Abstract
Background
Drug addiction, a leading health problem, is a chronic brain disease with a significant genetic component. Animal models and clinical studies established the involvement of glutamate and GABA neurotransmission in drug addiction. This study was designed to assess if 258 variants in 27 genes of these systems contribute to the vulnerability to develop drug addiction.
Methods
Four independent analyses were conducted in a sample of 1860 subjects divided according to drug of abuse (heroin or cocaine) and ancestry (African and European).
Results
A total of 11 SNPs in eight genes showed nominally significant associations (P < 0.01) with heroin and/or cocaine addiction in one or both ancestral groups but the associations did not survive correction for multiple testing. Of these SNPs, the GAD1 upstream SNP rs1978340 is potentially functional as it was shown to affect GABA concentrations in the cingulate cortex. In addition, SNPs GABRB3 rs7165224; DBI rs12613135; GAD1 SNPs rs2058725, rs1978340, rs2241164; and GRIN2A rs1650420 were previously reported in associations with drug addiction or related phenotypes.
Conclusions
The study supports the involvement of genetic variation in the glutamatergic and GABAergic systems in drug addiction with partial overlap in susceptibility loci between cocaine and heroin addiction.
Keywords: GAD1, glutamate, GABA, heroin, cocaine, addiction, African Americans
1. Introduction
Drug addiction, a leading health problem, is a chronic relapsing brain disease with a significant genetic component (Kendler et al., 2000; Kreek et al., 2012). Though most drugs of abuse increase dopamine (DA) release in the striatum, the diversity of drug effects is mediated by multiple neurotransmitters (Di Chiara et al., 2004). Opioids indirectly disinhibit DA neurons by inhibition of gamma-aminobutyric acid (GABA) release through binding opioid receptors on GABA interneurons in the ventral tegmental area (VTA) (Dilts and Kalivas, 1989; Johnson and North, 1992). Cocaine increases DA availability in the striatum through the blockade of transporter-mediated reuptake. Cocaine was shown to alter GABAA receptor subunit expression in the nucleus accumbens (NAc) through chromatin remodeling (Kennedy et al., 2013). Rodent's studies provided evidence for an essential role for the glutamatergic and the GABAergic systems in drug addiction and indicated an effect of genetic background and impairments in synaptic plasticity (Xi and Stein, 2000; Kalivas, 2009; Miguens et al., 2013; Schlussman et al., 2013; Gipson et al., 2014).
GABA and glutamate are the major inhibitory and excitatory neurotransmitters in the central nervous system, respectively. These systems are involved in memory, learning and synaptic plasticity, and they are modulated by drugs of abuse (Pinheiro and Mulle, 2008). GABA also inhibits the hypothalamic-pituitary-adrenal axis (HPA) responses to stress and may be important in addiction-associated stress and relapse (Herman et al., 2004). The GABA system is targeted as a potential pharmacotherapeutic target for the treatment of alcohol and drug abuse disorders (Addolorato et al., 2012). Glutamate receptors have a role in forming associative memories that are important for drug craving, and several glutamatergic medications have been investigated for reducing the vulnerability to relapse (Olive et al., 2012).
GABA acts via ionotropic (GABAA) and metabotropic (GABAB) receptors. GABAA receptors are ligand-gated chloride-ion channels that confer fast synaptic inhibition (Olsen and Sieghart, 2009). The GABAB receptor is linked to potassium and calcium channels via G-proteins (Pinard et al., 2010). Glutamate receptors can be divided into ionotropic glutamate receptors (iGluR; NMDA, AMPA, and kainate) and metabotropic receptors (mGluR). The AMPA receptors comprise four subunits (GluR1-4) that assemble in different combinations. NMDA receptors are heterotetramer composed of two NR1 subunits, and two of the four isoforms' NR2 subunits. There are five types of kainate receptor subunits (GluK1-5) that are arranged in different ways to form a tetramer. Activation of the metabotropic glutamate receptors (mGluR1-8) modulates ion channels and other signaling proteins through G-proteins. mGluR1 is predominantly expressed post-synaptically and modulates NMDA, AMPA and GABA receptor activity (Ferraguti et al., 2008). The group I mGluRs (mGluR1 and mGluR5) are associated with the synaptic scaffolding protein, HOMER1.
The HOMER family belong to a complex network that converges dopamine and glutamate signaling to appropriate nuclear targets (de Bartolomeis and Tomasetti, 2012). Cocaine withdrawal was shown to create an imbalance of Homer1/2 isoforms' expression, which disturbed glutamate function and heightened cocaine preference in rodents (Szumlinski et al., 2006; Ary et al., 2013).
