found to be ectopic CpG targets in other, differentiated tissues. A genetic-default model for PRC2 action was also suggested by Van Heeringen and colleagues, based on the observation that the pan-vertebrate conserved DNA sequence signatures of H3K27me3 are linked to a propensity for H3K27me3 across different cell types. This suggests that methylation of H3K27 is default at these regions and is actively prevented by cell type-specific factors (van Heeringen et al. 2014).
Besides the absence of particular transcription activators, PRC2 recruitment correlates also with the presence of specific TF motifs. Distinctive motif contributions were identified when comparing Ezh2-positive and -negative NMIs in ESCs. Ezh2-negative NMIs were marked by H3K4me3, and showed strong enrichment for motifs of transcriptional activators like NFY, Myc, and Ets1. In contrast, Ezh2-positive NMIs were mostly H3K27me3 enriched, and were associated with motifs for TFs that are known to be expressed in ESCs: NESF/REST, Cux1, and NFκB (Ku et al. 2008). In Xenopus, NMIs that gain H3K4me3 are enriched for motifs that bind housekeeping TFs. NMIs that gain H3K27me3, on the other hand, generally contain motifs for developmental regulators, like Sox and homeobox TFs (van Heeringen et al. 2014). Binding sites that were predicted to recruit PcG components in motif analyses, such as for Rest and Runx1, induced ectopic H3K27 methylation. Furthermore, their respective TFs were shown to physically interact with PcG proteins (Dietrich et al. 2012; Yu et al. 2012; Arnold et al. 2013). For example, regions that obtained H3K27me3 during neurogenesis were enriched for a specific set of motifs, among which binding sites for Rest and Snail. Insertion of Rest and Snail motifs was sufficient to ectopically induce H3K27 methylation in mouse ESCs (Arnold et al. 2013). More recently, a study in Xenopus showed that Snail2 cooperates with PRC2 via Ezh2 binding, which is important in modulating the expression of neural crest genes. Co-occupancy of Snail2 and Ezh2 was shown to be important for maintenance of H3K27me3 levels and expansion of the neural crest domain (Tien et al. 2015).
However, TFs can also be involved in both transcriptional activation or repression depending on the environmental context, which comprises CpG density and available co-factors (Arnold et al. 2013; Pinello et al. 2014). For example, Rest binding during neurogenesis was shown to increase trimethylation of H3K27 at CpG-rich loci, but to decrease trimethylation of H3K27 at CpG poor loci upon differentiation (Arnold et al. 2013). Environmental effects could also be a result of differential co-factor binding, which has been suggested to contribute to cell type-specific PcG recruitment (figure 2). A recent analysis of H3K27me3 profiles in 19 different cell lines identified regions with variable H3K27me3 deposition across cell-lines, so called high plasticity regions (HPRs). HPRs were found at both CGIs surrounding TSSs as well as distal elements. Motif analysis yielded 41 cell-type specific associations between TF motifs and distal HPRs. Genome-wide binding profiles showed that binding of these TFs was indeed enriched at HPRs. Tal1 binding correlated with HPRs in primary human erythroid progenitor cells, however, its capacity to recruit PRC2 was found to be determined by co-factor binding, rather than Tal1 binding itself. Inactive, H3K27me3 marked enhancers were generally occupied by Tal1-GFI1B, whereas Tal1-Gata1 was found at active, H3K27ac marked enhancers (Pinello et al. 2014).
These studies highlight the complex relationships between the binding of sequence-specific activators and repressors and the recruitment of PRC2 but fall short of establishing that PRE-like mechanisms of PRC2 recruitment also exist in vertebrates. TFs and cofactors can be used to separate NMIs targeted for transcription activation or repression. In addition to DNA binding factors, pre-existing histone modifications and chromatin structure are also important factors in proper PRC2 targeting, as is discussed in the next section.
5. Responsive PRC2 binding: management by modified histones
5.1. Nucleosome density
Chromatin structure can direct PRC2 binding in two ways, namely by nucleosome density and by crosstalk with histone modifications (figure 3). Binding sites for PcG and TrxG proteins have a relatively high histone replacement rate and a low nucleosome occupancy, as was shown at the homeotic gene clusters in fly (Mito et al. 2007). Contradictionary, PRC2 binding and activity was increased when comparing dinucleosomes with mononucleosomes (Martin et al. 2006). Despite the relatively high histone replacement rate for PcG proteins in fly, nucleosome turnover rate is higher in regions occupied by TrxG proteins compared to regions bound by PcG proteins (Deal et al. 2010).
Figure 3. PRC2 guidance by modified histones.
A) Multiple posttranslational modifications stimulate the recruitment of PRC2. PRC2 can bind to H3K27me3 and H2AK119ub. Binding to these marks or to trimethylated Jarid2-K119 stimulates its activity. On heterochromatic regions, PRC2 binding to H3K27me3 and HP1 binding to H3K9me3 cooperate to facilitate formation and maintenance of heterochromatic state. B) Histone modifications that inactivate PRC2 are H3K27ac, H3K4me3 and H3K36me3. These modifications inactivate PRC2 when they are located on the same histone tail as where the complex is located. H1K26me3 inactivates PRC2 after binding the complex. When H3S28ph is positioned next to H3K27me3, Ezh2 is repelled and exchanged for Ezh1.
Despite the diminished nucleosome density at CGIs prior to PRC2 recruitment, nucleosome compaction seems to increase at these loci just before PRC2 binding (Yuan et al. 2012). Yuan and colleagues tested whether the density of the substrate chromatin could regulate PRC2. They found that preventing transcription activation for the gene AYP26a1 in mouse ESCs by withdrawal of retinoic acid resulted in increased nucleosome density prior to H3K27me3 deposition (Yuan et al. 2012). CGIs that became PRC2 targets upon transcription inhibition in mouse ESCs, also showed lower nucleosome density prior to PRC2 binding, compared to CGIs that did not recruit PRC2 (Riising et al. 2014). Recently Tee and colleagues described how altering the chromatin accessibility upon Erk1/2 binding can stimulate PRC2 recruitment in ESCs (Tee et al. 2014). These studies indicate that PcG targets have a relatively low nucleosome density, which already becomes denser just before binding of the complex.
