Abstract
Insulin increases glucose transport in fat and muscle cells by stimulating the exocytosis of specialized vesicles containing the glucose transporter GLUT4. This process, which is referred to as GLUT4 translocation, increases the amount of GLUT4 at the cell surface.
Previous studies have provided evidence that insulin signaling increases the amount of Rab10-GTP in the GLUT4 vesicles and that GLUT4 translocation requires the exocyst, a complex that functions in the tethering of vesicles to the plasma membrane, leading to exocytosis. In the present study we show that Rab10 in its GTP form binds to Exoc6 and Exoc6b, which are the two highly homologous isotypes of an exocyst subunit, that both isotypes are found in 3T3-L1 adipocytes, and that knockdown of Exoc6, Exoc6b, or both inhibits GLUT4 translocation in 3T3-L1 adipocytes. These results suggest that the association of Rab10-GTP with Exoc6/6b is a molecular link between insulin signaling and the exocytic machinery in GLUT4 translocation.
Keywords: Exocyst, GLUT4, Insulin, Rab10
1. Introduction
A major physiological effect of insulin is to lower the blood glucose level. Insulin causes part of this effect by increasing the rate at which glucose enters fat and muscle cells. The increase in glucose transport is due to an insulin-elicited increase in the amount of the glucose transporter GLUT4 at the cell surface. This increase in GLUT4 at the plasma membrane is caused by the rapid exocytosis of specialized vesicles containing GLUT4 in response to insulin [1, 2]. The latter process is referred to as GLUT4 translocation. A challenge has been to elucidate the molecular pathway(s) linking insulin signaling to GLUT4 translocation. Considerable evidence now supports the following pathway in adipocytes: insulin activates the protein kinase Akt; Akt phosphorylates the Rab GTPase activating protein TBC1D4; phosphorylation of TBC1D4 suppresses its GTPase activating activity toward its target Rabs, which include Rab10; as a consequence Rab10, which is a component of the GLUT4 vesicles, converts from the GDP to the GTP form; then the elevated Rab10-GTP in the GLUT4 vesicles in some way initiates their exocytosis [3-7].
The exocyst is a complex of eight subunits that acts to tether exocytic vesicles to the plasma membrane. This engagement then leads onto fusion of the vesicles with the plasma membrane through association of their respective SNARE proteins [8-11]. Previous studies have implicated the exocyst as a participant in GLUT4 vesicle exocytosis in adipocytes [12-14]. The evidence includes the finding that knockdown of several exocyst subunits (Exoc 3, 4, and 7) partially inhibited insulin-stimulated glucose transport [13]. One way in which exocytic vesicles bind to the exocyst is through the association of a vesicle Rab in its GTP form with an exocyst subunit. In yeast this subunit is Sec15 [8, 9]. Animal cells contain two genes homologous to Sec15, Exoc6 and Exoc6b [15]. These two encode highly similar forms of this subunit; the amino acid sequences of the mouse proteins are 71 % identical. Earlier studies in animal cells identified Rab11 as a Rab that binds to Exoc6 [16] and participates in exocyst-dependent exocytosis in various cells [8, 9].
In the present study, we show that Rab10 in its GTP form binds to both Exoc6 and Exoc6b and that both Exoc6 and 6b participate in GLUT4 translocation in 3T3-L1 adipocytes. These findings thus suggest that the association of the elevated Rab10-GTP on GLUT4 vesicles with Exoc6/6b connects insulin signaling to the exocytosis of the vesicles.
2. Materials and Methods
2.1 Plasmids, antibodies, proteins, and siRNAs
The plasmid for mouse Rab10 was a generous gift from Dr. Mitsunori Fukuda. Plasmids for mouse Exoc6 and mouse Exoc6b were purchased from DNAForm and Open Biosystems, respectively. Plasmids in desired vectors were generated from these by PCR of the cDNA and ligation into appropriate restriction sites. Mutations were made by use of Agilent Technologies mutagenesis kits. All constructs were verified by DNA sequencing. An antiserum raised against a GST-fusion protein with amino acids 1–110 of mouse Exoc6 was a generous gift from Dr. Nancy C. Andrews [17]. Antibodies against phosphoserine 473 of Akt and phosphothreonine 642 of TBC1D4 were from Cell Signaling Technology, catalog numbers 9271 and 4288, respectively. Recombinant 3×Flag-tagged mouse Exoc6 and 6b were purified on anti-Flag beads (Sigma) from HEK293 cells transfected with the cDNAs for these proteins in the p3×FLAG-CMV-7.1 vector, as described in [18]. The control siRNA (catalog number 4390844) and the siRNAs for Exoc6 (catalog number 4390771, s98838) and Exoc6b were Silencer Select ones from Ambion/Life Technologies. The siRNA for Exoc6b was a custom one. Its sequence was: CCCGUUUCAAGAUAUAGAAtt.