Activation of the NMDA receptor leads to postsynaptic calcium entry that binds to calmodulin, which activates calcium calmodulin-dependent protein kinase (CaMKII). CaMKII is composed of four different chains and has an important role in the plasticity of glutamatergic synapses (Giese and Mizuno, 2013). Expression and phosphorylation of alpha-CaMKII (encoded by CaMK2A) is increased following cocaine exposure and a CaMK2A SNP was reported to confer rapid progression to severe cocaine use (Easton et al., 2014).
A number of studies have reported associations between polymorphisms in genes of these systems and drug addictions. We have reported an association of GRIN2A SNPs with heroin addiction (opioid dependence/OD) in a subsample of the current sample of African Americans (AA) (Levran et al., 2009). GRIN2A SNPs were also associated with OD in Han Chinese (Zhao et al., 2013; Zhong et al., 2014). GABRA2, GABRB3, GABRG2, and GAD1 SNPs were associated with OD in different populations (Loh et al., 2007; Enoch et al., 2010; Wu et al., 2012; Chen et al., 2014; Li et al., 2014; Yang et al., 2014). HOMER1 SNPs were associated with opiate abuse in Caucasians (Jacobs et al., 2013) and with cocaine dependence (CD) in AA (Dahl et al., 2005). Alcohol dependence (AD) was associated with SNPs in GRIN1, GRIN2B, GRIK1, GABRA1, GABRA2, GABRA6, GABRG1, GABRG2, and GAD2 SNPs in different populations (e.g.,(Kim et al., 2006; Kranzler et al., 2009; Enoch et al., 2010; Zintzaras, 2012; Li et al., 2014)).
This study focuses on 27 genes including eight glutamate and nine GABAA receptors' subunits, and ten related proteins. It was designed to determine whether polymorphism in these genes contribute to the susceptibility to heroin and/or cocaine addiction in two populations of distinct ancestry (European and African). The study is an extension of our previous studies of OD with a larger sample, modified SNP content and an additional cocaine group (Levran et al., 2008; Levran et al., 2009). The sample analyzed in the study was independently analyzed for genes in other systems (e.g. stress, dopaminergic) (Levran et al., 2014a; Levran et al., 2014b; Levran et al., 2014c; Levran et al., 2015).
2. Methods
2.1 Subjects
This study follows our previous studies (Levran et al., 2008; Levran et al., 2009), for which we added 481 new African American (AA) subjects and 465 new European/Middle Eastern (EA) subjects. The study included 1860 subjects (38% females) divided into two major ancestry groups. A subject was defined as EA if he/she shows >75% European, Middle Eastern (ME) or combined EA/ME ancestry contributions based on Structure analysis (see below). A subject was defined as AA if he/she shows >50% African ancestry contribution. Self-identified Hispanics and AA subjects with >25% contribution of any major ancestry other than European, Middle Eastern or African were not included.
Ascertainment of cases and controls was made by personal interview performed in a similar manner at the recruiting places, using several instruments: the Addiction Severity Index (McLellan et al., 1992); Kreek-McHugh-Schluger-Kellogg Scale (KMSK) (Kellogg et al., 2003); and Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). Drug addiction diagnosis was based on life-time DSM-IV criteria. Subjects with active major psychotic mental illnesses were excluded. Subjects were recruited at the Rockefeller University Hospital, the Manhattan campus of the VA NY Harbor Health Care System, and the Dr. Miriam and Sheldon G. Adelson Clinics for Drug Abuse Treatment and Research in Las Vegas, USA and Tel Aviv, Israel.
The two ancestry groups were further divided into five groups based on addiction status and preferred drug of abuse (heroin or cocaine) as follows: (1) EA heroin ± cocaine (OD±CD), (2) AA OD±CD, (3) AA cocaine (CD, without OD), (4) EA control, and (5) AA control (Table 1).
