Abstract
Behavioral testing of mouse models of Huntington's disease (HD) is a key component of preclinical assessment for potential pharmacological intervention. An open field with a force plate floor was used to quantify numerous spontaneous behaviors in a slowly progressing model of HD. CAG140 (+/+, +/−, −/−) male and female mice were compared in a longitudinal study from 6 to 65 weeks of age. Distance traveled, wall rears, wall rear duration, number of low mobility bouts, in-place movements, number of high velocity runs, and gait parameters (stride rate, stride length, and velocity) were extracted from the ground reaction forces recorded in 20-min actometer sessions. Beginning at 11 weeks, HD mice (both +/− and +/+) were consistently hypoactive throughout testing. Robust hypoactivity at 39 weeks of age was not accompanied by gait disturbances. By 52 and 65 weeks of age the duration of wall rears increased and in-place tremor-like movements emerged at 65 weeks of age in the +/+, but not in the +/− HD mice. Taken together, these results suggest that hypoactivity preceding frank motor dysfunction is a characteristic of CAG140 mice that may correspond to low motivation to move seen clinically in the premanifest/prediagnostic stage in human HD. The results also show that the force plate method provides a means for tracking the progression of behavioral dysfunction in HD mice beyond the stage when locomotion is lost while enabling quantification of tremor-like and similar in-place behaviors without a change in instrumentation. Use of force plate actometry also minimizes testing-induced enrichment effects when batteries of different tests are carried out longitudinally.
Keywords: Force plate, distance traveled, gait, Huntington's Disease, CAG140 knock-in mouse, power spectra
1. Introduction
It has been over two decades since publication of the seminal report establishing the genetic cause of Huntington's disease (HD): a CAG triplet repeat expansion in the gene that codes for the huntingtin protein [1]. Building on this genetic discovery, researchers have developed at least 56 distinct genetic mouse models of the disease [2]. These genetic models have led to a substantial and ongoing effort to identify phenotypic traits (behavioral, neurochemical, neurophysiological, neuropathological) that allow the charting of disease course and set the context for preclinically assessing the potential efficacy of pharmacological treatments. In the behavioral domain of this modeling effort, one of the most frequently used phenotyping tools is the open field test [2,3]. The central purpose of the work reported here was to show how a force plate actometer [4] can be used as an open field that, in addition to affording measurement of locomotor activity, it can be used concurrently to measure several other behavioral parameters with high potential relevance to the expression of HD-like symptoms in mice. These additional measures include number of wall rears, duration of wall rears, frequency of occurrence of bouts of non-locomotion episodes, frequency of occurrence of long, straight runs, selected gait parameters (e.g., stride rate, stride length, and run velocity), and in-place behaviors. These latter behaviors, such as tremor or grooming, occur during periods marked by the absence of locomotion. Recording of such behaviors with a force plate actometer is made possible by the instrument's capacity to sense movement in the vertical direction even when no locomotion is occurring [4].
Force plate actometry has been successfully used to quantify several HD-like behavioral abnormalities in the fast progressing, short-life span R6/2 transgenic mouse model of HD [5] and was successfully used to demonstrate the value of disrupting the binding of calmodulin to mutant huntingtin in the R6/2 mouse model [6]. However, the force plate measurement approach has not heretofore been used to examine any slowly progressing, relatively long-lived HD mouse models, mainly created with a CAG triplet repeat expansion in a full length gene for huntingtin (the mouse used in this study is known in the literature as CAG140, KI140, CAG140 KI, or Q140; for a summary, see [2]). Such knock-in models are genetically and phenotypically more like human HD than the transgenic mouse models and appear to be preferred in preclinical research on potential pharmacological or dietary HD interventions [2,7,8,9].
For the work presented here we chose the “CAG140” mouse [10]. This model was first developed with a CAG triplet repeat expansion of about 140, but the number of CAG repeats in the offspring is variable and has shown some shrinkage, yet still retains its CAG140 name and its HD-like behavioral phenotype [2, 11]. The CAG140 mouse has been reported to be hyperactive in the open field at 1 month of age [10], whereas others have reported hypoactivity at this age [8,9]. However, at about 2 months and older, hypoactivity and/or diminished frequency of rearing appear to be a consistent finding in both heterozygous and homozygous CAG140 mice of both sexes [8,9,10,11,12]. Gait disturbance in the form of shortened stride was reported for 12 month-old CAG140 homozygous mice[10], but, in contrast, Rising et al. [12] did not detect gait alterations in CAG140 mice (neither homozygous nor heterozygous) during monthly testing conducted for 18 months. With respect to in-place behaviors, Hickey et al.[7] observed that at 20-26 months of age homozygous CAG140 mice exhibited clearly discernable “tremor or shakiness” in their home cages.
Neuropathology studies have established abnormalities in several brain loci in CAG140 mice (striatum, nucleus accumbens, olfactory tubercle, cerebral cortex) that are prominently damaged in human HD [10, 11, 13]. In addition, electrophysiological methods using CAG140 mice have revealed disruption of corticostriatal circuitry function [14], as well as, dysfunction in synaptic transmission in medium-sized spiny neurons [15], a neuronal population that is particularly vulnerable to loss in HD. Thus, the HD-like phenotype of the CAG140 mouse is well documented at several levels of analysis and appears suitable for pushing forward refinements in behavior methodology pertinent to HD preclinical research.
2. Materials and Methods
2.1. Animals, husbandry, and genotyping
Three breeding pairs of CAG 140 knock-in heterozygote C57BL/6J mice were obtained from Drs. Marie-Francoise Chesselet and Michael Levine (David Geffen School of Medicine University of California Los Angeles). This knock-in mouse model was generated with a full-length mouse/human chimeric huntingtin gene with 140 CAG repeats in exon 1 [10]. We generated 11 litters from these breeding pairs which resulted in 36 females (8−/−, 19 +/−, 9+/+) and 37 males (11−/−, 17 +/−, 9+/+) that were used for behavioral testing. Mice were genotyped by PCR amplification of DNA extracted from tail biopsies. As previously reported, the CAG repeat length is unstable in successive generations in this knock-in model similar to other models resulting in variability in CAG repeat length. Quantitation of CAG triplet repeat length was performed by Laragen, Inc, Culver City, CA 90232. The results, based on 54 CAG140 mice (homozygous and heterozygous combined), indicated a mean CAG triplet repeat expansion of 135.8, a standard deviation of 6.9, with a range of 122-147. Mice were housed in a temperature-, humidity-, and light-controlled room (12h light/dark cycle, lights on 0600 h) and food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the University of Kansas Institutional Animal Care and Use Committee.
2.2. Behavioral methods
2.2.1. Apparatus
The apparatus was a force plate actometer with a square floor [4] measuring 42 cm on each side. A mouse was confined to the load plate by a clear polycarbonate cage with 30 cm high walls. The cage was suspended 2 mm above the load plate so that the mass of the cage itself would not be sensed by the force transducers that supported the load plate. This size of the load plate was sufficiently large to allow mice to express their running behavior in uninterrupted sequences of locomotion suitable for gait analysis, but small enough to eliminate galloping. Note that a square 42 cm on a side has a diagonal measurement of 59.4 cm. The 4 force transducers that supported the load plate at its corners were sampled 100 times/s (i.e.,temporal resolution of 0.01 s). Force resolution was 0.2 gram-force, and spatial resolution was about 2 mm. A Pascal program written in house directed the timing and data-logging processes via a LabMaster interface. Additional Pascal algorithms were used to extract the macrobehavioral variables (e.g., distance traveled), and a scrolling graphics program written in Visual Basic was used to extract selected gait parameters (e.g., velocity of runs, stride length and stride rate) from the data stream.
2.2.2. Procedure
A total of 73 mice (19 −/−, 36 +/−, and 18 +/+, as mentioned in section 2.1. above) were run in the force plate actometer located in a quiet, dimly lit (~2 lux) room. Mice were evaluated once at each age in 20-min recording sessions. The ages at testing were 6, 11, 26, 39, 52, and 65 weeks. Data were collected during the light phase of the daily 12-h light/dark cycle. Between recording sessions the actometer floor and walls were wiped down with 70% ethanol solution.
