Abstract
Background
Angiotensin converting enzyme (ACE) inhibitors such as lisinopril, represent the front line pharmacological treatment for heart failure, which is characterised by marked left ventricular (LV) dilatation and hypertrophy. This study sought to determine whether initiating treatment with ACE inhibitors at different stages in the remodelling process would alter the efficacy of treatment.
Methods
To this end, LV size and function were determined in the aortocaval (AV) fistula model of volume overload-induced heart failure. Sprague-Dawley rats were assigned to sham, untreated AV fistula (21 weeks), AV fistula treated with lisinopril (21 weeks), or AV fistula treated with lisinopril from six to 21 weeks post-fistula groups.
Results
Administration of lisinopril for the entire 21-week period prevented LV dilatation, attenuated myocardial hypertrophy and prevented changes in myocardial compliance and contractility, whereas delaying initiation of treatment until six weeks post-fistula attenuated LV dilatation and hypertrophy, however, the delayed onset of treatment had no beneficial effect on ventricular compliance or systolic function.
Conclusions
The results demonstrate differential effects that can occur with ACE inhibitors depending on the stage during the remodelling process at which treatment is administered.
Keywords: Angiotensin converting enzyme inhibitor, Volume overload, Hypertrophy, Heart failure
INTRODUCTION
Angiotensin converting enzyme (ACE) inhibitor therapy has become standard practice for the treatment of heart failure [1], in large part due to the success of clinical trials such as SOLVD, in which enalapril prevented left ventricular (LV) dilatation and improved systolic function in mild to moderate heart failure patients [2], and SAVE, where survival following myocardial infarction was improved by captopril treatment [3]. Previously, we demonstrated in the aortocaval (AV) fistula model of volume overload-induced heart failure, that treatment with the ACE inhibitor lisinopril, initiated at the time volume overload was imposed, prevented LV dilatation and maintained normal LV compliance [4]. The beneficial effects of lisinopril were attributable to its ability to inhibit MMP activity by directly binding to MMPs [4]. While this was an important observation, in clinical practice ACE inhibitor treatment is often not initiated until the patient becomes symptomatic, at which point the myocardial remodelling process is already well underway and frequently the heart has already begun to decompensate. Thus, this study sought to determine if the time at which ACE inhibitor treatment is initiated during the remodelling process may have differing effects and, thus, be critical to determining the degree of success. The current study used the AV fistula rat model of volume overload-induced congestive heart failure to compare the effects of lisinopril on LV structure and function, where lisinopril treatment was initiated either at the time of creation of volume overload (prevention), or six weeks after the creation of volume overload (regression). We hypothesised that lisinopril treatment initiated six weeks after the creation of volume overload would be less beneficial on myocardial remodelling and function than prevention treatment. Herein we report that both prevention and regression treatments with lisinopril markedly attenuated LV dilatation, as well as LV and right ventricular (RV) hypertrophy, while only preventative lisinopril treatment was able to prevent increased myocardial compliance and loss of intrinsic systolic function.
MATERIALS AND METHODS
All experiments were performed using adult male Sprague-Dawley (Hsd:SD) rats housed under standard environmental conditions and maintained on commercial rat chow and tap water ad libitum. All studies conformed to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was also approved by the University’s Animal Care and Use Committee. Anaesthesia for surgical procedures and subsequent euthanasia at the experimental endpoint was induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg). Post-operative analgesia was achieved by administration of buprenorphine HCl (0.025 mg/kg, s.c).
Experimental Design
These experiments were designed to determine whether lisinopril administered six weeks after the induction of a sustained cardiac volume overload could achieve improved structural and functional adaptations in a manner comparable to prevention treatment with lisinopril. Prior to surgery, rats were randomly divided into sham (n=10), 21-week untreated AV fistula (n=14), 21-week AV fistula continually treated with lisinopril (n=9, prevention) and AV fistula treated with lisinopril where treatment was initiated six weeks following the creation of an AV fistula and continued through to 21 weeks post-fistula (n=12, regression). Lisinopril (Sigma, St Louis Missouri) was administered in the drinking water at a concentration of 100 mg/L. The time-point of six weeks post-fistula was chosen to begin lisinopril treatment in the regression group because it represents a period in the remodelling process where there is already ventricular dilatation and increased myocardial compliance. Thus, this would represent a time where the hearts of patients are already decompensated when they first begin ACE inhibitor therapy.
Surgical Preparation
An AV fistula was created as previously described [5]. Briefly, the aorta and caudal vena cava were exposed via ventral abdominal laparotomy. Both vessels were occluded proximal and distal to the intended puncture site before an 18-gauge needle was inserted into the abdominal aorta and advanced through the medial wall of the vena cava and subsequently withdrawn. The ventral aortic puncture was sealed with cyanoacrylate and flow restored. Successful creation of a fistula was confirmed by the presence of pulsatile oxygenated blood flow in the vena cava. Incisions to the musculature and skin were closed with absorbable sutures and autoclips, respectively.