Table 1. Groups description.
| Ancestry | Heroin addiction (OD±CD) | Cocaine addiction (CD without OD) | Controls | Total |
|---|---|---|---|---|
|
| ||||
| n | n | n | n | |
| EA | 827 (1) | - | 232 (4) | 1059 |
| AA | 315 (2) | 279 (3) | 207 (5) | 801 |
| Total | 1142 | 279 | 439 | 1860 |
Group assigned numbers are in parenthesis
Subjects in groups 1 and 2 were former heroin addicts in methadone maintenance treatment with a history of at least one year of daily multiple uses of heroin and about half of them also had past or current cocaine addiction. The subjects in group 3 had past or current cocaine addiction, had no heroin addiction and about a third of them also had past or current alcohol addiction. The EA “CD without OD” group was not included in this study due to small sample size. Subject could not be defined as controls if they had (1) ≥1 instance of drinking to intoxication or any illicit drug use in the last month; (2) alcohol drinking to intoxication or illicit drug use, ≥2 times per week, for ≥6 consecutive months, and (3) cannabis use for ≥12 days in the previous month or past cannabis use for ≥2 times per week for ≥4 years.
The Institutional Review Boards of the Rockefeller University Hospital, the VA New York Harbor Healthcare System and the Tel Aviv Sourasky Medical Center (Helsinki Committee) approved the study. All subjects signed informed consent for genetic studies.
2.2 Genes and SNPs
A total of 27 genes were selected based on the original “addiction” array (Hodgkinson et al., 2008) with an addition of SNPs in six new genes (Table 2). After exclusion of 10 SNPs, in these genes, due to failure or low frequency in our previous studies, a total of 272 SNPs including tagging SNPs from the original array and 23 new SNPs that were added based on functionality or reported association with related phenotypes, were genotyped with a modified GoldenGate Custom Panel (GS0013101-OPA, Illumina, San Diego, CA) (Supplement Table 1). The X chromosome genes from these systems are included in the array (GRIA3, GABRA3, GABRE, and GABRQ) but were not included in the current analysis. Analysis was performed with BeadStudio software v2.3.43 (Illumina) and cluster plots were also visually inspected.
Table 2. Genes List.
| Symbol | Description | Chr. | # of SNP s | ||
|---|---|---|---|---|---|
| Glutamate Receptors subunits | |||||
| Ionotropic | GRIA1* | AMPA 1 | (GluR1) | 5 | 6 |
| GRIA2* | AMPA 2 | (GluR2) | 4 | 4 | |
| GRIN1 | NMDA 1 | (NR1) | 9 | 3 | |
| GRIN2A | NMDA 2A | (NR2A) | 16 | 16 | |
| GRIN2B | NMDA 2B | (NR2B) | 12 | 23 | |
| GRIN2C | NMDA 2C | (NR2C) | 17 | 3 | |
| GRIK1 | Kainate 1 | (GluK5) | 21 | 13 | |
| Metabotropic | GRM1 | Metabotropic 1 | 6 | 17 | |
| GABAA receptors subunits | |||||
| GABRA2 | alpha 2 | 4 | 11 | ||
| GABRA4 | alpha 4 | 4 | 10 | ||
| GABRA6 | alpha 6 | 5 | 4 | ||
| GABRB1 | beta 1 | 4 | 17 | ||
| GABRB2 | beta 2 | 5 | 15 | ||
| GABRB3 | beta 3 | 15 | 17 | ||
| GABRG2 | gamma 2 | 5 | 11 | ||
| GABRG3 | gamma 3 | 15 | 16 | ||
| GABRD | delta | 1 | 1 | ||
| Related enzymes, transporters and modulators | |||||
| CaMK2A* | calcium/calmodulin-dependent protein kinase II alpha | 5 | 2 | ||
| DBI | diazepam binding inhibitor (GABA receptor modulator) | 2 | 3 | ||
| GAD1 | glutamate decarboxylase 1 (67kDa) | 2 | 9 | ||
| GAD2 | glutamate decarboxylase 2 (65kDa) | 2 | 13 | ||
| HOMER 1* | homer homolog 1 (Drosophila) | 5 | 5 | ||
| HOMER 2* | homer homolog 2 (Drosophila) | 15 | 2 | ||
| HOMER 3* | homer homolog 3 (Drosophila) | 19 | 2 | ||
| SLC6A1 1 | solute carrier family 6 (neurotransmitter transporter), member 11 | 3 | 15 | ||
| SLC6A1 3 | solute carrier family 6 (neurotransmitter transporter), member 13 | 12 | 16 | ||
| SLC32A 1 | solute carrier family 32 (GABA vesicular transporter), member 1 | 20 | 4 | ||
These genes were not included in the original “addiction” array (Hodgkinson et al. 2008, Levran et al. 2008, 2009). The number of SNPs does not include the 14 SNP excluded from analyses.