2.2.3. Behavior quantitation
2.2.3.1. Distance traveled
Distance traveled was defined as the sum of the distances of adjacent x-y pairs of center of force locations taken every 0.50 s for each mouse. This time spacing between center of force locations was used in order to emphasize movements reflecting locomotion and to decrease the contribution to distance traveled of small high frequency movements that are detected when the temporal spacing is 0.01 s.
2.2.3.2. Force variability
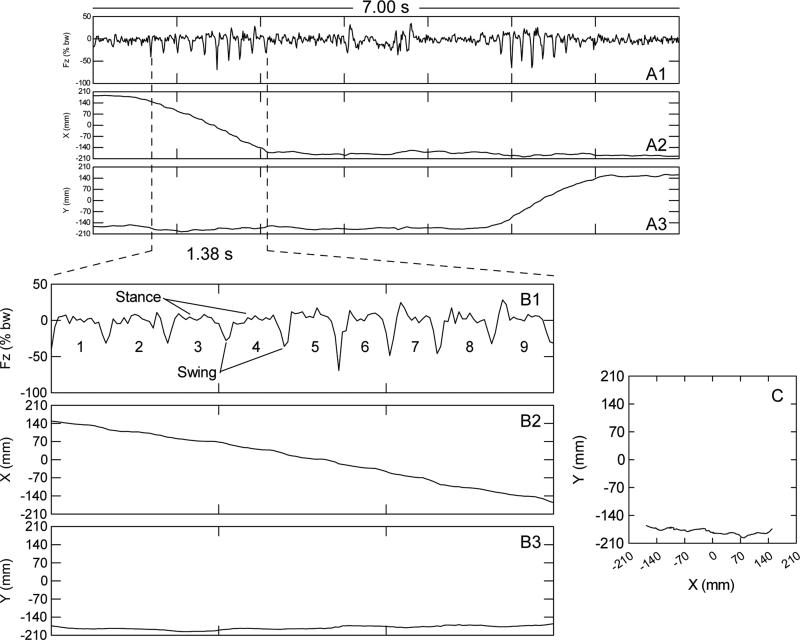
Force variability was defined as the root mean square of vertical force variations sampled at 100 samples/s by analog-to-digital conversion. In order to eliminate any influences of body weight on the force variable, force was expressed as a percent of body weight with the 100% offset removed. See Fig. 1A1 for a 7.00-s (700-samples) example of the resulting vertical force time series for two runs. The first run in Fig. 1A1 begins at the left most dashed vertical line; the second run begins at about 4.8 s. The runs are the frankly rhythmic sequences of force variation concurrent with rapid linear changes in the x-coordinate shown in Fig. 1A2 and accompanied by near constancy in the y-coordinate of the center of force (shown in Fig. 1A3). The root mean square (standard deviation) of the 700 consecutive force values shown in Fig. 1A1 is 11.92. In Fig. 1B1, a 1.38-s sub epoch has been enlarged to illustrate force variability of one run (see related text on gait, section 2.2.3.5.). The standard deviation of this 1.38-s subepoch of vertical force fluctuation is 14.47. The last 1.00 s of the 7.00-s record shown in Fig 1A1 has a standard deviation of 5.75 and illustrates a relatively low level of behavioral output. Thus, the force variability reflects the amplitude of vertical force of behavior-induced perturbations of the load plate over a defined time interval.
Figure 1.
Data for one representative mouse illustrating the kind of information that was used to extract selected gait parameters from continuously recorded ground reaction forces. This specific individual was a −/− female aged 39 weeks at the time of data collection. Fig. 1A1, Fig. 1A2, and Fig.1A3 are for a 7.00-s data segment that depicts ground reaction force, x coordinate, and y coordinate, respectively. Fig.1B1, Fig.1B2, and Fig.1B3 correspond to A1, A2, and A3, respectively but, with an expanded time scale that encompasses one run that lasted 1.38 s and comprised 9 half strides or 4.5 full strides. Fig.1C is the movement trajectory plot for the 1.38-s run. The run began at x-y coordinates 150,-171 and ended at x-y coordinates −156,−170 (i.e., the mouse moved from right to left and stayed near the front wall). During a run, diagonally opposite feet strike the load plate together (stance phase) while the other two feet are in the air (swing phase), and so on.
2.2.3.3. Low mobility bouts
Periods of no locomotion were identified by filtering the data according to both spatial and temporal constraints. Thus, a low mobility bout was defined as a 5.00-s period of continuous occupancy of a 15 mm radius virtual circle. If the 5.00-s criterion was met without a mouse's moving outside the circle, a new 5.00 s time period was begun and so on for the entire recording session. If the mouse moved beyond the boundaries of the virtual circle before the 5.00 s criterion was met, the time tally was zeroed and the virtual circle “followed” the mouse until the locomotion ceased at which time the duration tally was allowed to accumulate.
2.2.3.4. Power spectra of force variation during low mobility bouts
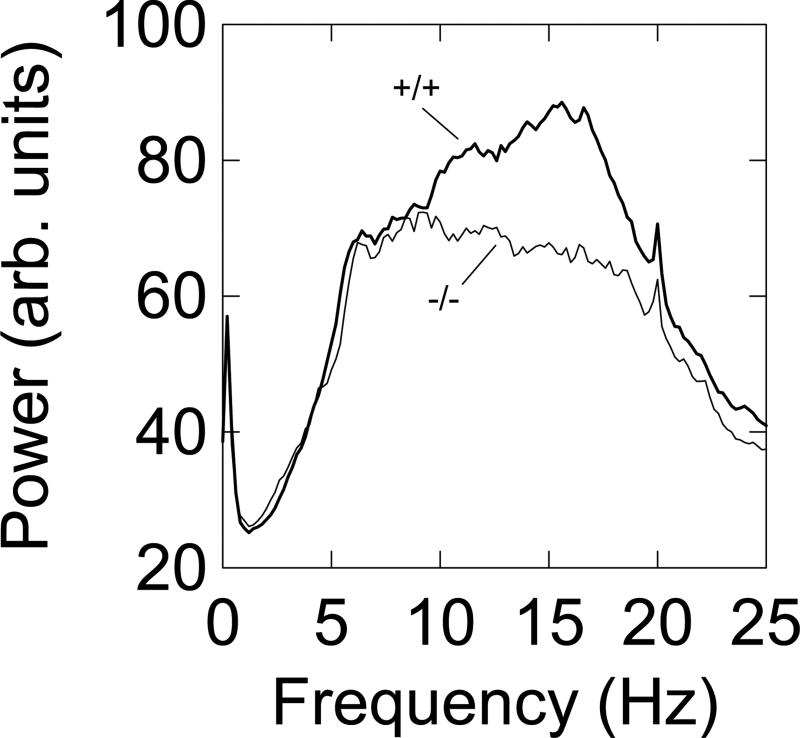
Power spectral analysis of time series was used to search for the presence of abnormal (meaning different from −/− mice) in-place movements such as tremor, during force variations that occurred in episodes of non-locomotion. For each qualifying bout of low mobility the 5.00-s force time series was saved to an open file as the low-mobility algorithm passed through a session's worth of data. Each force time series was subjected to Fourier transformation in MatLab after application of a Hanning data window. Next, the power spectra were averaged in the frequency domain to yield a single power spectral function for each mouse. Inspection of the group average power spectra for +/+ mice and −/− mice at week 65 showed that the main differences between groups (see Fig. 4) were manifest in the 10-20 Hz frequency band. The two groups were then compared statistically using a two way ANOVA (genotype-by-power estimates, of which there were 50 estimates in the 10-20 Hz band).
Figure 4.
Group average power spectra of ground reaction forces of low mobility bouts (periods of non-locomotion) for +/+ (n=18) and −/− (n=19) mice calculated for the recording session run when the mice were 65 week old. On the average −/− mice had 92.8 low mobility bouts in the 20-min session, while the +/+ mice averaged 111.3 bouts.