5.2. Stimulating PRC2 binding
Pre-existing histone modifications such as H3K27me3, H2AK119ub, and H3K9me3 can facilitate PRC2 recruitment (figure 3A). These epigenetic marks are partially transmitted during cell proliferation, and reconstituted by means of positive feedback. For example, PRC2 was shown to bind to its own catalytic product, H3K27me3, by the aromatic cage of Eed (Margueron et al. 2009; Xu et al. 2010). Eed was shown to recognize trimethylated histone peptides, with a particularly high affinity for H3K27me3, H1K26me3, and H3K9me3 (Xu et al. 2010). Furthermore, Eed binding to H3K27me3 results in allosteric activation of the complex and propagation of the mark, as was shown in vitro and in Drosophila (Margueron et al. 2009; Xu et al. 2010). In the absence of pre-existing H3K27me3, methylated Jarid2 was suggested to facilitate PRC2 recruitment. Interestingly, methylation of Jarid2 at lysine K116 is mediated by PRC2 itself. Jarid2-K116me3 is recognized by Eed, which in turn triggers an allosteric activation of PRC2’s enzymatic activity. Jarid2-K116me3, but not unmethylated Jarid2, was found to have a higher affinity for Eed compared to H3K27me3. Knockdown of Jarid2, or introduction of a methylation-deficient Jarid2 had no consequences for ESCs, but caused disturbed H3K27me3 patterns in differentiated embryoid bodies. This suggests that pre-existing H3K27me3 accounts for the maintenance of H3K27me3 during cell division, whereas the nucleation of new domains during cell differentiation is dependent on Jarid2-K116me3 (Sanulli et al. 2015).
H3K27me3 can also serve as a docking site for PRC1 component Cbx (Cao et al. 2002; Wang et al. 2004; Boyer et al. 2006). The Ring1 subunit of PRC1 can catalyse H2AK119 ubiquitylation (de Napoles et al. 2004), which in turn can serve as a docking site for PRC2 (Blackledge et al. 2014; Cooper et al. 2014; Kalb et al. 2014). PRC2 components were strongly enriched in affinity pull downs with either H2AK118ub or H2AK119ub using Drosophila or mouse ESC nuclear extracts, respectively. These studies demonstrate that ubiquitinated H2A serves as a binding site for Jarid2–Aebp2–containing PRC2 and promotes H3K27 trimethylation (Kalb et al. 2014). Binding of a MBD-Ring1b/Pcgf4 fusion protein to densely CpG methylated DNA resulted in H2AK119ub deposition in mouse. This was sufficient to establish H3K27me3 at paternal pericentric heterochromatin (PCH) domains (Cooper et al. 2014). In a separate study, Tet-repressor fusion proteins were used to recruit PRC1 to a Tet-operator site that was introduced in the mouse genome. The Tet-repressor was fused to Pcgf 1, 2, 3, 4, or 5, which are known to be present in different PRC1 complexes. Although Ring1b was recruited with every Pcgf fusion variant, profound ubiquitylation of H2AK119 only occurred in the presence of Pcgf1, 3, and 5. Fusion proteins that could mediate H2AK119ub enrichment, also recruited catalytically active PRC2 to the site (Blackledge et al. 2014). These studies suggest that PRC2 and PRC1 positively influence each other’s recruitment.
Methylated H3K9 is also associated with recruitment of PRC2. Proteome analysis in mouse ESCs uncovered that H3K9me3 and H3K27me3 are rarely found on the same peptide, but do co-occur in an asymmetric composition on different histone H3 tails (Voigt et al. 2012; Sidoli et al. 2014). Eed has strong affinity for H3K9me3, however, in vitro methylation assays showed that the binding of PRC2 to H3K9me3 substrates does not change the methyltransferase activity of Ezh2 (Xu et al. 2010). In HeLa and mouse ES cells, PRC2 and H3K9 methyltransferase G9a/GLP were shown to have a physical interaction, and genome-wide profiling of G9a/GLP binding revealed 25% overlap with PRC2 loci. H3K27me3 methylation at these shared binding sites was decreased in G9a and/or GLP deficient cells, independent of the derepession of these targets. Binding of G9a, but not of a G9a catalytically dead mutant, to an artificial docking site resulted in Ezh2 recruitment and trimethylation of H3K27. In addition, disturbed Ezh2 binding in G9a mutants ESCs could be rescued by wild type G9a, but not by a G9a catalytically inactive protein (Mozzetta et al. 2014).
Another way by which methylation of H3K9 recruits PRC2 is via the structural adaptor protein HP1 (Boros et al. 2014). In a pulldown experiment with H3 tail peptides methylated at H3K9 and/or H3K27, H3K27me3 was found to increase H3K9me3 dependent HP1 binding. Knockdown of Ezh2 in human fibrosarcoma cells caused proteasomal degradation of HP1, and overexpression of H3K27me2/3 demethylase resulted in removal of HP1 from chromatin, both independent of changes for H3K9me3. Hence PRC2 and H3K27me3 cooperate with H3K9me3 to facilitate heterochromatin formation and maintenance, by stabilizing HP1 binding (Boros et al. 2014).
5.3. PRC2 blockers
Histone modifications associated with transcription activation, such as H1K26me3, H3K27ac, H3S28ph, H3K36me3, and H3K4me3, are thought to inhibit PRC2 recruitment (figure 3B). PRC2 can be diverted from its target sites, via docking of the complex to H1K26me3 substrates. H1K26me3 competes with H3K27me3 and H3K9me3 for binding in the aromatic cage of Eed. Docking to trimethylated H1K26, however, decreases the enzymatic activity of PRC2 (Xu et al. 2010).