2.3 Assessment of ancestry contribution using ancestry informative markers (AIMs)
Biographic Ancestry Scores (e.g., fractions of affiliation of an individual in each cluster) were estimated by Structure 2.2 with seven clusters (K) using data from 155 AIMs with high quality (Hodgkinson et al., 2008). Each subject was anchored against genotypes of 1051 samples from 51 worldwide populations represented in the Human Genome Diversity Cell Line Panel (Ducci et al., 2009). The European and Middle-Eastern clusters were combined based on their low population differentiation (Tian et al., 2009; Atzmon et al., 2010). There was no evidence for substructure among the case/control subgroups for each ancestry.
2.4 Statistical analysis
Pairwise linkage disequilibrium (LD) (D′ and r2) was estimated using Haploview 4.2. LD blocks were identified using confidence intervals (Gabriel et al., 2002). Exact tests for deviation from Hardy-Weinberg equilibrium (HWE) were performed with the PLINK program in the control samples. Association analyses were conducted only for the genes of these two pathways using PLINK for each SNP separately by logistic regression, under dominant or recessive model assumptions. Association analyses were performed independently for EA OD±CD (1), AA OD±CD (2), AA CD (3), and AA OD±CD+CD (2+3). Correction for multiple testing was also performed by permutation test (n = 100,000) for each model of inheritance, using PLINK.
3. Results
Four case-control association analyses of selected SNPs in genes of the glutamatergic and GABAergic pathways were performed under two different models of inheritance (dominant or recessive) as follows: OD±CD in EA (1 vs. 4), OD±CD in AA (2 vs. 5), CD±AD in AA (3 vs. 5), and OD±CD+CD in AA (2+3 vs. 5) (Table 1). A total of 272 SNPs spanning 27 genes were genotyped in 1860 subjects (Tables 1, 2 & Supplement Table 1). Fourteen SNPs were excluded from analysis due to technical reasons and 258 SNPs were analyzed. In addition, 34 SNPs were excluded from the EA analysis and 10 SNPs were excluded from the AA analysis, based on low MAF (< 0.05) in the respective control samples, including one SNP (SLC6A11 rs11720592) that was excluded from both analyses (Supplement Table 1). Large deviation from HWE (P = 0.005) was detected for GABRB3 SNP rs7165224 (EA), SLC6A13 SNP rs10848623 (AA), and GABRA4 SNP rs3792208 (AA). LD analysis revealed 43 LD blocks including 15 SNP pairs and five triplets in almost complete LD (r2 > 0.95) in EA, of which five pairs are also in strong LD in AA, reducing the effective number of independent SNPs in the analyses (Supplement Table 1).
Of the 224 variants that passed quality control in the EA sample, 13 SNPs in ten genes showed nominal associations (P < 0.05) with OD±CD in EA, including GRIA1 SNP pair in strong LD (Supplement Table 2). Only the synonymous GRIN2C SNP rs689730 survived the P < 0.01 cutoff (Table 3). None of the signals survived correction for multiple testing.
Table 3. Association results (P < 0.01).
| Gene | SNP | Chr | Position | Location | Alleles | MAF | P | Model | OR (95 % CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| EA | AA | EA | AA | ||||||||||
|
|
|
||||||||||||
| OD±CD | OD±CD | CD | OD±CD+CD | ||||||||||
| DBI | rs12613135 | 2 | 119364764 | Upstreamb | C/T | 0.27 | 0.30 | 0.006 | (0.02) | R | 2.48 (1.3-4.7) | ||
| GABRB2 | rs3816596 | 5 | 161548326 | Upstream | C/T | 0.34 | 0.50 | 0.009 | (0.02) | R | 0.56 (0.4-0.9) | ||
| rs6882041 | 5 | 161515394 | Intron | C/T | 0.27 | 0.31 | (0.01) | 0.008 | R | 0.47 (0.3-0.8) | |||
| GABRB3 |
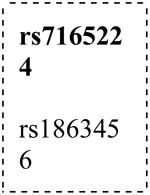
|
15 | 26779189 | Upstream | C/T | 0.07 | 0.21 | (0.04) | 0.003 | (0.04) | 0.005 | D | 1.71 (1.2-2.5) |
| 15 | 26728885 | Intron | A/G | 0.48 | 0.15 | 0.008 | (0.02) | D | 0.56 (0.4-0.9) | ||||
| GAD1 | rs1978340c | 2 | 170813611 | Upstream | C/T | 0.27 | 0.09 | 0.006 | (0.01) | D | 1.85 (1.2-2.9) | ||
| rs2058725 | 2 | 170833611 | Intron | A/G | 0.27 | 0.37 | 0.004 | (0.01) | 0.001 | R | 0.47 (0.3-0.7) | ||
| GRIN2A | rs1833161 | 16 | 9791170 | Intron | T/Ca | 0.32 | 0.42 | (0.04) | 0.009 | R | 1.77 (1.2-2.7) | ||
| GRIN2B | rs2284416 | 12 | 1376628 0 | Intron | T/G | 0.49 | 0.44 | (0.01) | (0.04) | 0.006 | R | 0.57 (0.4-0.9) | |
| GRIN2C | rs689730 | 17 | 74854994 | Ala33= | C/T | 0.09 | 0.21 | 0.004 | (0.03)d | D | 1.76 (1.2-2.6) | ||
| GRM1 | rs1997766 | 6 | 146180786 | Intron | T/C | 0.006 | 0.18 | 0.008 | (0.03) | 0.006 | D | 0.62 (0.4-0.9) | |
SNPs with P values < 0.01 in at least one analysis are listed. For these SNPs, P values < 0.05 in the other analyses are also listed.