Ventricular Function
At the conclusion of the study period, each rat was weighed, anaesthetised, fistula patency visually confirmed, and the heart removed and attached to a perfusion apparatus for evaluation of LV function. LV volume and function were assessed using a blood-perfused isolated heart preparation as previously described [4-10]. Briefly, the apparatus consisted of a pressurised (100-105 mmHg) perfusion reservoir and a collection reservoir connected in circuit with a support rat. LV volumes and pressures from un-paced hearts were recorded using a latex balloon inserted into the LV through the mitral valve orifice. Once the heart developed stable isovolumetric contractions, the balloon volume (V0) producing a LV end diastolic pressure (EDP) of 0 mmHg was determined. Balloon volume was then increased in 20 μl increments until an LVEDP of 25 mmHg was attained. The EDP and peak isovolumetric pressure, which were recorded following each increase in balloon volume, were then used to assess LV diastolic function and intrinsic contractility (i.e. slope of the linear isovolumetric pressure-volume relationship; Pmax-V) Following completion of the experiment the RV was dissected away and the LV plus septum and RV were weighed.
Statistical Analysis
Grouped data comparisons were made by one-way analysis of variance (ANOVA) using SPSS 11 software (SPSS Inc., Chicago, IL). When a significant F test (P≤0.05) was obtained, intergroup comparisons were analysed using Fisher’s protected least significant difference post-hoc testing.
RESULTS
Biometric Parameters
Body weight, LV weight and RV weight are reported in Table 1. Body weight was significantly increased in the 21-week untreated fistula group, indicative of oedema secondary to heart failure. This was prevented by lisinopril treatment over the entire 21-week period and significantly attenuated by regression therapy where lisinopril was initiated six weeks post-fistula. Imposition of volume overload induced bi-ventricular hypertrophy as evidenced by the significant increases in both LV and RV weights in the untreated fistula group, which were significantly attenuated in both lisinopril groups.
TABLE 1.
Morphometric parameters
| Body weight (g) |
LV weight (mg) |
RV weight (mg) |
|
|---|---|---|---|
| Sham | 356 ± 52 | 917.6 ± 108.7 | 257.8 ± 35.9 |
| 21wk AV fist | 545 ± 81* | 1874.6 ± 135.5* | 665.3 ± 119.2* |
| 21wk AV fist+lis | 400 ± 35† | 1235.0 ± 158.4*† | 438 ± 63.2*† |
| lis regression | 456 ± 29*†‡ | 1353.9 ± 129.4*† | 525.2 ± 95.6*† |
Values expressed as mean ± SD.
= p<0.5 compared to sham;
= p<0.05 compared to age-matched fistula;
= p<0.05 compared to 21 wk Fist+lis.
LV Structure and Function
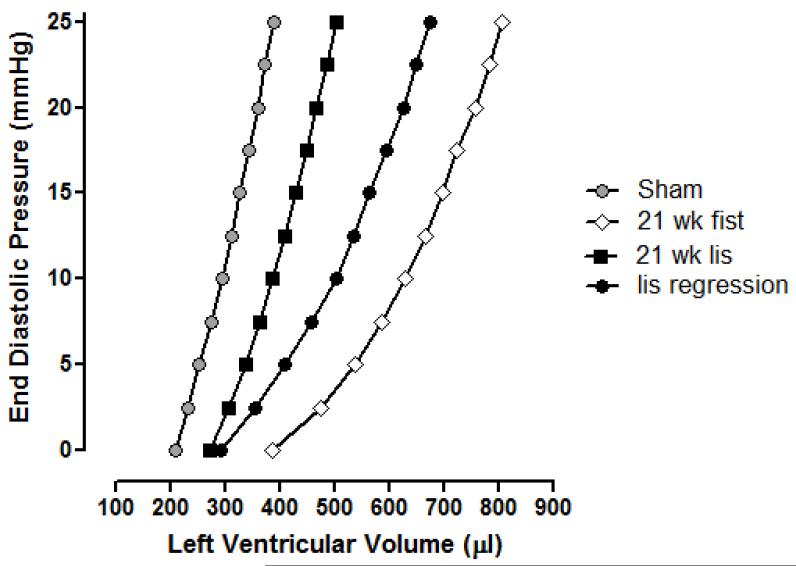
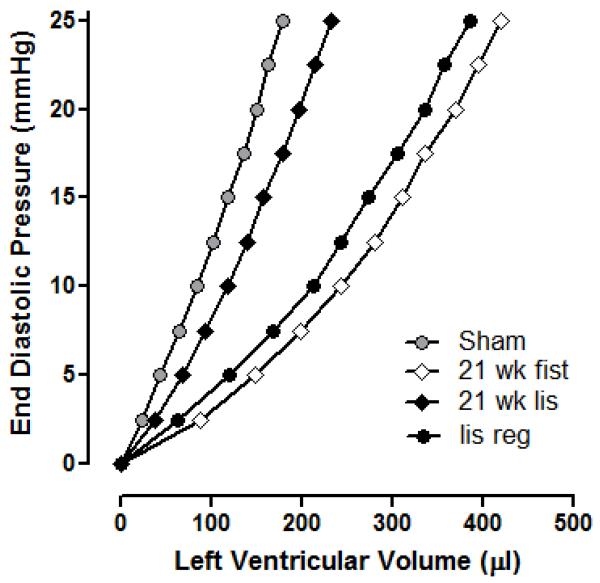
Table 2 contains the LV volumes over the EDP range of 0-25 mmHg, obtained using the blood-perfused isolated heart apparatus. The corresponding representative average LVEDP-end diastolic volume (EDV) curves are shown in Figure 1. Ventricular dilatation was assessed by comparing V0 values (LV volume at an EDP of 0 mmHg; Table 2), while ventricular compliance was ascertained from the volumes required to increase EDP from 0 to 25 mmHg (ΔV0-25; Table 2, Figure 2). There was a significant rightward shift in