Acetylation of H3K27 and methylation of the same residue are mutually exclusive but the two modifications could occur at separate histone H3 tails within the same nucleosome. However, H3K27me2/3 containing nucleosomes that also contain H3K27ac could hardly be detected by MS on mononucleosomes from mouse ESCs, mouse embryonic fibroblasts, and HeLa cells (Voigt et al. 2012). Genome-wide profiling in Drosophila embryos and mouse ESCs revealed that acetylation and methylation of H3K27 are inversely related; H3K27me3 was found to increase at loci where H3K27ac was decreased, and vice versa (Tie et al. 2009; Pasini et al. 2010). It was shown in mouse ESCs that NuRD-dependent deacetylation of H3K27 indeed led to recruitment of catalytically active PRC2 (Reynolds et al. 2011). In Drosophila embryos several histone modifying enzymes are in proximity to nascent DNA already 5 minutes after replication, including the ortholog of Ezh2 (E(z)), the H3K27 acetyltransferase CPB, and H3K27 demethylase UTX. Acetylation of H3K27 was achieved within 10 minutes after replication. In contrast, H3K27me3 could not be detected until one hour after replication (Petruk et al. 2013). The balance between acetylation and methylation of H3K27 changed upon treatment with inhibitors for CPB or UTX, showing trimethylation of H3K27 15 minutes after replication, together with a decreased acetylation of H3K27. This suggests that acetylation and demethylation of H3K27 are important to prevent aberrant deposition and accumulation of H3K27me3 (Petruk et al. 2013).
Acetylation of H3K27 might be facilitated by phosphorylation of the flanking serine residue S28. Targeting the H3S28 phosphatase Msk1 to the endogenous promoter of α-globulin in HEK293 cells resulted in transcription activation of the gene. At the α-globulin promoter both H3S28Ph and H3K27ac levels were increased and present on the same histone tail, while H3K27me3 levels were decreased (Lau and Cheung 2011). In HeLa cells stress activation led to increased phosphorylation of H3S28 on histone tails that were also trimethylated on H3K27, resulting in decreased binding of Cbx8 and Suz12 (Gehani et al. 2010). A separate study on PRC2 binding at the myogenin promoter during skeletal muscle cell differentiation showed that increased Msk1 and H3S28ph binding during transcriptional activation resulted in displacement of Ezh2, but not Ezh1, at the promoter (see Box 1) (Stojic et al. 2011). Similar results were obtained in affinity-purification experiments from extracts of differentiated myotubes using histone H3 tail peptides that were unmodified, or modified with K27me3 or K27me3/S28ph. Ezh1 bound with comparable affinity to both K27me3 and K27me3/S28ph modified peptides, whereas Ezh2 binding was significantly weakened in the presence of S28ph (Stojic et al. 2011).
In the fly, trimethylated H3K4 and H3K36, catalysed by Trx and Ash respectively (Mll and Setd2 in mammals), antagonize PcG-mediated silencing. Affinity assays showed that the binding of Su(z)12 in complex with Nurf55 (Suz12 and Rbbp4/RbAp48, Rbbp7/RbAp46 in mammals) to H3 peptides could significantly be reduced if the H3 peptides were methylated on lysine K4. In absence of Nurf55, H3-Su(z)12 binding was not affected, however, H3K4me3 and H3K36me3 did inhibit the catalytic activity of PRC2. Inhibition of di- and trimethylation by PRC2 was observed on H3 tails also trimethylated on K4 or K36, but not when these modifications were present on separate peptides (Schmitges et al. 2011). Though, in vivo trimethylation of H3K4 and H3K36 is rarely detected on H3 tails that are also tri-methylated for H3K27 (Sidoli et al. 2014; Yuan et al. 2011).
However, co-occurrence of H3K27me3 and H3K4me3 on different H3 tails in the same nucleosome has been reported (Voigt et al. 2012). MS on H3K4me3- containing mononucleosomes showed the presence of H3K27me3 and H3K4me3 within the same nucleosome, which was higher in mouse ESCs (approximately 15% of H3K4me3-containing nucleosomes) compared to mouse embryonic fibroblasts (Voigt et al. 2012). In Drosophila and Xenopus, significant co-occurrence of H3K27me3 and H3K4me3 within the same nucleosomal DNA population could not be detected (Akkers et al. 2009; Schuettengruber et al. 2009; Gan et al. 2010). In addition, when ESCs were cultured in 2i medium instead of serum, trimethylation levels of H3K27, and consequently the H3K27me3/K4me3 bivalent state, reduced dramatically (Marks et al. 2012). However, various studies showed that PRC2 can be recruited to actively transcribed genes via Polycomb-like (PCL) proteins which can bind to H3K36me3 (Ballaré et al. 2012; Musselman et al. 2012a; Cai et al. 2013). PCL protein Phf19 not only interacts with PRC2 but also interacts with H3K36me3 demethylase NO66; therefore, PCL proteins might recruit PRC2 to set up repression (Brien et al. 2012).
6. RNA regulated recruitment
Despite the repressive effect of H3K36me3 and H3K4me3 on PcG mediated silencing, PRC2 recruitment has also been positively associated with active transcription. Highly expressed genes showed monomethylated H3K27, which was dependent on H3K36me3, whereas lowly expressed genes accumulated dimethylation at H3K27 throughout the gene bodies (Ferrari et al. 2014). Knockdown of H3K36 methyltransferase Setd2 resulted in a loss of both H3K36me3 and H3K27me1, in addition to accumulation of H3K27me2 at these intergenic regions. Loss of PCR2 reduced accumulation of both H3K27me1 and H3K27me2, but not of H3K36me3. Furthermore, Eed deletion led to transcriptional upregulation of H3K27me2-marked genes and downregulation of H3K27me1-marked genes. MS data on H3K36me3 purified histones confirmed the presence of both K27me1 and K36me3 on the same H3 peptide (Ferrari et al. 2014). These results indicate that the methylation state of H3K36 regulates PRC2 action and subsequently determines methylation of H3K27.
These results suggest a role for PRC2 in actively transcribed genes, even though the presence of stable PRC2-binding could not be detected at these regions. One way by which PRC2 could be recruited to active genes is through interaction with RNA molecules. Multiple studies have reported binding of specific RNAs to PRC2, including non-coding (nc, lnc) RNAs such as Xist repA ncRNA in X-chromosome silencing (Zhao et al. 2008; da Rocha et al. 2014), and HOTAIR ncRNA in silencing of hox genes in human (Rinn et al. 2007; Tsai et al. 2010). In addition, lncRNAs were recently shown to function as scaffolds, stabilizing the binding between various PRC2 subunits such as Ezh2 and Jarid2 (Kaneko et al. 2014a).