2.2 Yeast two-hybrid analyses
Initially a yeast two-hybrid screen was performed with mouse Rab10 Q68L as the bait and a cDNA library derived from a differentiated human brown adipocyte cell line. Rab10 Q68L is a mutant that primarily binds GTP [19, 20]. In addition the carboxy terminal CysCys residues of Rab10, which are sites of lipid modification, were mutated to AlaAla, so that lipidation and binding to membranes could not occur. This bait was in the pB27 vector (derived from pBTM116), which leads to expression of the LexA DNA binding domain fused to the amino terminus of Rab10. The prey cDNAs were in the pP6 plasmid (derived from pGADGH), which results in the Gal4 activation domain linked to the amino terminus of the translated cDNA fragments. Bait and prey constructs were transformed into yeast haploid cells, respectively L40ΔGal4 (mata) and YHGX13 (Y187ade2-101::loxP-kanMX-loxP, matα) strains. The diploid cells were obtained using a mating protocol with both yeast strains [21]. Diploid yeast containing both bait and prey plasmids grow on a selective medium without tryptophan and leucine. Yeast in which the bait and prey interact can additionally grow in a selective medium without histidine due to expression of the HIS3 gene. Prey plasmids in the yeast growing on histidine-deficient selective medium were sequenced to identify the interacting preys.
We subsequently examined the interaction of the Rab10 Q68L bait with the carboxy terminal portions of Exoc6 and Exoc6b as preys and the interaction of the carboxy terminal portion of Exoc6b as prey with Rab10 Q68L, wild-type Rab10, and Rab10 T23N as baits. The same method as described for the screen was used. The three Rab baits contained the CysCys to AlaAla mutation. In these analyses, diploid yeast were also grown in the selective medium without tryptophan, leucine, and histidine in the presence of various concentrations of 3-aminotriazole, an inhibitor of the his3 enzyme. Yeast growth in the presence of higher concentrations of 3-aminotriazole indicates a stronger association of the bait and prey.
2.3 Cell culture and assay for cell surface GLUT4
3T3-L1 adipocytes expressing HA-GLUT4-GFP were generated as previously described [4]. On day four of differentiation siRNA was introduced into the cells by the nucleofection with an Amaxa/Lonza Nucleofector 2b device according to the protocol for these cells developed by this company. The total siRNA concentration used for nucleofection was 2 μM. The four conditions used were control siRNA alone, a mixture of the control siRNA plus an equal concentration of the siRNA for Exoc6 or Exoc6b, and a mixture of equal concentrations of the siRNAs for Exoc6 and Exoc6b. Three days later the cells were assayed for HA-GLUT4-GFP at the cell surface by the flow cytometry method described in [4]. In brief, in this assay HA-GLUT4-GFP at the cell surface is detected by immunofluorescence due to the binding of a fluorophore-labeled secondary anti-mouse antibody to mouse anti-HA bound to the extracellular HA tag on GLUT4. The signal from the HA tag is normalized to the total amount of HA-GLUT4-GFP in the cell by dividing by the GFP fluorescence of the cell. Adipocytes were put in serum-free medium with 1 mg per ml bovine serum albumin for 1.5 h, treated with 160 nM insulin for 30 min or not, and then chilled to 4° C for the assay.
2.4 Immunoblotting
SDS samples of 3T3-L1 adipocytes were prepared from duplicate wells at the same time as the assay of cell surface GLUT4, and the protein concentrations thereof were measured, as described previously [4]. SDS PAGE and immunoblotting were performed as in [4]. Mouse Exoc6 and Exoc6b are very similar in size (804 and 810 amino acids, respectively). Separation of the two on SDS PAGE required running the proteins toward the bottom of a 12-cm long, 8 % gel.