Blank cells represent P > 0.05.
The complete results (P < 0.05) are listed in Supplement Table 2
Alleles are listed with the major allele (in EA) first.
The model and OR refers to the minor allele. OR is listed for the lowest P value (in bold) but the other analyses for the same SNP showed the same direction, model and similar OR range unless indicated. OR > 1 represent risk effect of the minor allele (in bold), OR < 1 represent protective effect of the minor allele.
The bolded SNP was previously associated with OD in AA (Levran et al. 2009) and in Han Chinese (Zhao et al. 2013, Zhong et al. 2014)
Box represnet LD (D′ = 1, r2 < 0.1 in EA and AA)
the minor allele in AA is the major allele in EA
This SNP is also located at an intron of C2orf76 (chromosome 2 open reading frame 76).
This SNP showed an effect on GABA levels in prefrontal cortex
The association of OD±CD in AA was in the opposite direction comapre to the OD±CD in EA (OR (95% CI) 0.66 (0.45-0.96)). Chr, Chromosome; MAF, minor allele frequency; EA, European/Middle Eastern ancestry; AA, African Americans; OD, opioid dependence; CD Cocaine dependence; OR, Odds ratio; CI, confidence interval; D, dominant; R, recessive
Of the 248 variants that passed quality control in the AA sample, 34 SNPs in 15 genes showed nominal associations (P < 0.05) with OD±CD and/or CD (Supplement Table 2), including two nonsynonymous SNPs (GRIK1 rs363504 and GAD1 rs769402). The 10 SNPs with the most stringent associations (P < 0.01) are listed in Table 3. The two GABRB3 SNPs indicated are in strong LD (D′ = 1, r2 < 0.1 in EA and AA). GRIN2C SNP rs689730 that showed significant associations in EA showed less stringent associations with OD±CD in AA in the opposite direction (P = 0.032, OR = 0.66, Table 3). The upstream GABRB3 rs7165224 that showed significant associations in AA showed less stringent association in EA (P = 0.035, Table 3). None of the signals survived correction for multiple testing.
Under the more stringent cutoff (P < 0.01) there was no gene or SNP in common for EA and AA. Under the less stringent cutoff (P < 0.05) there were eight genes and two SNPs (GRIN2C rs689730 and GABRB3 rs7165224) in common although the association of GRIN2C rs689730 was in the opposite direction suggesting a protection effect in AA (Supplement Table 2, Table 3). Comparison of the results for the different AA addiction groups (OD±CD, CD or both) revealed three SNPs (GAD1 rs2058725, GRM1 rs1997766, GABRB3 rs7165224) that showed associations (P < 0.01) in the same direction and model, in more than one analyses (Table 3).
4. Discussion
The study suggests specific genetic contributions to heroin and cocaine addictions in the GABA and glutamate systems. This study follows our studies of heroin addiction in EA and AA (Levran et al., 2008; Levran et al., 2009) and was conducted after the recruitment of additional subjects to approximately double the size of the cohort. We have also added a cocaine addiction AA group, modified the SNP content and changed the analysis process to include only one or two pathways at a time instead of an analysis of the whole array, to reduce multiple testing. The study follows our recent independent studies of other pathways (e.g. stress, dopaminergic) in the current sample (Levran et al., 2014a; Levran et al., 2014b; Levran et al., 2014c; Levran et al., 2015).