In addition to sequence specific RNA-binding, PRC2 was also reported to bind RNA molecules in a nonselective manner. RNA immunoprecipitation in ESCs showed PRC2 to associate with thousands of different RNA molecules (Zhao et al. 2010; Kaneko et al. 2013). Quantitative electrophoretic mobility shift assays (EMSA) of reconstituted human PRC2 with various RNA molecules revealed that PCR2 binding is size-dependent rather than sequence-dependent, with lower affinity for shorter RNA molecules (Davidovich et al. 2013). The majority of the PRC2-bound RNA sequences corresponded to the 5′-regions of genes that were transcriptionally active. ChIP-sequencing data from various mouse cell lines revealed that the genes belonging to these PRC2 bound-RNAs were positively associated with Ezh2 recruitment and trimethylation of H3K4 and H3K36, but were depleted of H3K27me3 (Davidovich et al. 2013; Kaneko et al. 2013). Interestingly, H3K27me3 on Ezh2-RNA genes was more pronounced in differentiated mouse embryonic fibroblasts, as compared to pluripotent ESCs (Kaneko et al. 2013). RNA binding was shown to suppress the histone methyltransferase activity of Ezh2, although the RNA binding affinity of Ezh2 was reduced when bound to other PRC2 subunits (Cifuentes-Rojas et al. 2014). Di- and trimethylation of H3K27 on Ezh2-RNA genes could be induced by CRISPR-mediated truncation of the 5′-end these genes (Kaneko et al. 2014b). Together, these studies support a model in which PCR2 uses RNA binding to scan the genome, sensing the transcriptional activity of genes and deploying or redistributing the complex accordingly (figure 2B).
7. Conclusion and perspective
A growing body of evidence indicates that RNA transcripts, pre-existing histone modifications, and transcription factors together define a local chromatin state which controls accurate, cell-type specific epigenetic silencing by PRC2. Genetic sequence sets the fate for potential PRC2 targets, but the timing of stable PRC2-binding at these loci is influenced by TFs. Forming complexes with the different Ezh paralogs can result in different outcomes with respect to PRC2’s function in transcription regulation. This suggests that lineage-specific TFs are involved in determining the transcriptional output of potential PRC2 targets by modulating both the complex composition and the recruitment of the complex. Exactly which TFs are involved in regulating the expression of PcG target genes and in guiding of PcG proteins towards their targets remains one of the key questions to be addressed. Further studies are needed to uncover how TFs and their cofactors influence PRC2 regulation.
PRC2 also senses pre-existing histone modifications and binds to nascent RNA molecules, so that the complex can respond appropriately to different cellular states. The exact order of molecular events that specify these cellular states and their interplay remain to be elucidated. Resolving these molecular mechanisms will be both important and rewarding, as PcG mediated transcriptional repression is essential for maintenance of cellular identity.
Acknowledgments
This work has been supported by the US National Institutes of Health (NICHD, grant R01HD069344).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou El, Hassan M, Yu T, Song L, Bremner R. Polycomb Repressive Complex 2 Confers BRG1 Dependency on the CIITA Locus. J Immunol. 2015 Apr 10; doi: 10.4049/jimmunol.1403247. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25862816. [DOI] [PubMed]
- Akkers RC, van Heeringen SJ, Jacobi UG, Janssen-Megens EM, Françoijs K-J, Stunnenberg HG, et al. A hierarchy of H3K4me3 and H3K27me3 acquisition in spatial gene regulation in Xenopus embryos. Dev Cell. 2009 Sep;17(3):425–34. doi: 10.1016/j.devcel.2009.08.005. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2746918&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia L, Di Stefano B, Di Croce L. Polycomb complexes in stem cells and embryonic development. Development. 2013 Jun 15;140(12):2525–34. doi: 10.1242/dev.091553. Available from: http://dev.biologists.org/content/140/12/2525.long. [DOI] [PubMed] [Google Scholar]
- Arnold P, Schöler A, Pachkov M, Balwierz PJ, Jørgensen H, Stadler MB, et al. Modeling of epigenome dynamics identifies transcription factors that mediate Polycomb targeting. Genome Res. 2013 Jan 1;23(1):60–73. doi: 10.1101/gr.142661.112. Available from: http://genome.cshlp.org/content/23/1/60.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré C, Lange M, Lapinaite A, Martin GM, Morey L, Pascual G, et al. Phf19 links methylated Lys36 of histone H3 to regulation of Polycomb activity. Nat Struct Mol Biol. 2012 Dec;19(12):1257–65. doi: 10.1038/nsmb.2434. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3926938&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell. 2010 Oct 29;143(3):470–84. doi: 10.1016/j.cell.2010.10.012. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3640253&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste D, Sonntag H-J, Sanguinetti G, Sproul D. Transcription factor binding predicts histone modifications in human cell lines. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13367–72. doi: 10.1073/pnas.1412081111. Available from: http://apps.webofknowledge.com.proxy.ubn.ru.nl/full_record.do?product=WOS&search_mode=CitingArticles&qid=4&SID=2DtEWwFDZEe9GqSoB63&page=1&doc=1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Blackledge NP, Farcas AM, Kondo T, King HW, McGouran JF, Hanssen LLP, et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014 Jun 5;157(6):1445–59. doi: 10.1016/j.cell.2014.05.004. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4048464&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic O, Long SW, van Heeringen SJ, Brinkman AB, Gómez-Skarmeta JL, Stunnenberg HG, et al. Temporal uncoupling of the DNA methylome and transcriptional repression during embryogenesis. Genome Res. 2011 Aug 1;21(8):1313–27. doi: 10.1101/gr.114843.110. Available from: http://genome.cshlp.org/content/21/8/1313.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros J, Arnoult N, Stroobant V, Collet J-F, Decottignies A. Polycomb repressive complex 2 and H3K27me3 cooperate with H3K9 methylation to maintain heterochromatin protein 1α at chromatin. Mol Cell Biol. 2014 Oct 1;34(19):3662–74. doi: 10.1128/MCB.00205-14. Available from: http://mcb.asm.org.proxy.ubn.ru.nl/content/34/19/3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006 May 18;441(7091):349–53. doi: 10.1038/nature04733. Available from: http://www.nature.com.proxy.ubn.ru.nl/nature/journal/v441/n7091/full/nature04733.html. [DOI] [PubMed] [Google Scholar]