3. Results
3.1 Association of Rab10 with Exoc6b and Exoc6
In order to find adipocyte proteins that associate with the GTP form of Rab10, a yeast two-hybrid screen with the constitutively active Rab10 Q68L as the bait and a library from human brown adipocytes was performed. Table 1 lists twelve Rab10 interactors found with high confidence. Among this group, the associations of Rab10 with Ehbp1, Evi5, Mical1, Micall1, Micall2, and Myo5A have also been detected previously by various methods [22-24]. In addition, Akap10, Optn, and Rabep1 have been described to interact with other Rabs [23, 25].
Table 1.
Interactors with Rab10 Q68L
Rab10 Q68L was used as the bait with a library from human brown adipocytes in the yeast two-hybrid analysis, as described in the Materials and Methods. The number of independent colonies and the amino acid numbers of the smallest interacting fragment are given for each interactor.
| Interactor | Colonies | Fragment |
|---|---|---|
| AKAP10 (NM_007202) | 3 | 301-662 |
| EHBP1 (NM_015252) | 3 | 853-1210 |
| EVI5 (NM_005665) | 18 | 460-629 |
| EXOC6B (NM_015189) | 11 | 393-806 |
| FNTA (NM_002027) | 12 | 26-244 |
| MAP4K3 (NM_003618) | 4 | 491-894 |
| MICAL1 (NM_022765) | 8 | 978-1067 |
| MICALL1 (NM_033386) | 35 | 656-836 |
| MICALL2 (NM_182924) | 4 | 656-901 |
| MYO5A (NM_000259) | 2 | 1125-1450 |
| OPTN (NM_001008213) | 29 | 82-214 |
| RABEP1 (NM_001083585) | 9 | 180-369 |
One of the Rab10 interactors found was the Exoc6b subunit of the exocyst complex (Table 1). Previously, drosophila Rab11 in its GTP form [26] has been shown to bind to the carboxy terminal half of drosophila Exoc6. Our finding that Rab10 Q68L interacts with a fragment of human Exoc6b containing the carboxy terminal part of the protein (amino acids 393-806 out of 811 amino acids) agrees with the identification of this region as the Rab binding portion of the protein.
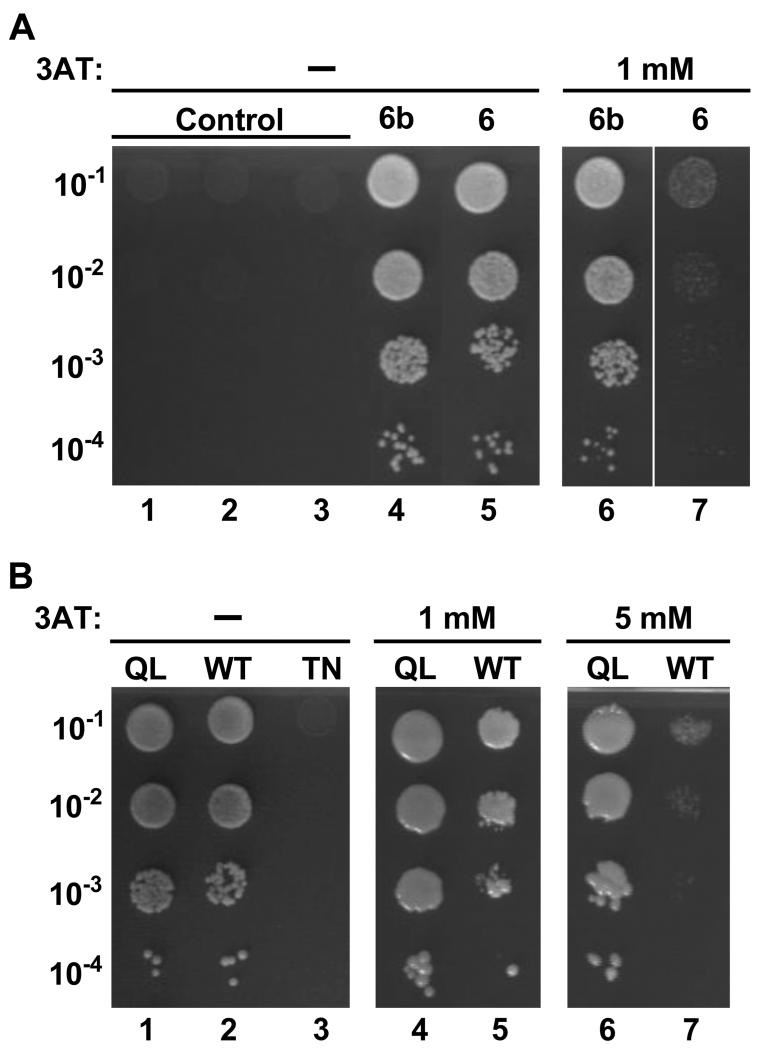
Since both Exoc6 and 6b are expressed in 3T3-L1 adipocytes (see below), we determined whether Exoc6 also interacts with Rab10 Q68L. In order to do so, we constructed a prey plasmid expressing amino acids 384-797 of human Exoc6 (NP_061926), which is the region homologous to the smallest fragment of Exoc6b found to interact with Rab10, and tested its ability to support growth with the Rab10 Q68L bait plasmid in the yeast two-hybrid system (Fig. 1A). Exoc6 also bound to Rab10 (Fig 1A, lane 5). Fig. 1A also shows in the interaction between the corresponding Exoc6b fragment and Rab10 (lane 4).