2.2.3.5. Derivation of selected gait parameters from force plate data
A scrolling graphics program written in-house was used to visualize, count, and record force-time variations that constituted half strides (see Fig. 1; also see [16] for an example of these methods applied to rats). Temporally aligned on the same screen with the force data were the mouse's location coordinates, which allowed the program user to identify and record only rhythmic, long, straight, fast, continuous runs and their associated force time series. In Fig. 1B, each numbered cycle is a half stride. The numbers 1 through 9 give the temporal order of half stride production. A rhythmic sequence of five or more half strides constituted a run where diagonally opposite feet act in unison to produce alternating stance and swing phases (see Fig. 1A1 and 1B1). In order to be considered suitable for quantitation, five or more half strides (i.e., two and a half full strides was the minimum) had to be discerned by the program user. Additional criteria for including a run in the gait data set were: i) A run had to be reasonably straight. This was judged by the program user by noting the lack of curvature in X(t) and/or Y(t) during the run sequence of half strides. This criterion ensured that variation in rhythm and other gait parameters would not be the result of changing direction of locomotion at corners of the cage that confined the mouse to the load plate, etc. ii) A run had to be expressed as a continuous sequence of half strides without any pausing in locomotion. iii) Rhythmic sequences of force variation, such as those produced by grooming or scratching were easily excluded from scoring because these in-place behaviors were not accompanied by locomotion. Qualifying locomotor sequences of force oscillations were saved in an Excel file and were subjected to further processing in SYSTAT in order to calculate the number of runs, duration of runs, stride length (run distance/number of strides), stride rate (strides/second), and velocity. Each of these parameters was then averaged across runs for each mouse. The initial plan was to score 20 runs per mouse; however, occasionally even after 20-min of time in the actometer fewer than 20 qualifying runs were made. Table 1 shows the actual group means and ranges of the number of runs that had gait parameters quantified for each group at the age of 39 weeks.
Table 1.
Mean and range of the number of separate runs for which selected gait parameters were estimated for week 39.
| Number of mice | Genotype | Mean # runs | Range |
|---|---|---|---|
| 18 | +/+ | 14.50 | 6-20 |
| 34 | +/− | 15.79 | 4-20 |
| 19 | −/− | 18.28 | 6-20 |
2.2.3.6. Wall rears
During a wall rear, a mouse supports its body with its hind limbs and tail and rests one or both fore limbs on the actometer wall. When the forelimb(s) contact the wall, a portion of the body weight is off-loaded from the load plate onto the wall. This results in a measureable diminution of force which can be detected by appropriate algorithms. In the current context a wall rear was defined as an off-load force greater than 5% and less than 40% of body weight that endured for 0.25 s or longer. These force-time criteria were selected to exclude force variations that accompany runs and leaps. See [5,17] for more detail on wall rears and video cross validation. Lengthening of wall rear duration has been interpreted as a motor phenomenon (response slowing or bradykinesia) [5,17] or as a reflection of non-selective attention [18].
2.2.3.7. Long straight runs
Conceptually, this behavioral measure is the inverse of a low mobility bout; it is a high mobility bout [5]. For this report a “long, straight, fast” run was defined as an episode of horizontal movement on the load plate that covered an approximately straight-line distance of 200 mm or more in 2.00 s. Thus, it provides an estimate of the number of relatively fast bouts (velocity of 100 mm/s or more) of sustained locomotion. The quantity is calculated by an algorithm that distinguishes movement velocity across a fixed-width time window (2.00 s) without being able to identify the beginning and end of individual runs. However, the gait analysis method illustrated in Fig. 1 does have the precision to discern individual runs, but with the disadvantage of having a much higher labor cost to obtain the data than using a hands-free algorithm. The long-straight-runs algorithm covered the entire 20-min session, but runs subjected to gait analysis were a subsample of the recording session.
2.3. Statistical methods
Repeated measures analysis of variance (ANOVA) with age as a within-groups factor and genotype (−/−, +/−, and +/+) as a between-groups factor was the main hypothesis testing tool. Post hoc tests were conducted as deemed appropriate for comparing the mutant mice with the −/− mice. In instances where results for a dependent variable exhibited evidence of a skewed distribution and/or lack of homogeneity of variance, data were Log10 transformed prior to hypothesis testing as noted in the results section.
3. Results
3.1. Body weight
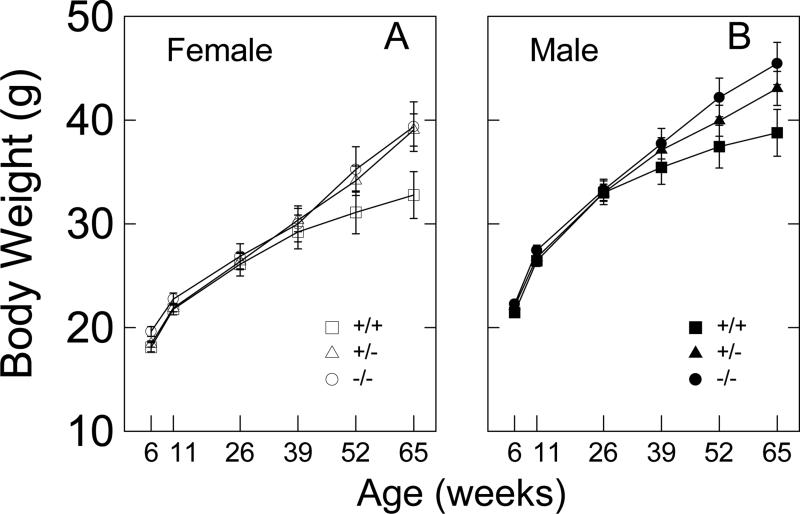
Plotted in Fig. 2 are the body weights as a function of age for both female and male CAG140 mice. Because group means and standard deviations were highly correlated (e.g., female: r=0.982, and male: r=0.984), data were log10 transformed prior to multivariate repeated measures ANOVA. The main effect of sex showed, as expected, that males had significantly higher body weights than females (F1,67=45.342, p<.001). The main effect of genotype approached significance (F2,67=2.807, p=0.068), and sex did not interact with genotype (F2,67=0.026, p=0.975). The main effect of age was significant (F5,335=712.232, p<0.001), and age interacted significantly with sex (F5,335=2.981) and genotype (F10,335=4.279, p<0.001). The 3-way interaction was not significant (F10,335=0.651, p=0.769). A post hoc univariate ANOVA for body weight at 65 weeks of age indicated that both female and male +/+ mice were significantly lower in body weight than −/− mice (p<.05 in both sexes).
Figure 2.
Body weight for female (A) and male (B) CAG140 mice as a function of age and HD-related genotype. ♀,+/+: n=9; ♀,+/−: n=19; ♀,−/−: n=8; ♂,+/+: n=9; ♂,+/−: n=17; ♂,−/−: n=11. Error bars are SEMs.
3.2. Distance traveled and vertical force variability
3.2.1. Preliminary analysis
Initially, a 4-way multivariate repeated measures ANOVA (sex X genotype X age X 4-min time block) was conducted on the distance traveled data in order to determine if sex main effects or sex-by-treatment interactions were present in the data. The aim was to reduce, if appropriate, the complexity of subsequent analyses and to help control the type I error rate that inflates as the number of F tests increases. Given that the number of F-tests for this 4-way design was 15, a Bonferroni correction was used for rejection of the null hypothesis (i.e., protection at the 0.05 level requires a computed p<0.003). This analysis identified genotype (F2,67=7.472, p=0.001), age (F5,63=41.737, p<0.001), time block (F6,64=61.864, p<0.001), and time block X genotype (F8,128=5.128, p<0.001) as significant effects. The sex effect and its related interactions were not significant. The same analysis was carried out for vertical force. Significant effects were detected for age X genotype (F10,126=3.055, p=0.002), age (F5,63=77.915, p<0.001), time block (F4,64=36.536, p<0.001), and age X time block (F20,48=5.044, p<0.001). Neither measure of behavior was significantly affected by the sex of the mouse. Therefore, in all subsequent analyses, female and male mice were pooled for each of the three genotype groups.