Since the associations were only nominally significant and did not survive correction for multiple testing, they should be considered tentative until further verification. Nevertheless, a hypothesis-driven study of genes with known or potential addiction-related functionality may not require as stringent a threshold for significance as a hypothesis-free study.
One of the main finding is the association of SNP rs1978340 upstream of GAD1 with CD in AA. This SNP was previously associated with drug addictions and related phenotypes (Kuo et al., 2009; Wu et al., 2012; Yang et al., 2014) and showed an effect on GABA concentrations in the cingulate cortex (Marenco et al., 2010). Glutamic acid decarboxylase (GAD67, encoded by GAD1) is one of two major isoforms of GAD that converts glutamate to GABA and is critical for the maintenance of GABA reserves. It has an important role in the pathogenesis of anxiety disorders and decreased expression of GAD1 in hippocampus GABAergic interneurons was observed in subjects with schizophrenia and bipolar disorder that was associated with cognitive deficits (Benes et al., 2007). Studies have shown that chronic administration of drugs of abuse alters the expression and activity of GAD1 in the brain (Enoch et al., 2012).
The study corroborates the association of SNPs GAD1 rs2058725, DBI rs12613135, and GABRB3 rs7165224 that were identified in our previous study of OD in a subsample of the AA group (Levran et al., 2009). GRIN2A SNP rs1650420 that was indicated in this study in association with OD in AA by the less stringent cutoff (P = 0.019), was previously associated with OD in Han Chinese (Zhao et al., 2013; Zhong et al., 2014) and in our previous study of in AA (Levran et al., 2009).
Some of the findings may be population-specific. Comparison of the results obtained in EA and AA reveals no SNP in common under the more stringent cutoff and only two SNPs in common under the less stringent cutoff, one of them with an opposite effect. Interestingly, the upstream GABRB3 SNP rs7165224 indicated in the current study, was not detected in Han Chinese in a study that reported association with another upstream GABRB3 SNP rs4906902 with OD (Chen et al., 2014). GABRB3 SNP rs4906902 is very rare in African populations and was not included in the current study. This data suggests a potentially different profile of the GABRB3 regulatory region across different populations.
Comparison of the results obtained in the analyses of the two addictions (CD and OD), in AA, reveals several genes and SNPs in common that support the existence of both shared and specific vulnerability between the two addictions. For example, the P values for the associations of SNPs GAD1 rs1978340 and GABRB3 rs1863456 were lower in the analysis of CD in AA than in the combined OD±CD in AA, in spite of the smaller sample size, suggesting a cocaine-specific vulnerability. The P values for the associations of SNPs DBI rs12613135 and GABRB2 rs3816596 were lower in the OD±CD analysis in AA than in the combined analysis suggesting a heroin-specific vulnerability. However, this comparison is limited due to substance-related comorbidity, and a limited power to detect small effects.
The GABA system is a potential pharmacotherapeutic target for the treatment of drug abuse disorders (Addolorato et al., 2012) and variants in genes of this system may also affect the response to treatment, as was shown for GABRA2 genotype and the treatment of alcohol drinking (Bauer et al., 2007).
4.1 Conclusions
This study suggests numerous potential susceptibility loci (or markers that tag them) in the glutamatergic and GABAergic pathways for heroin and cocaine addiction, in subjects of African and European ancestry, with partial overlap in susceptibility loci between populations and between addictions to different drugs. Future studies are required to corroborate the results and to assess the relevance of the findings for diagnosis and treatment.
Supplementary Material
Highlights.
Association study of heroin and cocaine addictions with glutamate and GABA genes
Samples of African and European ancestries
Eleven SNPs in eight genes showed nominally significant associations
Corroboration of previous association studies
GAD1 upstream SNP rs1978340 was previously shown to affect GABA concentration
Acknowledgments
We thank all the clinical staff including S Linzy, DNP, E Ducat, NP and B Ray, NP. We are grateful to P-H Shen, PhD, and D Goldman, PhD from the NIH/NIAAA for STRUCTURE analysis. We thank C Zhao, PhD, and B Zhang, from the Rockefeller Genomic Resource Center, for their excellent assistance in genotyping.
Role of funding source: This work was supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Clinical and Translational Science Award UL1RR024143 from the National Center for Advancing Translational Sciences of the National Institutes of Health (B. Coller) and NSFC grant 31470070 from the Chinese Government (J. Ott).