Fig 1.
Association of Rab10 with Exoc 6 and 6b. Growth on selective medium of several dilutions (10−1 to 10−4) of the various diploid yeast cells, from the same number of cells in the stocks, in the absence and presence of 3-aminotriazole (3AT) is shown. Part A. The diploid cells (lanes 1 – 7) contained the following plasmid pairs: 1, empty bait vector and Gal activation domain (GAD)-Exoc6 carboxy terminus (CT); 2, empty bait vector and GAD-Exoc6b CT; 3, LexA-Rab10 Q68L and empty prey vector; 4 and 6, LexA-Rab10 Q68L and GAD-Exoc6b CT; 5 and 7 LexA-Rab10 Q68L and GAD-Exoc6 CT. Part B. The diploid cells contained the GAD-Exoc6b CT prey plasmid together with LexA-Rab10 Q68L (lanes 1, 4, 6), LexA-Rab10 wild-type (lanes 2, 5, 7), or LexA-Rab10 T23N (lane 3). In both parts A and B growth on 1, 5, 10, and 50 mM 3AT was determined, but for ease of presentation the results with the higher concentrations are not shown. 50 mM 3AT was required to inhibit completely the growth of cells containing LexA-Rab10 Q68L and GAD-Exoc6b CT.
To obtain a qualitative estimate of the relative affinities of Rab10 Q68L for Exoc6b and Exoc6, we compared the growth of the yeast containing the bait Rab10 plasmid and prey Exoc6b or Exoc6 plasmid in the presence of various concentrations of 3-aminotriazole, an inhibitor of the histidine biosynthetic enzyme required for yeast growth on the selective medium. 3-aminotriazole at a concentration of 1 mM was sufficient to suppress growth with the Exoc6 fragment, but not with the Exoc6b fragment (Fig. 1A, lanes 6, 7). Hence, Exoc6b binds more strongly than Exoc6 to Rab10 Q68L. Titration of the 3-aminotriazole showed that suppression of growth in the presence of the Exoc6b plasmid required 50 mM (see legend of Fig. 1).
To determine the relative affinities of Rab10 in the GDP and GTP forms for the Exoc6b carboxy terminal fragment, we examined growth of yeast cells containing this Exoc6b prey plasmid and the bait plasmid for Rab10 Q68L, wild-type Rab10, or Rab10 T23N. Wild-type Rab10 is expected to be a mixture of largely GDP form with some GTP form [20], and Rab10 T23N is expected to be only in the GDP form [27]. Wild-type Rab10 supported growth, whereas Rab10 T23N did not (Fig 1B, lanes 2, 3). 3-aminotriazole in the range of 1 to 5 mM suppressed growth with wild-type Rab10, whereas 50 mM was required to suppress growth with Rab10 Q68L (Fig. 1B, lanes 4-7 and legend). These results indicate that Exoc6b binds most strongly to Rab10 in the GTP form.