3.2.2. Distance traveled
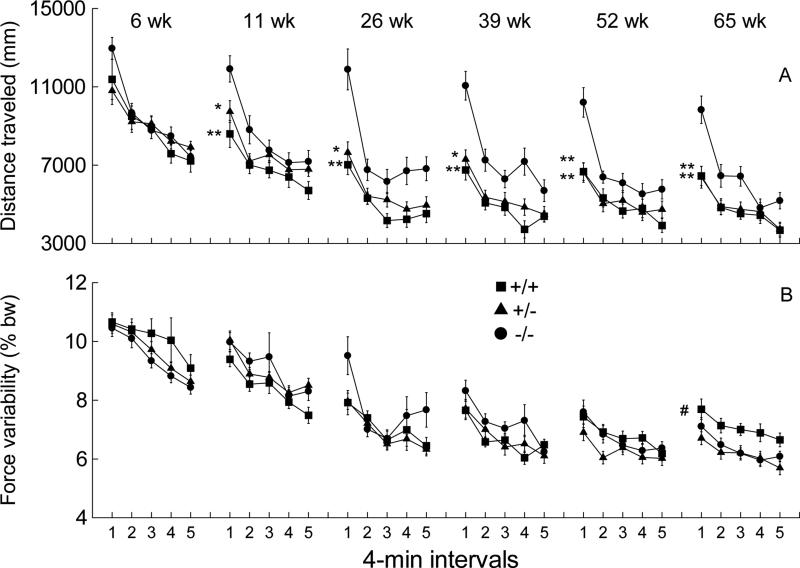
The distance traveled decreased with age and was lower in CAG +/− and +/+ mice than the −/− mice during the first four minutes of testing. Fig. 3A shows the effects of genotype, age, and time block on distance traveled. Repeated measures ANOVA indicated significant main effects of genotype (F2,70=8.143, p<0.001), age (F5,66=38.898, p<0.001), and time block (F4,67=62.661, p<0.001). These statistical results confirm that distance traveled declined as a function of mutation, age, and time block. Time block interacted significantly with genotype (F8,134=5.228, p<0.01). Post hoc contrasts showed that this interaction effect was attributable to consistently greater distances traveled by −/− compared to +/+ or +/− mice during the first 4 min of the recording session for all but the youngest age tested. .
Figure 3.
Distance traveled (A) and vertical force variability (B) for the six sessions across the 65 weeks of force plate actometer measurements shown in 4-min time blocks of the 20-min recording sessions. +/+: n=18; +/−: n=36; −/−: n=19. Error bars are SEMs. Asterisks indicate significant differences in the first time block between −/− and +/+ or between −/− and +/− mice (*p<0.05; **p<0.01). In B, # indicates a significant difference between +/+ and −/− mice across the five time blocks.
3.2.3. Variability in ground reaction forces
Distance traveled as a dependent variable emphasizes movement in the x-y plane and is mathematically independent of the vertical force amplitude at any given instant. In contrast, variability of ground reaction forces as a dependent variable (see Fig. 3B) includes force variance that arises from both locomotor and “in-place” behaviors. Examples of in-place behaviors are grooming, tremor, myoclonic jerks, leaps, rears, dyskinesias, and in-place stereotypies. The force variability measure for a given mouse is the standard deviation of the Fz forces (expressed as percent of body weight with offset removed); Fz is the sum of the forces from the four force transducers at any instant. Repeated measures ANOVA detected significant age-related decline in force variability (F5,66=74.875, p<0.001), a time-block-related decline (F4,67=36.050, p<0.001), and an age-by-time block interaction (F20,51=3.989, p<0.001). In addition, a significant age-by-genotype interaction (F10,132=3.058, p<.001) was detected. This interaction plus inspection of the data for age 65 weeks suggested that force variability in the +/+ mice was higher than both −/− and +/− mice across the 5 time blocks of week 65 (see Fig. 3B). A post hoc univariate ANOVA for week 65 confirmed that +/+ mice exhibited higher force variability than −/− mice (F1,35=5.852, p=0.021), while +/− mice did not differ from −/− mice (F1,53=0.437, p=0.511). By referring to data in Fig. 3A at age 65 weeks, one can see that distance traveled by +/+ mice was substantially and significantly lower than the −/− mice, but the force variability measure (Fig.3B) showed the reverse (i.e., +/+ mice greater than −/− mice). This lack of concordance between distance traveled and vertical force variability suggests that locomotion, which is accompanied by both high and low ground reaction forces (and therefore high variance), is not responsible for the higher force variability observed for the +/+ mice at 65 weeks of age. One way to investigate the occurrence of the comparatively high forces by the +/+ mice at 65 weeks of age is to calculate the force variability only for episodes of non-locomotion (bouts of low mobility), as explained in the next paragraph.
3.3. Power spectra for ground reaction forces during bouts of low mobility
When mice are not locomoting, they may be engaging in a variety of behaviors, including grooming (licking paws or flank), scratching, probing with the snout, exhibiting tremor, shuddering, jerking, or rearing. These behaviors often have a rhythmic or semi-rhythmic force-time signature as measured by the force sensors that support the load plate. A prominent example of rhythmic in-place behavior is harmaline-induced tremor, which is used as a laboratory model of essential tremor. The preferred method for quantifying this tremor is power spectral analysis [4], and its application in this setting detects a robust 13 Hz rhythm. Therefore, spectral analysis was used to determine if the character of the force-time recordings from the HD model mice during low mobility bouts differed between for the −/− and +/+ mutant mice at age 65 weeks. Power spectra of force variations were calculated for each low mobility bout of each +/+ and −/− mouse, and an average spectrum was computed for each mouse. Then group mean power spectra were calculated and plotted (group mean spectra are shown in Fig. 4). Repeated measures ANOVA applied to the power values in the 10 - 20 Hz frequency band, indicated a significant genotype effect (F1,35=13.900, p<0.001) and a significant quadratic interaction effect (F1,35=38.591, p<0.001). These data support the hypothesis that the +/+ mice exhibited higher power ground reaction forces at higher frequencies than the −/− mice independently of locomotion. Of course, further work is needed to characterize the topographical nature of these movements. Nevertheless, such in-place movements may be concomitants of the tremor or shakiness observed by others in +/+ CAG140 mice beyond one year of age [7]. Power spectra for +/− mice were not calculated because overall force variability measures for −/− mice and +/− mice were statistically indistinguishable (see Fig. 3B at 65 weeks).
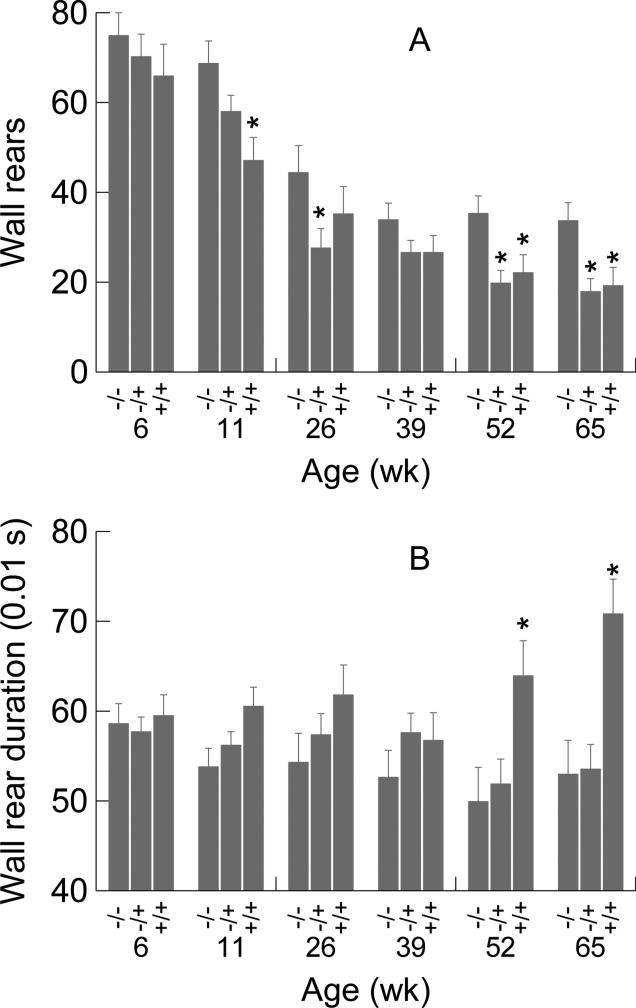
3.4. Wall rears
Number of wall rears (see Fig. 5A) was significantly affected by genotype (F2,70=5.811, p=0.005) and declined significantly with age (F5,350=65.758, p<0.001); the interaction was not significant. Results of post hoc contrasts at each age are reported in Fig. 5A; +/− and +/+ groups each had significantly fewer rears than −/− mice at ages of 52 and 65 weeks. The data shown in Fig. 5A at week 11 indicated a significant reduction in number of wall rears for the +/+ group, but not for the +/− mice. The reverse was seen for week 26 (i.e., +/− lower than −/−, but not lower than control for the+/+ mice). On week 39 both +/− and +/+ were graphically lower than −/−, but not significantly so. A conservative interpretation is that random statistical fluctuations near the threshold of significance could account for this pattern of results for weeks 11, 26, and 39, however other explanations could be possible. The two-way ANOVA for rear duration (see Fig. 5B) detected a significant genotype effect (F2,70=6.420, p=.003), no age effect, and a significant age-by-genotype effect (F10,350=2.042, p=0.029). Post hoc analysis revealed that +/+ mice had longer duration wall rears at 52 and 65 weeks than −/− mice, but +/− mice did not differ significantly from −/− mice at these older ages. Number of wall rears and duration of wall rears yielded different results in regard to zygosity. Thus the wall rear data reveal that some aspects of the motor phenotype of the CAG140 mouse are more severe in the homozygous than in the heterozygous mutants at the 52 and 65 week time points.