Footnotes
Author contributions: O. Levran: project design, data collection, analysis and interpretation, manuscript writing; M.J Kreek: principal investigator who oversaw all aspects of the study including review of the final manuscript; M. Randesi sample preparation and data acquisition; J. Ott and J. C. da Rosa: statistical analysis. E. Peles, M. Adelson, and J. Rotrosen: subjects' ascertainment, study samples providers. All authors have approved the final manuscript.
Disclosure: None of the authors have any conflicts of interest to declare with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addolorato G, Leggio L, Hopf FW, et al. Novel therapeutic strategies for alcohol and drug addiction: Focus on gaba, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology. 2012;37:163–77. doi: 10.1038/npp.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary AW, Lominac KD, Wroten MG, et al. Imbalances in prefrontal cortex cc-homer1 versus cc-homer2 expression promote cocaine preference. J Neurosci. 2013;33:8101–13. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Hao L, Pe'er I, et al. Abraham's children in the genome era: Major jewish diaspora populations comprise distinct genetic clusters with shared middle eastern ancestry. Am J Hum Genet. 2010;86:850–9. doi: 10.1016/j.ajhg.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, et al. Variation in gabra2 predicts drinking behavior in project match subjects. Alcohol Clin Exp Res. 2007;31:1780–7. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, et al. Regulation of the gaba cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–9. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Huang CC, Liao DL. Association analysis of gabrb3 promoter variants with heroin dependence. PLoS One. 2014;9:e102227. doi: 10.1371/journal.pone.0102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JP, Kampman KM, Oslin DW, et al. Association of a polymorphism in the homer1 gene with cocaine dependence in an african american population. Psychiatr Genet. 2005;15:277–83. doi: 10.1097/00041444-200512000-00010. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A, Tomasetti C. Calcium-dependent networks in dopamine-glutamate interaction: The role of postsynaptic scaffolding proteins. Mol Neurobiol. 2012;46:275–96. doi: 10.1007/s12035-012-8293-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, et al. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Kalivas PW. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–27. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- Ducci F, Roy A, Shen PH, et al. Association of substance use disorders with childhood trauma but not african genetic heritage in an african american cohort. Am J Psychiatry. 2009;166:1031–40. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton AC, Lourdusamy A, Havranek M, et al. Alphacamkii controls the establishment of cocaine's reinforcing effects in mice and humans. Transl Psychiatry. 2014;4:e457. doi: 10.1038/tp.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan Q, et al. The influence of gabra2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry. 2010;67:20–7. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Zhou Z, Kimura M, et al. Gabaergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol-naive p and np rats. PLoS One. 2012;7:e29369. doi: 10.1371/journal.pone.0029369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Crepaldi L, Nicoletti F. Metabotropic glutamate 1 receptor: Current concepts and perspectives. Pharmacol Rev. 2008;60:536–81. doi: 10.1124/pr.108.000166. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Giese KP, Mizuno K. The roles of protein kinases in learning and memory. Learn Mem. 2013;20:540–52. doi: 10.1101/lm.028449.112. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76 Pt B:276–86. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of gaba and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, et al. Addictions biology: Haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–15. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MM, Okvist A, Horvath M, et al. Dopamine receptor d1 and postsynaptic density gene variants associate with opiate abuse and striatal expression levels. Mol Psychiatry. 2013;18:1205–10. doi: 10.1038/mp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, et al. The kreek-mchugh-schluger-kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–50. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, et al. Illicit psychoactive substance use, heavy use, abuse, and dependence in a us population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–9. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Feng J, Robison AJ, et al. Class i hdac inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16:434–40. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park M, Yang SY, et al. Association study of polymorphisms in n-methyl-d-aspartate receptor 2b subunits (grin2b) gene with korean alcoholism. Neurosci Res. 2006;56:220–3. doi: 10.1016/j.neures.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, Anton RF, et al. Association of markers in the 3' region of the glur5 kainate receptor subunit gene to alcohol dependence. Alcohol Clin Exp Res. 2009;33:925–30. doi: 10.1111/j.1530-0277.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, et al. Opiate addiction and cocaine addiction: Underlying molecular neurobiology and genetics. J Clin Invest. 