- Brien GL, Gambero G, O’Connell DJ, Jerman E, Turner SA, Egan CM, et al. Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation. Nat Struct Mol Biol. 2012 Dec;19(12):1273–81. doi: 10.1038/nsmb.2449. Available from: http://dx.doi.org/10.1038/nsmb.2449. [DOI] [PubMed] [Google Scholar]
- Brinkman AB, Gu H, Bartels SJJ, Zhang Y, Matarese F, Simmer F, et al. Sequential ChIP-bisulfite sequencing enables direct genome-scale investigation of chromatin and DNA methylation cross-talk. Genome Res. 2012 Jun;22(6):1128–38. doi: 10.1101/gr.133728.111. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3371717&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Mucci D, Whiteley M, Dirksen M-L, Kassis JA. The Drosophila Polycomb Group Gene pleiohomeotic Encodes a DNA Binding Protein with Homology to the Transcription Factor YY1. Mol Cell. 1998 Jun;1(7):1057–64. doi: 10.1016/s1097-2765(00)80106-9. Available from: http://www.sciencedirect.com/science/article/pii/S1097276500801069. [DOI] [PubMed] [Google Scholar]
- Cai L, Rothbart SB, Lu R, Xu B, Chen W-Y, Tripathy A, et al. An H3K36 methylation-engaging Tudor motif of polycomb-like proteins mediates PRC2 complex targeting. Mol Cell. 2013 Mar 7;49(3):571–82. doi: 10.1016/j.molcel.2012.11.026. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3570589&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of Histone H3 Lysine 27 Methylation in Polycomb-Group Silencing. Science. 2002 Nov;1(5595):298. doi: 10.1126/science.1076997. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12351676. [DOI] [PubMed] [Google Scholar]
- Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004 Jul 2;15(1):57–67. doi: 10.1016/j.molcel.2004.06.020. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15225548. [DOI] [PubMed] [Google Scholar]
- Cifuentes-Rojas C, Hernandez AJ, Sarma K, Lee JT. Regulatory interactions between RNA and polycomb repressive complex 2. Mol Cell. 2014 Jul 17;55(2):171–85. doi: 10.1016/j.molcel.2014.05.009. Available from: http://www.sciencedirect.com/science/article/pii/S1097276514004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Dienstbier M, Hassan R, Schermelleh L, Sharif J, Blackledge NP, et al. Targeting polycomb to pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. Cell Rep. 2014 Jun 12;7(5):1456–70. doi: 10.1016/j.celrep.2014.04.012. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4062935&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol. 2013 Oct;20(10):1147–55. doi: 10.1038/nsmb.2669. Available from: http://dx.doi.org/10.1038/nsmb.2669. [DOI] [PubMed] [Google Scholar]
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila Enhancer of Zeste/ESC Complexes Have a Histone H3 Methyltransferase Activity that Marks Chromosomal Polycomb Sites. Cell. 2002 Oct;111(2):185–96. doi: 10.1016/s0092-8674(02)00975-3. Available from: http://www.sciencedirect.com/science/article/pii/S0092867402009753. [DOI] [PubMed] [Google Scholar]
- Davidovich C, Zheng L, Goodrich KJ, Cech TR. Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol. 2013 Nov;20(11):1250–7. doi: 10.1038/nsmb.2679. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3823624&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010 May 28;328(5982):1161–4. doi: 10.1126/science.1186777. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2879085&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich N, Lerdrup M, Landt E, Agrawal-Singh S, Bak M, Tommerup N, et al. REST-mediated recruitment of polycomb repressor complexes in mammalian cells. PLoS Genet. 2012 Jan;8(3):e1002494. doi: 10.1371/journal.pgen.1002494. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3291536&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan IM. Polycomblike: a gene that appears to be required for the normal expression of the bithorax and antennapedia gene complexes of Drosophila melanogaster. Genetics. 1982 Sep;102(1):49–70. doi: 10.1093/genetics/102.1.49. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1201924&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcas AM, Blackledge NP, Sudbery I, Long HK, McGouran JF, Rose NR, et al. KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. Elife. 2012 Jan 18;1:e00205. doi: 10.7554/eLife.00205. Available from: http://elifesciences.org/content/1/e00205.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari KJ, Scelfo A, Jammula S, Cuomo A, Barozzi I, Stützer A, et al. Polycomb-Dependent H3K27me1 and H3K27me2 Regulate Active Transcription and Enhancer Fidelity. Mol Cell. 2014 Jan 9;53(1):49–62. doi: 10.1016/j.molcel.2013.10.030. Available from: http://www.sciencedirect.com/science/article/pii/S109727651300796X. [DOI] [PubMed] [Google Scholar]
- Gan Q, Schones DE, Ho Eun S, Wei G, Cui K, Zhao K, et al. Monovalent and unpoised status of most genes in undifferentiated cell-enriched Drosophila testis. Genome Biol. 2010 Jan;11(4):R42. doi: 10.1186/gb-2010-11-4-r42. Available from: http://genomebiology.com/2010/11/4/R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang J, Bonasio R, Strino F, Sawai A, Parisi F, et al. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol Cell. 2012 Feb 10;45(3):344–56. doi: 10.1016/j.molcel.2012.01.002. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3293217&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehani SS, Agrawal-Singh S, Dietrich N, Christophersen NS, Helin K, Hansen K. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol Cell. 2010 Sep 24;39(6):886–900. doi: 10.1016/j.molcel.2010.08.020. Available from: http://www.sciencedirect.com/science/article/pii/S1097276510006301. [DOI] [PubMed] [Google Scholar]