Figure 5.
Group mean number of wall rears (A) and group means of the median rear durations (B) as a function of age and genotype. Error bars are SEMs. Asterisks denote significant differences between +/+ or +/− from −/− mice based on post hoc univariate F tests (*p<0.05). Numbers of mice per group are the same as those shown in the caption for Fig. 3.
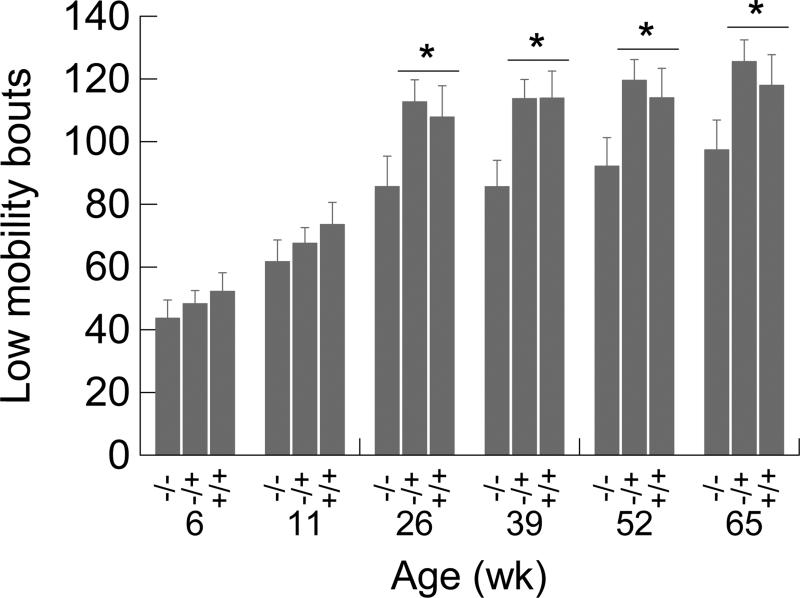
3.5. Low mobility bouts
There were more low mobility bouts in the +/− and +/+ mice compared to −/− mice starting at 26 weeks of age. ANOVA detected a significant genotype effect (F2,70=3.564, p=0.034) and a significant increase in low mobility bouts with age (F5,350=69.323, p<0.001), while the genotype-by- age-interaction was not significant (see Fig. 6). For data spanning the time period from 26 to 65 weeks, post hoc contrasts indicated that low mobility bouts occurred more frequently in the +/− and +/+ mice than the −/− mice.
Figure 6.
Low mobility bouts as a function of age and genotype. The asterisks indicate significant (*p<0.05) post hoc contrasts between the −/− group and groups +/− and +/+ combined. Error bars are SEMs.
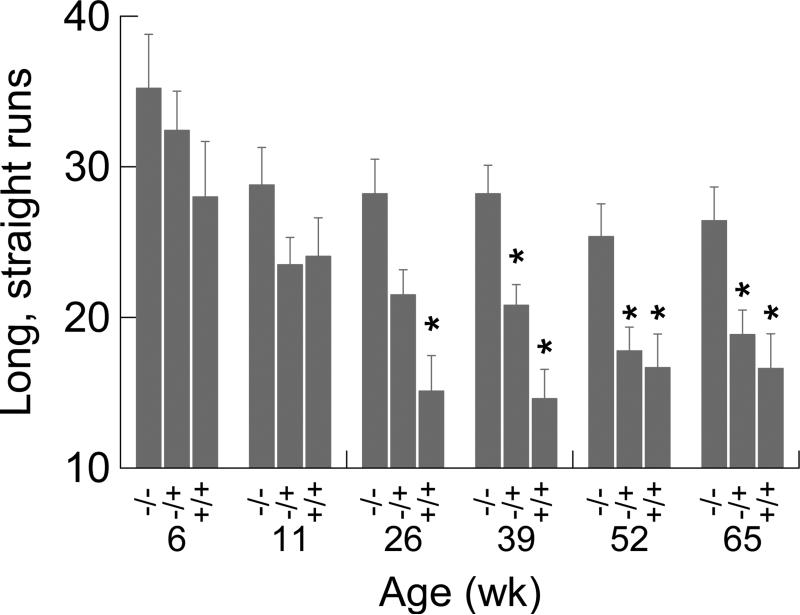
3.6. Number of long, straight, fast runs
The number of long, straight, fast runs was lower in the +/− and +/+ compared to −/− as the mice aged. For this dependent variable (see Fig. 7), there was a significant genotype effect (F2,70=8.530, p<0.001) and a significant reduction in number of runs as mice aged (F5,350=16.985, p<0.001). The interaction term was not significant. Post hoc contrasts showed that +/− mice had significantly fewer runs than −/− mice at 39, 52, and 65 weeks; +/+ mice exhibited significantly fewer runs than −/− mice at 26, 39, 52, and 65 weeks. This pattern of results for week 26 and week 39 suggests an earlier onset of significantly lower number of long runs in the +/+ mice than in the +/− mice.
Figure 7.
Mean number of long, straight, fast runs as a function of genotype and age. Asterisks identify significant (p<.05) differences between wild type and mutant mice. Error bars are SEMs.
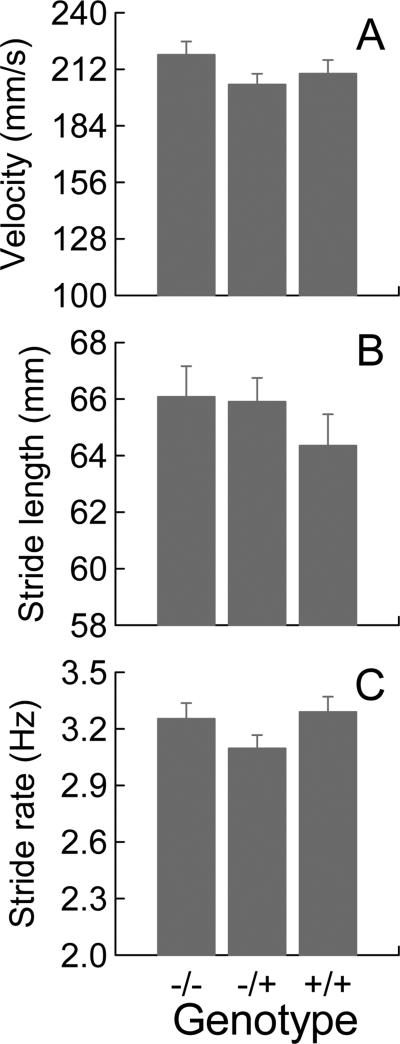
3.7. Gait measurements
Procedures described in the Methods section were used to extract selected gait measures from the force plate data collected for mice at 39 weeks of age. Week 39 was chosen for analysis because it was the age when there was a robust effect of the HD genotype on distance traveled, but before body weight began to be affected. In addition, data for long runs (Fig. 7) on week 39 showed a monotonic relation to zygosity where +/− mice occupied an intermediate position between −/− and +/+ mice. Thus, gait data at this time point would afford an interesting comparison between a variable that measures tendency to engage in locomotion (i.e., number of long runs) and a triad of gait variables that quantitate the motor execution of locomotion. Velocity, stride length, and stride rate were the gait parameters selected for quantification because these are measures often used in preclinical [10] and clinical studies of HD [19]. A one-way ANOVA with genotype as the independent variable was applied to each of the three dependent variables (see Fig.8). At 39 weeks of age, there were no differences in the three gait variables between the HD genotypes; ANOVA did not detect any genotype effect on any of the three calculated gait parameters.