3.2 Participation of Exoc6 and Exoc6b in GLUT4 translocation
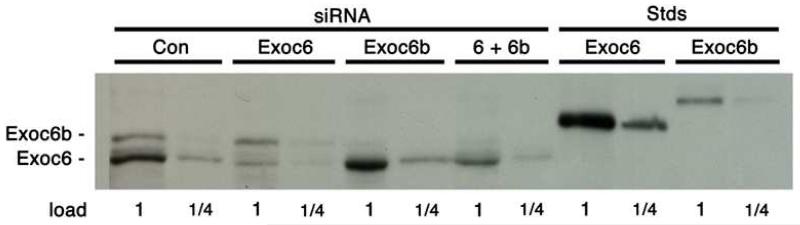
We were able to detect both mouse recombinant Flag-tagged Exoc6 and Exoc6b by immunoblotting with an antibody made against a GST fusion protein with an amino terminal fragment of mouse Exoc6 (Fig. 2, Stds lanes). The signal for Exoc6 is about four times stronger than that for the same amount of Exoc6b. 3T3-L1 adipocytes contain both Exoc6 and Exoc6b (Fig. 2, siRNA Con lanes). Based on visual comparison of the intensities of the Exoc6 and Exoc6b signals with those of the recombinant standards, the relative amounts of Exoc6 and 6b in 3T3-L1 adipocytes are approximately 1 to 2. A siRNA for Exoc6 knocked down the Exoc6 by about 75 %, without any effect on expression of Exoc6b (Fig. 2, compare the siRNA Con and Exoc6 lanes). Similarly, a siRNA for Exoc6b knocked down the Exoc6b by about 75 %, without any effect on Exoc6 expression (Fig. 2, compare the siRNA Con and Exoc6b lanes). The combination of both siRNAs resulted in about 50 % knockdown of Exoc6 and 75 % knockdown of Exoc6b (Fig. 2, compare siRNA Con and 6+6b lanes).
Fig 2.
siRNA knockdown of Exoc 6 and 6b. The immunoblot for Exoc6/6b was performed with SDS samples of 3T3-L1 adipocytes containing control siRNA alone (Con), siRNA for Exoc6 or Exoc6b plus Con, or a mixture of siRNAs for Exoc 6 and 6b. The 1× load was 20 μg protein. The standards (Stds) were purified recombinant 3×Flag-tagged Exoc 6 or 6b. The 1 load for the standards was 1 ng. The 3×Flag tag on the standards decreased their mobility slightly. A replicate of this immunoblot with SDS samples from a separate experiment gave similar results.
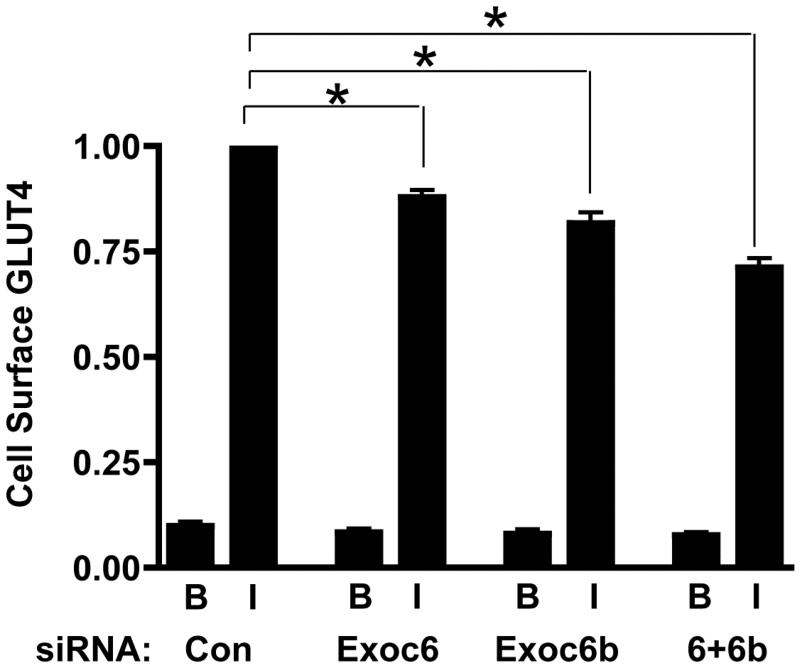
We determined the effect of these knockdowns on the insulin-stimulated translocation of GLUT4 to the cell surface, assayed with HA-GLUT-GFP (Fig. 3). The control showed the expected 10-fold increase in GLUT4 at the cell surface upon insulin treatment. Knockdown of Exoc6 reduced the amount of GLUT4 at the cell surface to 88 % of the control value. Knockdown of Exoc6b reduced the amount to 81 % of the control value. The combined knockdown caused a reduction to 71 % of the control value. Hence, both Exoc6 and 6b participate in GLUT4 translocation.