- Van Heeringen SJ, Akkers RC, van Kruijsbergen I, Arif MA, Hanssen LLP, Sharifi N, et al. Principles of nucleation of H3K27 methylation during embryonic development. Genome Res. 2014 Mar 1;24(3):401–10. doi: 10.1101/gr.159608.113. Available from: http://genome.cshlp.org/content/24/3/401.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermann P, Hoerner L, Burger L, Schübeler D. Short sequences can efficiently recruit histone H3 lysine 27 trimethylation in the absence of enhancer activity and DNA methylation. Proc Natl Acad Sci U S A. 2014 Aug 19;111(33):E3415–21. doi: 10.1073/pnas.1400672111. Available from: http://www.pnas.org.proxy.ubn.ru.nl/content/111/33/E3415.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb R, Latwiel S, Baymaz HI, Jansen PWTC, Müller CW, Vermeulen M, et al. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat Struct Mol Biol. 2014 Jun;21(6):569–71. doi: 10.1038/nsmb.2833. Available from: http://dx.doi.org/10.1038/nsmb.2833. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Bonasio R, Saldaña-Meyer R, Yoshida T, Son J, Nishino K, et al. Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol Cell. 2014a Jan 23;53(2):290–300. doi: 10.1016/j.molcel.2013.11.012. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4026005&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Son J, Bonasio R, Shen SS, Reinberg D. Nascent RNA interaction keeps PRC2 activity poised and in check. Genes Dev. 2014b Oct 15;28(18):1983–8. doi: 10.1101/gad.247940.114. Available from: http://genesdev.cshlp.org.proxy.ubn.ru.nl/content/28/18/1983.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Son J, Shen SS, Reinberg D, Bonasio R. Nat Struct Mol Biol. 11. Vol. 20. Nature Publishing Group; 2013. Nov 20, PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells; pp. 1258–64. Available from: http://www.nature.com.proxy.ubn.ru.nl/nsmb/journal/v20/n11/full/nsmb.2700.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis JA, Brown JL. Polycomb group response elements in Drosophila and vertebrates. Adv Genet. 2013 Jan;81:83–118. doi: 10.1016/B978-0-12-407677-8.00003-8. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4157523&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. van Steensel B, editor. PLoS Genet. 2008 Oct;4(10):e1000242. doi: 10.1371/journal.pgen.1000242. Available from: http://dx.plos.org/10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed]
- Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002 Nov 15;16(22):2893–905. doi: 10.1101/gad.1035902. Available from: http://genesdev.cshlp.org/content/16/22/2893.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PNI, Cheung P. Histone code pathway involving H3 S28 phosphorylation and K27 acetylation activates transcription and antagonizes polycomb silencing. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2801–6. doi: 10.1073/pnas.1012798108. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3041124&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006 Apr 21;125(2):301–13. doi: 10.1016/j.cell.2006.02.043. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3773330&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–70. doi: 10.1038/276565a0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/103000. [DOI] [PubMed] [Google Scholar]
- Long HK, Blackledge NP, Klose RJ. ZF-CxxC domain-containing proteins, CpG islands and the chromatin connection. Biochem Soc Trans. 2013a Jun 1;41(3):727–40. doi: 10.1042/BST20130028. Available from: http://www.biochemsoctrans.org/bst/041/0727/bst0410727.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HK, Sims D, Heger A, Blackledge NP, Kutter C, Wright ML, et al. Epigenetic conservation at gene regulatory elements revealed by non-methylated DNA profiling in seven vertebrates. Elife. 2013b Jan 26;2:e00348. doi: 10.7554/eLife.00348. Available from: http://elifesciences.org/content/2/e00348.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MD, Smith AJH, De Gobbi M, Flenley M, Hughes JR, Vernimmen D, et al. An interspecies analysis reveals a key role for unmethylated CpG dinucleotides in vertebrate Polycomb complex recruitment. EMBO J. 2012 Jan 18;31(2):317–29. doi: 10.1038/emboj.2011.399. Available from: http://emboj.embopress.org/content/31/2/317.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Justin N, Ohno K, Sharpe ML, Son J, Drury WJ, et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009 Oct 8;461(7265):762–7. doi: 10.1038/nature08398. Available from: http://www.nature.com.proxy.ubn.ru.nl/nature/journal/v461/n7265/full/nature08398.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008 Nov 21;32(4):503–18. doi: 10.1016/j.molcel.2008.11.004. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3641558&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 2012 Apr 27;149(3):590–604. doi: 10.1016/j.cell.2012.03.026. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3398752&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Cao R, Zhang Y. Substrate preferences of the EZH2 histone methyltransferase complex. J Biol Chem. 2006 Mar 31;281(13):8365–70. doi: 10.1074/jbc.M513425200. Available from: http://www.jbc.org/content/281/13/8365.abstract?ijkey=8988efe670b376d3c45abc6ba0307e6078bc58e8&keytype2=tf_ipsecsha. [DOI] [PubMed] [Google Scholar]
- Mendenhall EM, Koche RP, Truong T, Zhou VW, Issac B, Chi AS, et al. GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells. PLoS Genet. 2010;6(12):1–10. doi: 10.1371/journal.pgen.1001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito Y, Henikoff JG, Henikoff S. Histone replacement marks the boundaries of cis-regulatory domains. Science. 2007 Mar 9;315(5817):1408–11. doi: 10.1126/science.1134004. Available from: http://www.sciencemag.org.proxy.ubn.ru.nl/content/315/5817/1408.long. [DOI] [PubMed] [Google Scholar]
- Mousavi K, Zare H, Wang AH, Sartorelli V. Polycomb protein Ezh1 promotes RNA polymerase II elongation. Mol Cell. 2012 Jan 27;45(2):255–62. doi: 10.1016/j.molcel.2011.11.019. Available from: http://www.sciencedirect.com/science/article/pii/S1097276511009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzetta C, Pontis J, Fritsch L, Robin P, Portoso M, Proux C, et al. The histone H3 lysine 9 methyltransferases G9a and GLP regulate polycomb repressive complex 2-mediated gene silencing. Mol Cell. 2014 Jan 23;53(2):277–89. doi: 10.1016/j.molcel.2013.12.005. Available from: http://www.sciencedirect.com/science/article/pii/S1097276513008733. [DOI] [PubMed] [Google Scholar]
- Müller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, et al. Histone Methyltransferase Activity of a Drosophila Polycomb Group Repressor Complex. Cell. 2002 Oct;111(2):197–208. doi: 10.1016/s0092-8674(02)00976-5. Available from: http://www.sciencedirect.com/science/article/pii/S0092867402009765. [DOI] [PubMed] [Google Scholar]
- Musselman CA, Avvakumov N, Watanabe R, Abraham CG, Lalonde M-E, Hong Z, et al. Molecular basis for H3K36me3 recognition by the Tudor domain of PHF1. Nat Struct Mol Biol. 2012a Dec;19(12):1266–72. doi: 10.1038/nsmb.2435. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3603146&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman CA, Lalonde M-E, Côté J, Kutateladze TG. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol. 2012b Dec;19(12):1218–27. doi: 10.1038/nsmb.2436. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3645987&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell. 2004 Nov;7(5):663–76. doi: 10.1016/j.devcel.2004.10.005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15525528. [DOI] [PubMed] [Google Scholar]