Figure 8.
Group average data for velocity (A), stride length (B), and stride rate (C) quantified for force plate data when the mice were 39 weeks old. Error bars are SEMs. ANOVA did not detect any significant effects of genetic status.
For an empirical summary of the results, please see Table 2.
Table 2.
Results summary showing statistically significant differences between −/− mice and +/− mice or between −/− mice and +/+ mice.
| Age of mice in weeks | ||||||||
|---|---|---|---|---|---|---|---|---|
| Figure | Variable | Type | 6 | 11 | 26 | 39 | 52 | 65 |
| 3A | Distance traveled First 4 min | +/− | → | ↓ | ↓ | ↓ | ↓ | ↓ |
| +/+ | → | ↓ | ↓ | ↓ | ↓ | ↓ | ||
| 3B | Force variability | +/− | → | → | → | → | → | → |
| +/+ | → | → | → | → | → | ↑ | ||
| 5A | Number of Wall rears | +/− | → | → | ↓ | → | ↓ | ↓ |
| +/+ | → | ↓ | → | → | ↓ | ↓ | ||
| 5B | Wall rear duration | +/− | → | → | → | → | → | → |
| +/+ | → | → | → | → | ↑ | ↑ | ||
| 6 | Low mobility bouts | +/− | → | → | ↑ | ↑ | ↑ | ↑ |
| +/+ | → | → | ↑ | ↑ | ↑ | ↑ | ||
| 7 | Number of long straight runs | +/− | → | → | → | ↓ | ↓ | ↓ |
| +/+ | → | → | ↓ | ↓ | ↓ | ↓ | ||
| 8 | Gait velocity | +/− | → | |||||
| +/+ | → | |||||||
| 8 | Gait stride length | +/− | → | |||||
| +/+ | → | |||||||
| 8 | Gait stride rate | +/− | → | |||||
| +/+ | → | |||||||
Down arrows indicate mutants lower than controls and up arrows indicate mutants higher than controls, while rightward pointing arrows signal no difference. Blank cells mean data not extracted.
4. Discussion
Among the behavioral measures presented here, distance traveled stood out as the earliest, the most consistent, and the most enduring discriminator between the −/− mice and both +/− and +/+ mutants. The current results are consistent with published work showing that beyond 1 mo of age, both +/− and +/+ CAG140 mice have been reported to be hypoactive in an open field setting compared to −/− mice [8, 10]. Several transgenic HD model mice, such as the R6/2 also exhibit this hypoactivity phenotype [20], and we previously reported significant hypoactivity in R6/2 mice compared to wild-type littermates [5,6]. Few if any studies on CAG140 mice have tracked locomotor activity across as broad a longitudinal age span as we have here (six repeated assessments starting at 6 weeks and ending at 65 weeks). However, the zQ175 knock-in mouse with its 188 CAG repeats, is closely related to the CAG140 mouse, and it has been examined in an unusually thorough locomotor activity assessment comprising 9 30-min sessions administered monthly from 4 to 36 weeks of age [3]; see Fig 3 in the cited paper and compare it to our Fig. 3A). Both our CAG140 mice and the zQ175 mice displayed the typical pattern of within session changes in distance traveled, namely, a maximum amount of activity during the first time block followed by a marked decline in activity in subsequent time blocks. In both studies the first time block provided the clearest evidence for significant hypoactivity in the HD mutant mice compared to controls. However, the two studies differed in that the zQ175 mice exhibited evidence for a zygosity effect on distance traveled (i.e., +/− mice's impairment fell between +/+ and −/− mice) that began at about 20 weeks of age and continued to the end of testing (36 weeks). In our study, +/− and +/+ mutants were not different from each other in locomotor activity assessed (as measured by distance traveled) at ages beyond 11 weeks. This discrepancy in zygosity between CAG140 and zQ175 mice may be important in developing an understanding of why the longer CAG repeat length of the zQ175 mouse weakens the penetrance of heterozygosity in this knock-in model.
With regard to “early” manifestations of motor disturbances in CAG140 mice, a 2003 report [10] indicated that at 1 month of age these mutant mice exhibited increased rearing and lines crossed in an open field. This report has been cited frequently (e.g., [7, 31, 32]) as evidence for an early-occurring hyperkinesia in CAG140 mice. However, two subsequent studies [8,9] reported that CAG140 mice at 1 month of age were significantly hypoactive compared to −/− mice. These two contradictory studies used open field arenas and rearing as the measure of activity. One of the two studies [9] also indicated significantly lower distance traveled by +/+ mice compared to −/− mice. Data from [8,9,10] were all collected in the dark phase of the light/dark cycle, and the same video-base scoring methods were used in each study. Because studies [9] and [10] both used the same 15 min sessions for assessment and closely similar measurement methodologies, it was possible to compare directly the number of rears from these two studies. These data are shown in Table 3. For the CAG140 group (+/+ mice in Table 3), the number of rears for the two studies were in fairly close agreement; however, for the WT mice there is a large discrepancy between the two studies: an average of 13 rears reported in [10], in contrast to 62 rears reported in [9]. Such a large discrepancy between the WT mice suggests a sampling error or misclassification error in study [10]. A more recent report that included locomotor activity assessment in zQ175 mice at 1 month of age [3], did not detect hyperactivity in these HD model mice which are closely similar to the CAG140 model. Taken together, these observations from the published literature raise the possibility that the often cited 1-month hyperactivity by CAG140 mice is not a reliable effect.
Table 3.
Group mean number of rears in 15 min made by −/− and +/+ mice in small open fields from two separate studies that tested CAG140 mice at 1 month of age.
Number of wall rears and duration of wall rears were included in the analysis because these behavioral measures describe a motor response that cannot be performed during locomotion and thereby may be underpinned by neural processes different from or in addition to those processes brought into play during mouse locomotion in the horizontal plane. Moreover, a wall rear is a discrete motor act that has a definable beginning and end and affords the opportunity to define bradykinesia in terms of slowing of response execution. Compared to control mice, number of wall rears were significantly and consistently reduced at 52 and 65 weeks of age in +/− and +/+ mice (see Fig 5A). These results are congruent with a previous report that showed diminished wall rearing in transgenic R6/2 HD model mice [5]. In the current study, the number of wall rears did not temporally parallel the distance traveled data; that is, for distance traveled significant hypoactivity in the HD mutants first emerged at week 11, but diminished number of wall rears was not consistently expressed by the HD mutants until 52 and 65 week of age. This discrepancy suggests that wall rearing and distance traveled are not interchangeable estimators of locomotor activity. In regard to slowing of wall rear duration, Fig. 5B shows that significant rear-duration-lengthening effects were detected only for the +/+ mice at week 52 and 65. This latter result suggests a more severe motor phenotype in the homozygous than in the heterozygous mice at these ages. In a previous report that used force plate methods to behaviorally phenotype D2 dopamine receptor knock-out mice [17], the authors of that paper suggested that lengthened wall rear duration in D2 knockouts reflects a Parkinson's disease-like bradykinesia and/or a retardation in the initiation of the “standing-down” of the wall rearing response. The initial report that D2 receptor knockout mice exhibit some Parkinson-like behavioral features was made in [30] and later supported in [17]. Bradykinesia is a currently acknowledged feature of human HD [21,22,23].
Low mobility bouts were increased in +/− and +/+ mice compared to the −/− mice at 29-65 weeks, and the two types of mutants were closely similar to each other over this time span. However, the difference between mutants and controls was much less than expected based on a previous study that used an identical force plate actometer (and software) to assess the behavior of R6/2 HD model mice [5]. In the cited study, the R6/2 mice had 3.3 times as many low mobility bouts as the WT mice, where as in the present study the CAG140 mice had only 0.3 times more low mobility bouts than controls. This discrepancy between the two studies is likely, in part, attributable to the milder HD-like phenotype of the CAG140 mouse compared to the relatively fast progressing, short lived R6/2 model.