Fig 3.
Effects of Exoc 6 and 6b knockdown on GLUT4 at the cell surface. 3T3-L1 adipocytes containing control siRNA (Con) or siRNA for Exoc6 or Exoc6b plus Con or the mixture of siRNAs for Exoc 6 and 6b were analyzed for HA-GLUT4-GFP at the cell surface in the basal (B) or insulin (I) state, as described in the Experimental Procedures. For each experiment the values were normalized to 1.0 for the control siRNA in the insulin state. The results are the means +/− the standard deviation for seven separate experiments. * designates p<0.05 for the comparison with the control insulin value.
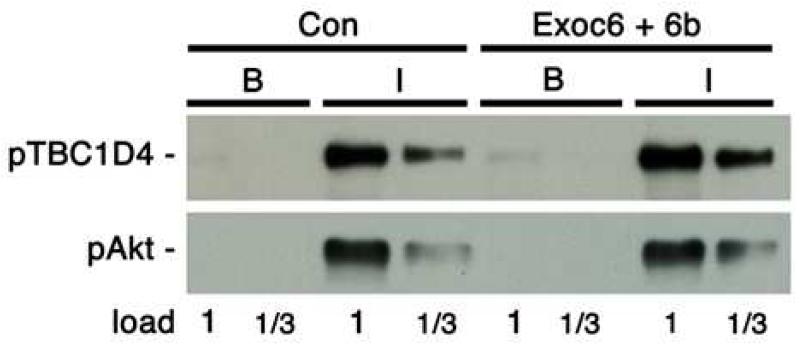
It is most likely that the inhibitory effects of the knockdowns are due to interference with exocyst function in GLUT4 vesicle exocytosis. An alternate explanation is that in some way the knockdowns inhibit the insulin signaling pathway leading to GLUT4 translocation, which proceeds through the kinase Akt and its substrate the Rab10 GTPase activating protein TBC1D4. To check this possibility, we immunoblotted SDS samples of basal and insulin-treated 3T3-L1 adipocytes for Akt activation, as assessed by phosphorylation on Ser473, and for Akt phosphorylation of TBC1D4 on Thr642. Both the sites showed robust phosphorylation, which was not decreased by knockdown of Exoc6/6b (Fig. 4). Thus, the knockdown did not inhibit insulin signaling to GLUT4 translocation.
Fig 4.
Effect of Exoc6/6b knockdown on insulin signaling. 3T3-L1 adipocytes containing control siRNA (Con) or the mixture of siRNAs for Exoc6 and Exoc6b were treated with insulin (I) or left in the basal state (B). SDS samples of these were immunoblotted for the phosphothreonine 642 on TBC1D4 or phosphoserine 473 on Akt. The 1 load was 20 μg protein. A replicate of this experiment gave the same results.
4. Discussion
The results herein show that Exoc6/6b is an effector for Rab10 in its GTP form and that Exoc6/6b participates in GLUT4 translocation in adipocytes. In conjunction with the earlier studies indicating that insulin elevates Rab10-GTP in GLUT4 vesicles and implicating the exocyst complex in GLUT4 translocation, these findings support the proposal that this interaction acts as a link between insulin signaling and GLUT4 vesicle exocytosis.