- Nekrasov M, Wild B, Müller J. Nucleosome binding and histone methyltransferase activity of Drosophila PRC2. EMBO Rep. 2005 Apr;6(4):348–53. doi: 10.1038/sj.embor.7400376. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1299286&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004 Oct 13;23(20):4061–71. doi: 10.1038/sj.emboj.7600402. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=524339&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Malatesta M, Jung HR, Walfridsson J, Willer A, Olsson L, et al. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res. 2010 Aug;38(15):4958–69. doi: 10.1093/nar/gkq244. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2926606&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, et al. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009 Dec 24;139(7):1290–302. doi: 10.1016/j.cell.2009.12.002. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2911953&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelly AR, Copur Ö, Jäckle H, Herzig A, Müller J. A histone mutant reproduces the phenotype caused by loss of histone-modifying factor Polycomb. Science. 2013 Feb 8;339(6120):698–9. doi: 10.1126/science.1231382. Available from: http://www.sciencemag.org.proxy.ubn.ru.nl/content/339/6120/698.long. [DOI] [PubMed] [Google Scholar]
- Petruk S, Black KL, Kovermann SK, Brock HW, Mazo A. Stepwise histone modifications are mediated by multiple enzymes that rapidly associate with nascent DNA during replication. Nat Commun. 2013 Jan;4:2841. doi: 10.1038/ncomms3841. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3874871&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinello L, Xu J, Orkin SH, Yuan G-C. Analysis of chromatin-state plasticity identifies cell-type-specific regulators of H3K27me3 patterns. Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):E344–53. doi: 10.1073/pnas.1322570111. Available from: http://www.pnas.org/content/111/3/E344.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds N, Salmon-Divon M, Dvinge H, Hynes-allen A, Balasooriya G, Leaford D, et al. NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression. EMBO J. 2011 Nov;:1–13. doi: 10.1038/emboj.2011.431. Available from: http://dx.doi.org/10.1038/emboj.2011.431. [DOI] [PMC free article] [PubMed]
- Riising EM, Comet I, Leblanc B, Wu X, Johansen JV, Helin K. Gene silencing triggers polycomb repressive complex 2 recruitment to CpG islands genome wide. Mol Cell. 2014 Aug 7;55(3):347–60. doi: 10.1016/j.molcel.2014.06.005. Available from: http://www.cell.com/article/S1097276514004870/fulltext. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Rehmsmeier M, Dura J-M, Paro R. Genome-wide prediction of Polycomb/Trithorax response elements in Drosophila melanogaster. Dev Cell. 2003 Nov;5(5):759–71. doi: 10.1016/s1534-5807(03)00337-x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14602076. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann Sa, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007 Jun 29;129(7):1311–23. doi: 10.1016/j.cell.2007.05.022. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2084369&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Rocha ST, Boeva V, Escamilla-Del-Arenal M, Ancelin K, Granier C, Matias NR, et al. Jarid2 Is Implicated in the Initial Xist-Induced Targeting of PRC2 to the Inactive X Chromosome. Mol Cell. 2014 Jan 23;53(2):301–16. doi: 10.1016/j.molcel.2014.01.002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24462204. [DOI] [PubMed] [Google Scholar]
- Sanulli S, Justin N, Teissandier A, Ancelin K, Portoso M, Caron M, et al. Jarid2 Methylation via the PRC2 Complex Regulates H3K27me3 Deposition during Cell Differentiation. Mol Cell. 2015 Jan; doi: 10.1016/j.molcel.2014.12.020. Available from: http://www.sciencedirect.com/science/article/pii/S1097276514009988. [DOI] [PMC free article] [PubMed]
- Schmitges FW, Prusty AB, Faty M, Stützer A, Lingaraju GM, Aiwazian J, et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol Cell. 2011 May 6;42(3):330–41. doi: 10.1016/j.molcel.2011.03.025. Available from: http://www.cell.com/article/S1097276511002875/fulltext. [DOI] [PubMed] [Google Scholar]
- Schneider TD, Arteaga-Salas JM, Mentele E, David R, Nicetto D, Imhof A, et al. Stage-specific histone modification profiles reveal global transitions in the Xenopus embryonic epigenome. PLoS One. 2011 Jan;6(7):e22548. doi: 10.1371/journal.pone.0022548. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3142184&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B, Ganapathi M, Leblanc B, Portoso M, Jaschek R, Tolhuis B, et al. Functional anatomy of polycomb and trithorax chromatin landscapes in Drosophila embryos. PLoS Biol. 2009 Jan 13;7(1):e13. doi: 10.1371/journal.pbio.1000013. Available from: http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B, Oded Elkayam N, Sexton T, Entrevan M, Stern S, Thomas A, et al. Cooperativity, specificity, and evolutionary stability of Polycomb targeting in Drosophila. Cell Rep. 2014 Oct 9;9(1):219–33. doi: 10.1016/j.celrep.2014.08.072. Available from: http://www.sciencedirect.com/science/article/pii/S2211124714007645. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Kahn TG, Nix DA, Li X, Bourgon R, Biggin M, et al. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat Genet. 2006;38(6):700–5. doi: 10.1038/ng1817. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16732288. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. A new world of Polycombs: unexpected partnerships and emerging functions. Nat Rev Genet. 2013 Dec;14(12):853–64. doi: 10.1038/nrg3603. Available from: http://dx.doi.org/10.1038/nrg3603. [DOI] [PubMed] [Google Scholar]
- Sidoli S, Schwämmle V, Ruminowicz C, Hansen TA, Wu X, Helin K, et al. Middle-down hybrid chromatography/tandem mass spectrometry workflow for characterization of combinatorial post-translational modifications in histones. Proteomics. 2014 Oct;14(19):2200–11. doi: 10.1002/pmic.201400084. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25073878. [DOI] [PubMed] [Google Scholar]