The force variability data (see Fig. 3B and Fig. 4) demonstrated that the force plate actometer provides a method for quantifying in-place mouse behaviors that may correspond to involuntary movements that emerge in the later stages of HD. Investigators have noted the occurrence of tremor, shakiness, or shuddering in HD model mice [3,7,24]. For example, working with the zQ175 mouse (both +/+ and +/−), Menalled et al [3] reported significantly more body tremor at 33 weeks of age in their +/+ mice (68 % expressed tremor) compared to +/− mice (13% expressed tremor). Using +/+ CAG140 mice 20-26 mo old, Hickey et al.[7] observed 12.5% of −/− mice exhibited tremor while 60% of the +/+ mice showed tremor. In the Menalled et al.[3] study and in the present work, greater amounts of tremor-like behavior in +/+ than in +/− mice supports the hypothesis that some in-place behaviors reflect the action of zygosity effects. A major advantage of the force plate methodology is that it provides a way to track HD progression beyond the stage when locomotion is lost. Also, this approach enables the quantification of tremor and similar behaviors without a change in instrumentation.
As shown in Fig.7, the number of long, fast runs was significantly diminished in +/+ mice beginning at week 26 and continuing through week 65. A similar trend was observed for the +/− mice, but it occurred at least 13 weeks later than seen in the +/+ mice. The difference between +/+ mice and +/− mice was not observed at week 52 and 65, while the difference between mutants and controls remained robust at these times. This pattern suggests a zygosity effect in which there was a greater than 13-week-wide window of time during which +/− mice were less affected than the +/+ mice. Fig. 3A of this report does not show any significant gene dose effect for distance traveled beyond 11 weeks of age. Taken together, the results depicted in Fig. 3A and Fig. 7 imply that distance traveled is not solely a reflection of the number of long, fast runs. In addition, it should be kept in mind that the occurrence of even a few high velocity runs shows the preservation of a relatively high degree of motor competence.
At 39 weeks of age, the gait analysis did not detect any significant differences between −/− mice and mutants (see Fig. 8). Stride shortening in HD model mice is well established for fast progressing R6/2 HD model mice [20,24,25]. On the other hand, in slower progressing transgenic and knock-in models, stride shortening has been reported to occur by one year [10] or later [20] or not at all [12]). The lack of effect of the HD mutation on gait at 39 weeks reported here shows that deficits in the distance traveled in the +/− and +/+ mice at week 39 are not governed by locomotor incapacity per se. With the methods used here one cannot rule out the possible effect that fatigue or metabolic dysfunctions may have on the observed hypoactivity seen in the HD mutant mice. However, since the deficits in distance traveled occurred during the first four minutes of each 20-minute session, fatigue is less likely a factor underlying the hypoactivity. At week 39, the number of long straight runs (see Fig. 7) was related to zygosity (i.e., +/− mice made about half the number of runs produced by −/− mice, and +/+ mice made about half as many runs as +/− mice). In contrast, −/−, +/−, and +/+ were statistically indistinguishable in gait velocity, stride length and stride rate at the same age for the same mice (Fig. 8). Therefore, when one compares the data for week 39 in Figs. 3A, 7, and 8, the results imply that the open field hypoactivity exhibited by the HD mutant mice is not a reflection of limited ability to perform motor execution during locomotion, but, instead, is a result of fewer runs made by the mutants compared to the −/− mice. These data suggest that HD-related hypoactivity is attributable, at least in part, to disruption of cognitive and/or motivation processes that guide behavior. Recent data collected on humans with HD shows that “apathy” or lack of motivation to initiate and persist in interacting with the environment is an early and progressive symptom of HD which begins to be observed in the premanifest phase and continues to worsen through the mild, moderate, and late stages of the disease [23,26]. Motivational deficits would be expected to have substantially deleterious effects on the performance of a wide range of tasks used to evaluate the behavioral capacities of HD model mice, regardless of whether a specific behavioral test is more or less heavily weighted to reveal cognitive or motor dysfunctions.
It is well documented that laboratory rodents, in response to enhanced environmental stimulation, exhibit substantial molecular, cellular, and behavioral gain-of-function effects [27] compared to rodents reared and/or maintained in “standard”, less complex environments. Investigators have begun to explore the potential of environmental enrichments as treatments to counteract pathological neurodegeneration, and several groups have reported therapeutic effects in some HD rodent models [28,29]. Such results raise the possibility that repeated exposure to the multiple behavioral assays used to track the course of the disease are themselves altering the course of the disease via unintentional enrichment. In slowly progressing HD mouse models, such as the CAG140 mouse, longitudinal testing may last for 18 months [12] and employ as many as seven or more separate assay procedures [20] with repeated testing occurring up to eight times. A priori this amount of experience conferred by repeated and varied kinds of testing with different instruments in a longitudinal design makes the occurrence of enrichment effects seem plausible. However, experimentally parsing out the degrees of functional changes wrought by repeated behavioral testing in various test paradigms would be prohibitively expensive. A distinct advantage of the force plate actometer is its ability to minimize testing-induced enrichment effects without sacrificing the advantage of using multiple behavioral markers of dysfunction.
5. Conclusion
This longitudinal study revealed that CAG140 mice exhibit hypoactivity preceding frank motor dysfunction. The earliest sign of significant behavioral abnormality in the CAG140 mouse was hypoactivity that occurred at 11 weeks of age and continued to be manifest through 65 weeks, which was the oldest age tested. A gait analysis conducted at 39 weeks of age, detected no abnormalities in velocity, stride length, or stride rate. This negative effect for the gait variables in the face of hypoactivity, suggests that the observed hypoactivity was, at least in part, the result of lack of motivation to move and not a problem with motor competence per se at 39 weeks. Motor dysfunction in the form of longer duration wall rears was seen at 52 and 65 weeks of age in the homozygous HD mice, and evidence for tremor-like movement was also seen for these same mice at 65 weeks of age. When combined with force plate technology the open field/actometer assessment provided a means to track HD signs of dysfunction in the CAG140 mouse model from the premanifest phase and into manifest phases when motor dysfunctions become more apparent (e.g., increased duration of wall rears, expression of tremor-like movements when not locomoting). The approach to behavioral measurement illustrated here may be especially useful in preclinical animal research devoted to the evaluation of experimental drugs where relatively high through put, low labor costs, a high degree of objectivity, and the measurement of several different behaviors are needed.
Highlights.
A force plate actometer quantified behavior of CAG140HD model mice aged 6 to 65 wk
From 11 wk, HD mice were hypoactive, and tremor-like movements emerged at 65 wk
At 39 wk, gait parameters (stride rate and stride length) were normal
Lengthening of wall rear durations was observed at 52 and 65 wk
Zygosity effects were seen for several behaviors, but not for distance traveled
Acknowledgements
S.C.F. was supported in part by NIH Grant P30 HD02528. The authors thank Paul Kimble for his help with record keeping and data logging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72(6):971–83. doi: 10.1016/0092-8674(93)90585-e. PMID: 8458085. [DOI] [PubMed] [Google Scholar]
- 2.Menalled L, Lutz C, Ramboz S, Brunner D, Lager B, Noble S, Park L, Howland D. A field guide to working with mouse models of Huntington's Disease. 2014 Chdifoundation.org.