The combined knockdowns of Exoc 6 and 6b inhibited GLUT4 translocation by 30 %. One explanation for the fact that the inhibition was not larger may be that the knockdowns were not complete. The remaining amount of Exoc6/6b subunit may be sufficient to support the reduced rate of GLUT4 vesicle exocytosis. A second possible explanation is that a portion of the GLUT4 exocytosis proceeds independently of the exocyst. In a previous study [13] knockdowns of Exoc 3, 4 and 7 resulted in 37, 29, and 57 % inhibition of insulin-stimulated glucose transport, which reflects GLUT4 translocation.
The role of the exocyst in exocytosis has been studied most extensively in budding yeast. In this system exocytic vesicles interact in part with the exocyst through binding of the vesicular yeast Rab Sec4 to the exocyst subunit, Sec15, which is the yeast homolog of Exoc6/6b [8, 9]. Among the 60 mammalian Rabs, Rab10 and the closely related Rabs 8 and 13 are the ones most similar to Sec4. Thus, the association of Rab10-GTP with Exoc6/6b seemed likely, but it has not been previously described. An earlier study reported the co-immunoprecipitation of Rab10 with another subunit of the exocyst, Exoc4, from renal epithelial cells [28]. This co-immunoprecipitation may involve the association of Rab10 with Exoc6/6b in the exocyst complex. In muscle cells the insulin signaling pathway to GLUT4 translocation proceeds through elevation of Rab8-GTP, rather than Rab10-GTP [2]. Drosophila Exoc6 has been reported to bind to GST-Rab8 in the GTP form [26]. Thus, the exocyst may also participate in GLUT4 translocation in muscle cells.
Two other subunits of the exocyst, Exoc2 and Exoc8, bind to the small G protein Ral in its GTP form [8, 9]. Insulin treatment of adipocytes elevates the GTP form of Ral, and this process has been implicated in GLUT4 translocation [1, 7]. While our study was in progress, it was reported that Rab10-GTP binds Rlf, a guanine nucleotide exchange factor for Ral, and that this association contributes to the increase of Ral-GTP seen in response to insulin [7]. Since GLUT4 vesicles contain Ral as well as Rab10, it was proposed that Rlf is a Rab10 effector that links insulin signaling to the binding of GLUT4 vesicles to the exocyst via Ral-GTP. In light of our results it is likely that the role of vesicular Rab10-GTP in the association of GLUT4 vesicles with the exocyst consists of both its direct association with Exoc6/6b and its role in generating vesicular Ral-GTP. Since each GLUT4 vesicle almost certainly contains a number of Rab10 molecules, this dual role is feasible.
In the future, it will be important to determine how the interaction of the exocyst with GLUT4 vesicles develops in response to insulin. In yeast it appears that all the subunits of the exocyst except Sec3p (analogous to animal Exoc1) and Exo70p (analogous to animal Exoc7) associate first with the exocytic vesicle; Sec3p and Exo70p are found on the plasma membrane and join the complex there [29]. A second unanswered question is how the tethering of vesicles to the plasma membrane by the exocyst leads on to fusion.
Supplementary Material
Highlights.
Insulin stimulates the fusion of vesicles containing GLUT4 with the plasma membrane.
This requires vesicular Rab10-GTP and the exocyst plasma membrane tethering complex.
We find that Rab10-GTP associates with the Exoc6 subunit of the exocyst.
We find that knockdown of Exoc6 inhibits fusion of GLUT4 vesicles with the membrane.
The interaction of Rab10-GTP with Exoc6 potentially links signaling to exocytosis.