2012;122:3387–93. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo PH, Kalsi G, Prescott CA, et al. Associations of glutamate decarboxylase genes with initial sensitivity and age-at-onset of alcohol dependence in the irish affected sib pair study of alcohol dependence. Drug Alcohol Depend. 2009;101:80–7. doi: 10.1016/j.drugalcdep.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, et al. Genetic susceptibility to heroin addiction: A candidate gene association study. Genes Brain Behav. 2008;7:720–9. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, et al. Heroin addiction in african americans: A hypothesis-driven association study. Genes Brain Behav. 2009;8:531–40. doi: 10.1111/j.1601-183X.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Randesi M, et al. Dopaminergic pathway polymorphisms and heroin addiction: Further support for association of csnk1e variants. Pharmacogenomics. 2014a;15:2001–9. doi: 10.2217/pgs.14.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Randesi M, et al. Stress-related genes and heroin addiction: A role for a functional fkbp5 haplotype. Psychoneuroendocrinology. 2014b;45:67–76. doi: 10.1016/j.psyneuen.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Randesi M, da Rosa JC, et al. Overlapping dopaminergic pathway genetic susceptibility to heroin and cocaine addictions in african americans. Ann Hum Genet. 2015;79:188–98. doi: 10.1111/ahg.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Randesi M, Li Y, et al. Drug addiction and stress-response genetic variability: Association study in african americans. Ann Hum Genet. 2014c;78:290–8. doi: 10.1111/ahg.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Sulovari A, Cheng C, et al. Association of gamma-aminobutyric acid a receptor alpha2 gene (gabra2) with alcohol use disorder. Neuropsychopharmacology. 2014;39:907–18. doi: 10.1038/npp.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh EW, Tang NL, Lee DT, et al. Association analysis of gaba receptor subunit genes on 5q33 with heroin dependence in a chinese male population. Am J Med Genet B Neuropsychiatr Genet. 2007;144:439–43. doi: 10.1002/ajmg.b.30429. [DOI] [PubMed] [Google Scholar]
- Marenco S, Savostyanova AA, van der Veen JW, et al. Genetic modulation of gaba levels in the anterior cingulate cortex by gad1 and comt. Neuropsychopharmacology. 2010;35:1708–17. doi: 10.1038/npp.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miguens M, Botreau F, Olias O, et al. Genetic differences in the modulation of accumbal glutamate and gamma-amino butyric acid levels after cocaine-induced reinstatement. Addict Biol. 2013;18:623–32. doi: 10.1111/j.1369-1600.2011.00404.x. [DOI] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, et al. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–10. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. Gaba a receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–8. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard A, Seddik R, Bettler B. Gabab receptors: Physiological functions and mechanisms of diversity. Adv Pharmacol. 2010;58:231–55. doi: 10.1016/S1054-3589(10)58010-4. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: Physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–36. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Buonora M, Brownstein AJ, et al. Regional mrna expression of gabaergic receptor subunits in brains of c57bl/6j and 129p3/j mice: Strain and heroin effects. Brain Res. 2013;1523:49–58. doi: 10.1016/j.brainres.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: Implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16:251–7. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Tian C, Kosoy R, Nassir R, et al. European population genetic substructure: Further definition of ancestry informative markers for distinguishing among diverse european ethnic groups. Mol Med. 2009;15:371–83. doi: 10.2119/molmed.2009.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhu YS, Li SB. Polymorphisms in the glutamate decarboxylase 1 gene associated with heroin dependence. Biochem Biophys Res Commun. 2012;422:91–6. doi: 10.1016/j.bbrc.2012.04.112. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Increased mesolimbic gaba concentration blocks heroin self-administration in the rat. J Pharmacol Exp Ther. 2000;294:613–9. [PubMed] [Google Scholar]
- Yang Y, Chen Y, Wu J, et al. association study of cnr1, gad1 and bdnf polymorphisms with male heroin dependence in the dai population in yunnan. Yi Chuan. 2014;36:888–96. doi: 10.3724/SP.J.1005.2014.0888. [DOI] [PubMed] [Google Scholar]
- Zhao B, Zhu Y, Wang W, et al. Analysis of variations in the glutamate receptor, n-methyl d-aspartate 2a (grin2a) gene reveals their relative importance as genetic susceptibility factors for heroin addiction. PLoS One. 2013;8:e70817. doi: 10.1371/journal.pone.0070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong HJ, Huo ZH, Dang J, et al. Functional polymorphisms of the glutamate receptor n-methyl d-aspartate 2a gene are associated with heroin addiction. Genet Mol Res. 2014;13:8714–21. doi: 10.4238/2014.October.27.12. [DOI] [PubMed] [Google Scholar]
- Zintzaras E. Gamma-aminobutyric acid a receptor, alpha-2 (gabra2) variants as individual markers for alcoholism: A meta-analysis. Psychiatr Genet. 2012;22:189–96. doi: 10.1097/YPG.0b013e328353ae53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