- 3.Menalled LB, Kudwa AE, Miller S, Fitzpatrick J, Watson-Johnson J, Keating N, Ruiz M, Mushlin R, Alosio W, McConnell K, Connor D, Murphy C, Oakeshott S, Kwan M, Beltran J, Ghavami A, Brunner D, Park LC, Ramboz S, Howland D. Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington's disease: zQ175. PLoS One. 2012;7(12):e49838. doi: 10.1371/journal.pone.0049838. PMID: 23284626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107(1-2):107–24. doi: 10.1016/s0165-0270(01)00359-4. PMID: 11389948. [DOI] [PubMed] [Google Scholar]
- 5.Fowler SC, Miller BR, Gaither TW, Johnson MA, Rebec GV. Force-plate quantification of progressive behavioral deficits in the R6/2 mouse model of Huntington's disease. Behav Brain Res. 2009;202(1):130–7. doi: 10.1016/j.bbr.2009.03.022. PMID: 19447289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai Y, Dudek NL, Li Q, Fowler SC, Muma NA. Striatal expression of a calmodulin fragment improved motor function, weight loss, and neuropathology in the R6/2 mouse model of Huntington's disease. J Neurosci. 2009;29(37):11550–9. doi: 10.1523/JNEUROSCI.3307-09.2009. PMID: 19759302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience. 2008;157(1):280–95. doi: 10.1016/j.neuroscience.2008.08.041. (2008) PMID: 18805465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickey MA, Zhu C, Medvedeva V, Lerner RP, Patassini S, Franich NR, Maiti P, Frautschy SA, Zeitlin S, Levine MS, Chesselet MF. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington's disease. Mol Neurodegener. 2012;7:12. doi: 10.1186/1750-1326-7-12. PMID: 22475209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickey MA, Zhu C, Medvedeva V, Franich NR, Levine MS, Chesselet MF. Evidence for behavioral benefits of early dietary supplementation with CoEnzymeQ10 in a slowly progressing mouse model of Huntington's disease. Mol Cell Neurosci. 2012;49(2):149–57. doi: 10.1016/j.mcn.2011.10.007. PMID: 22044764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington's disease with 140 CAG repeats. J Comp Neurol. 2003;465(1):11–26. doi: 10.1002/cne.10776. PMID: 12926013. [DOI] [PubMed] [Google Scholar]
- 11.Deng YP, Wong T, Wan JY, Reiner A. Differential loss of thalamostriatal and corticostriatal input to striatal projection neuron types prior to overt motor symptoms in the Q140 knock-in mouse model of Huntington's disease. Front Syst Neurosci. 2014;8:198. doi: 10.3389/fnsys.2014.00198. PMID: 25360089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rising AC, Xu J, Carlson A, Napoli VV, Denovan-Wright EM, Mandel RJ. Longitudinal behavioral, cross-sectional transcriptional and histopathological characterization of a knock-in mouse model of Huntington's disease with 140 CAG repeats. Exp Neurol. 2011;228(2):173–82. doi: 10.1016/j.expneurol.2010.12.017. PMID: 21192926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng YP, Wong T, Bricker-Anthony C, Deng B, Reiner A. Loss of corticostriatal and thalamostriatal synaptic terminals precedes striatal projection neuron pathology in heterozygous Q140 Huntington's disease mice. Neurobiol Dis. 2013;60:89–107. doi: 10.1016/j.nbd.2013.08.009. PMID: 23969239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy-Nakhnikian A, Dorner JL, Fischer BI, Bower-Bir ND, Rebec GV. Abnormal burst patterns of single neurons recorded in the substantia nigra reticulata of behaving 140 CAG Huntington's disease mice. Neurosci Lett. 2012;512(1):1–5. doi: 10.1016/j.neulet.2011.12.040. PMID: 22327034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings DM, Cepeda C, Levine MS. Alterations in striatal synaptic transmission are consistent across genetic mouse models of Huntington's disease. ASN Neuro. 2010;2(3):e00036. doi: 10.1042/AN20100007. PMID: 20585470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanford JA, Shuler JM, Fowler SC, Stanford KG, Ma D, Bittel DC, Le Pichon JB, Shapiro SM. Hyperactivity in the Gunn rat model of neonatal jaundice: age-related attenuation and emergence of gait deficits. Pediatr Res. Epub ahead of print. 2014 doi: 10.1038/pr.2014.199. PMID: 25518009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler SC, Zarcone TJ, Vorontsova E, Chen R. Motor and associative deficits in D2 dopamine receptor knockout mice. Int J Dev Neurosci. 2002;20(3-5):309–21. doi: 10.1016/s0736-5748(02)00009-6. PMID: 12175868. [DOI] [PubMed] [Google Scholar]
- 18.Fresiello A, Grammatikopoulos G, Pignatelli M, Sadile AG. Environmental factors during postnatal period modify activity and non-selective attention in the Naples High-Excitability rat. Behav Brain Res. 2002;130(1-2):111–5. doi: 10.1016/s0166-4328(01)00426-0. PMID: 11864726. [DOI] [PubMed] [Google Scholar]
- 19.Koller WC, Trimble J. The gait abnormality of Huntington's disease. Neurology. 1985;35(10):1450–4. doi: 10.1212/wnl.35.10.1450. PMID: 3162109. [DOI] [PubMed] [Google Scholar]
- 20.Menalled L, El-Khodor BF, Patry M, Suárez-Fariñas M, Orenstein SJ, Zahasky B, Leahy C, Wheeler V, Yang XW, MacDonald M, Morton AJ, Bates G, Leeds J, Park L, Howland D, Signer E, Tobin A, Brunner D. Systematic behavioral evaluation of Huntington's disease transgenic and knock-in mouse models. Neurobiol Dis. 2009;35(3):319–36. doi: 10.1016/j.nbd.2009.05.007. PMID: 19464370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delval A, Krystkowiak P, Blatt JL, Labyt E, Dujardin K, Destée A, Derambure P, Defebvre L. Role of hypokinesia and bradykinesia in gait disturbances in Huntington's disease: a biomechanical study. J Neurol. 2006;253(1):73–80. doi: 10.1007/s00415-005-0929-2. PMID: 16096818. [DOI] [PubMed] [Google Scholar]
- 22.Walker FO. Huntington's disease. Lancet. 2007;369(9557):218–28. doi: 10.1016/S0140-6736(07)60111-1. PMID: 17240289. [DOI] [PubMed] [Google Scholar]
- 23.Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10(4):204–16. doi: 10.1038/nrneurol.2014.24. PMID: 24614516. [DOI] [PubMed] [Google Scholar]
- 24.Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19(8):3248–57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. PMID: 10191337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pallier PN, Drew CJ, Morton AJ. The detection and measurement of locomotor deficits in a transgenic mouse model of Huntington's disease are task- and protocol-dependent: influence of non-motor factors on locomotor function. Brain Res Bull. 2009;78(6):347–55. doi: 10.1016/j.brainresbull.2008.10.007. PMID: 19010400. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JC, Harris J, Sollom AC, Stopford CL, Howard E, Snowden JS, Craufurd D. Longitudinal evaluation of neuropsychiatric symptoms in Huntington's disease. J Neuropsychiatry Clin Neurosci. 2012;24(1):53–60. doi: 10.1176/appi.neuropsych.11030057. PMID: 22450614. [DOI] [PubMed] [Google Scholar]
- 27.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–8. doi: 10.1038/35044558. PMID: 11257907. [DOI] [PubMed] [Google Scholar]
- 28.van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington's in mice. Nature. 2000;404(6779):721–2. doi: 10.1038/35008142. PMID: 10783874. [DOI] [PubMed] [Google Scholar]
- 29.Mazarakis NK, Mo C, Renoir T, van Dellen A, Deacon R, Blakemore C, Hannan AJ. ‘Super-Enrichment’ Reveals Dose-Dependent Therapeutic Effects of Environmental Stimulation in a Transgenic Mouse Model of Huntington's Disease. J Huntingtons Dis. 2014;3(3):299–309. doi: 10.3233/JHD-140118. PMID: 25300333. [DOI] [PubMed] [Google Scholar]
- 30.Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377(6548):424–428. doi: 10.1038/377424a0. PMID: 7566118. [DOI] [PubMed] [Google Scholar]
- 31.Heng MY, Detloff PJ, Albin RL. Rodent genetic models of Huntington disease. Neurobiol Dis. 2008;2(1):1–9. doi: 10.1016/j.nbd.2008.06.005. PMID: 18638556. [DOI] [PubMed] [Google Scholar]
- 32.Ferrante RJ. Mouse models of Huntington's disease and methodological considerations for therapeutic trials. Biochim Biophys Acta. 2009;92(6):506–20. doi: 10.1016/j.bbadis.2009.04.001. PMID: 19362590. [DOI] [PMC free article] [PubMed] [Google Scholar]