Acknowledgements
We are grateful to Dr. Petra Tafelmeyer at Hybrigenics for overseeing the yeast two-hybrid screen of the brown adipocyte library and to Kyoko Sano for technical assistance. This research was supported by NIH grant DK025336 to G.E.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- [2].Klip A, Sun Y, Chiu TT, et al. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am. J. Physiol. Cell Physiol. 2014;306:C879–886. doi: 10.1152/ajpcell.00069.2014. [DOI] [PubMed] [Google Scholar]
- [3].Sakamoto K, Holman GD. Emerging role for As160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 2008;295:E29–37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sano H, Peck GR, Kettenbach AN, et al. Insulin-stimulated GLUT4 translocation in adipocytes requires the Rab10 guanine nucleotide exchange factor Dennd4C. J. Biol. Chem. 2011;286:16541–16545. doi: 10.1074/jbc.C111.228908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen Y, Wang Y, Zhang J, et al. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J. Cell. Biol. 2012;198:545–560. doi: 10.1083/jcb.201111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sadacca LA, Bruno J, Wen J, et al. Specialized sorting of GLUT4 and its recruitment to the cell surface are independently regulated by distinct Rabs. Mol. Biol. Cell. 2013;24:2544–2557. doi: 10.1091/mbc.E13-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karunanithi S, Xiong T, Uhm M, et al. A Rab10:RalA G protein cascade regulates insulin-stimulated glucose uptake in adipocytes. Mol. Biol. Cell. 2014;25:3059–3069. doi: 10.1091/mbc.E14-06-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].He B, Guo W. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luo G, Zhang J, Guo W. The role of Sec3p in secretory vesicle targeting and exocyst complex assembly. Mol. Cell Biol. 2014;25:3813–3822. doi: 10.1091/mbc.E14-04-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rivera-Molina F, Toomre D. Live-cell imaging of the exocyst links its spatiotemporal dynamics to various stages of vesicle fusion. J. Cell Biol. 2013;201:673–680. doi: 10.1083/jcb.201212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Inoue M, Chang L, Hwang J, et al. The exocyst complex is required for targeting of GLUT4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- [13].Inoue M, Chiang S, Chang L, et al. Compartmentalization of the exocyst complex in lipid rafts controls GLUT4 vesicle tethering. Mol. Biol. Cell. 2006;17:2303–2311. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ewart M, Clarke M, Kane S, et al. Evidence for a role of the exocyst in insulin-stimulated Glut4 trafficking in 3T3-L1 adipocytes. J. Biol. Chem. 2005;280:3812–3816. doi: 10.1074/jbc.M409928200. [DOI] [PubMed] [Google Scholar]
- [15].Brymora A, Valova VA, Larsen MR, et al. The brain exocyst complex interacts with RalA in a GTP-dependent manner. J. Biol. Chem. 2001;276:29792–29797. doi: 10.1074/jbc.C100320200. [DOI] [PubMed] [Google Scholar]
- [16].Zhang X, Ellis S, Sriratana A, et al. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- [17].Lim JE, Jin O, Bennett C, et al. A mutation in Sec15l1 causes anemia in hemoglobin deficit (hbd) mice. Nat. Genet. 2005;37:1270–1273. doi: 10.1038/ng1659. [DOI] [PubMed] [Google Scholar]
- [18].Chavez J, Roach WG, Keller SR, et al. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J. Biol. Chem. 2008;238:9187–9195. doi: 10.1074/jbc.M708934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sano H, Eguez L, Teruel MN, et al. Rab10, a target of the AS160 GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [20].Sano H, Roach WG, Peck GR, et al. Rab10 in insulin-stimulated GLUT4 translocation. Biochem. J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- [21].Fromont-Racine M, Rain JC, Legrain P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat. Genet. 1997;16:277–282. doi: 10.1038/ng0797-277. [DOI] [PubMed] [Google Scholar]
- [22].Shi A, Chen CC, Banerjee R, et al. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol. Biol. Cell. 2010;21:2930–2943. doi: 10.1091/mbc.E10-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fukuda M, Kanno E, Ishibashi K, et al. Large scale screening for novel rab effectors reveals unexpected broad rab binding specificity. Mol. Cell. Proteomics. 2008;7:1031–1042. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- [24].Roland JT, Lapierre LA, Goldenring JR. Alternative splicing in class V myosins determines association with Rab10. J. Biol. Chem. 2009;284:1213–1223. doi: 10.1074/jbc.M805957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vitale G, Rybin V, Christoforidis S, et al. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu S, Mehta SQ, Pichaud F, et al. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol. 2005;12:879–895. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- [27].Lee MG, Mishra A, Lambright DG. Structural mechanisms for regulation of membrane traffic by Rab GTPases. Traffic. 2009;10:1377–1389. doi: 10.1111/j.1600-0854.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Babbey CM, Bacallao RL, Dunn KW. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am. J. Physiol. Renal Physiol. 2010;299:F495–506. doi: 10.1152/ajprenal.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boyd C, Hughes T, Pypaert M, et al. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. M. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.