- Signolet J, Hendrich B. The function of chromatin modifiers in lineage commitment and cell fate specification. FEBS J. 2015 May;282(9) doi: 10.1111/febs.13132. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25354247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Chiang A, Bender W, Shimell MJ, O’Connor M. Elements of the drosophila bithorax complex that mediate repression by polycomb group products. Developmental biology. 1993:158. doi: 10.1006/dbio.1993.1174. [DOI] [PubMed] [Google Scholar]
- Smits AH, Jansen PWTC, Poser I, Hyman AA, Vermeulen M. Stoichiometry of chromatin-associated protein complexes revealed by label-free quantitative mass spectrometry-based proteomics. Nucleic Acids Res. 2013 Jan 7;41(1):e28. doi: 10.1093/nar/gks941. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3592467&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen PA, Ringrose L. What are memories made of? How Polycomb and Trithorax proteins mediate epigenetic memory. Nat Rev Mol Cell Biol. 2014 May;15(5):340–56. doi: 10.1038/nrm3789. Available from: http://dx.doi.org/10.1038/nrm3789. [DOI] [PubMed] [Google Scholar]
- Stojic L, Jasencakova Z, Prezioso C, Stützer A, Bodega B, Pasini D, et al. Chromatin regulated interchange between polycomb repressive complex 2 (PRC2)-Ezh2 and PRC2-Ezh1 complexes controls myogenin activation in skeletal muscle cells. Epigenetics Chromatin. 2011 Jan;4:16. doi: 10.1186/1756-8935-4-16. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3180244&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares L, Dimitrova E, Oxley D, Webster J, Poot R, Demmers J, et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell. 2012 Feb 17;148(4):664–78. doi: 10.1016/j.cell.2011.12.029. Available from: http://www.cell.com/article/S009286741200027X/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W-W, Shen SS, Oksuz O, Narendra V, Reinberg D. Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs. Cell. 2014 Feb 13;156(4):678–90. doi: 10.1016/j.cell.2014.01.009. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4006806&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, et al. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009 Sep;136(18):3131–41. doi: 10.1242/dev.037127. Available from: http://dev.biologists.org/content/136/18/3131.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien C-L, Jones A, Wang H, Gerigk M, Nozell S, Chang C. Snail2/Slug cooperates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development. 2015 Jan 23;142(4):722–31. doi: 10.1242/dev.111997. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25617436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, Wit E, De Muijrers I, Teunissen H, Talhout W, Van Steensel B, et al. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat Genet. 2006;38(6):694–700. doi: 10.1038/ng1792. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16628213. [DOI] [PubMed] [Google Scholar]
- Tsai M-C, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010 Aug 6;329(5992):689–93. doi: 10.1126/science.1192002. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2967777&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SA, Bracken AP. A “complex” issue: deciphering the role of variant PRC1 in ESCs. Cell Stem Cell. 2013 Feb 7;12(2):145–6. doi: 10.1016/j.stem.2013.01.014. Available from: http://www.sciencedirect.com/science/article/pii/S1934590913000179. [DOI] [PubMed] [Google Scholar]
- Vizán P, Beringer M, Ballaré C, Di Croce L. Role of PRC2-associated factors in stem cells and disease. FEBS J. 2015 May;282(9):1723–35. doi: 10.1111/febs.13083. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25271128. [DOI] [PubMed] [Google Scholar]
- Voigt P, LeRoy G, Drury WJ, Zee BM, Son J, Beck DB, et al. Asymmetrically modified nucleosomes. Cell. 2012 Sep 28;151(1):181–93. doi: 10.1016/j.cell.2012.09.002. Available from: http://www.sciencedirect.com/science/article/pii/S0092867412010677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter E, Quante T, Merusi C, Arczewska A, Stewart F, Webb S, et al. Synthetic CpG islands reveal DNA sequence determinants of chromatin structure. Elife. 2014 Sep 26;3:e03397. doi: 10.7554/eLife.03397. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4204011&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown JL, Cao R, Zhang Y, Kassis JA, Jones RS. Hierarchical recruitment of polycomb group silencing complexes. Mol Cell. 2004 Jun 4;14(5):637–46. doi: 10.1016/j.molcel.2004.05.009. Available from: http://www.sciencedirect.com/science/article/pii/S1097276504002928. [DOI] [PubMed] [Google Scholar]
- Xu C, Bian C, Yang W, Galka M, Ouyang H, Chen C, et al. Binding of different histone marks differentially regulates the activity and specificity of polycomb repressive complex 2 (PRC2) PNAS. 2010;107(45):19266–71. doi: 10.1073/pnas.1008937107. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20974918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shao Z, Li D, Xie H, Kim W, Huang J, et al. Developmental Control of Polycomb Subunit Composition by GATA Factors Mediates a Switch to Non-Canonical Functions. Mol Cell. 2015 Jan; doi: 10.1016/j.molcel.2014.12.009. Available from: http://www.sciencedirect.com/science/article/pii/S1097276514009587. [DOI] [PMC free article] [PubMed]
- Yu M, Mazor T, Huang H, Huang H-T, Kathrein KL, Woo AJ, et al. Direct recruitment of polycomb repressive complex 1 to chromatin by core binding transcription factors. Mol Cell. 2012 Feb 10;45(3):330–43. doi: 10.1016/j.molcel.2011.11.032. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3278717&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Wu T, Fu H, Dai C, Wu H, Liu N, et al. Dense chromatin activates Polycomb repressive complex 2 to regulate H3 lysine 27 methylation. Science. 2012 Aug 24;337(6097):971–5. doi: 10.1126/science.1225237. Available from: http://www.sciencemag.org.proxy.ubn.ru.nl/content/337/6097/971.long. [DOI] [PubMed] [Google Scholar]
- Yuan W, Xu M, Huang C, Liu N, Chen S, Zhu B. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J Biol Chem. 2011 Mar 11;286(10):7983–9. doi: 10.1074/jbc.M110.194027. Available from: http://www.jbc.org/content/286/10/7983.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau DJ, Sarma K, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010 Dec 22;40(6):939–53. doi: 10.1016/j.molcel.2010.12.011. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3021903&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin Ja, Song J-J, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008 Oct 31;322:750–6. doi: 10.1126/science.1163045. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2748911&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]