the LV EDP-EDV relationship for untreated AV fistula rats relative to controls. This shift in the P-V relationship was due to both ventricular dilatation, since V0 was significantly increased and to an overall increase in ventricular compliance. These changes were prevented in AV fistula rats treated with lisinopril for 21 weeks. Conversely, a differential effect was seen in AV fistula rats treated with lisinopril from six weeks post-fistula. In these animals V0 was returned to normal, however, this occurred without a concomitant reduction in compliance as observed in the lisinopril prevention group.
TABLE 2.
Isolated heart diastolic function.
| V0, μl | ΔV0-25, μl | |
|---|---|---|
| Sham | 209 ± 13 | 188 ± 12 |
| 21wk AV fist | 387 ± 24* | 393 ± 40* |
| 21wk AV fist+lis | 270 ± 23† | 261 ± 15† |
| lis regression | 257 ± 35† | 429 ± 50* |
Values expressed as mean ± SEM. V0, volume at EDP of 0 mmHg; ΔV0-25, change in LV volume between EDP of 0 and 25 mmHg. For statistical purposes all age-matched control groups were combined into one group.
= p<0.05 compared to sham;
= p<0.05 compared to age matched fistula.
Figure 1.
Effect of lisinopril treatment on left ventricular end diastolic pressure and left ventricular end diastolic volume relationships in the rat AV fistula model of heart failure. Each curve represents the average values for that group. Sham group (n=10) P-V relationship is significantly different from the 21wk AV fistula group (n=14; p<0.05). P-V relationship for the 21wk lisinopril group (n=9) was not significantly different from shams, but was significantly different to the 21wk AV fistula group (P<0.05). P-V relationship for the lisinopril regression group (n=12) was significantly different from the sham group (P<0.05), but was not different from the 21wk AV fistula group.
Figure 2.
The P-V relationship normalised for LV volume that produced an EDP of 0 mmHg to illustrate the effect of lisinopril on changes in ventricular compliance. Each curve represents the average values for that group. Sham group (n=10) P-V relationship is significantly different from the 21wk AV fistula group (n=14; p<0.05). P-V relationship for the 21wk lisinopril group (n=9) was not significantly different from shams, but was significantly different to the 21wk AV fistula group (P<0.05). P-V relationship for the lisinopril regression group (n=12) was significantly different from the sham group (P<0.05), but was not different from the 21wk AV fistula group.
The relationship between peak isovolumetric pressure and EDV, a measure of intrinsic systolic contractility, was highly linear, as evidenced by the range of correlation coefficients (Table 3). The slope for the Pmax-V relationship was significantly decreased in the 21-week untreated fistula indicating decreased intrinsic myocardial contractility and therefore systolic dysfunction. This depression in contractility was prevented by lisinopril treatment over the entire study period, but not by lisinopril treatment initiated six weeks post-fistula.
TABLE 3.
Isolated heart systolic intrinsic contractility.
| Slope-Pmax−V, mmHg/μl | rPmax−V Range | |
|---|---|---|
| Sham | 0.378 ± 0.073 | 0.94 ± 0.03 |
| 21 wk Fist | 0.172 ± 0.019* | 0.96 ± 0.01 |
| 21 wk Fist+lis | 0.312 ± 0.065 | 0.97 ± 0.01 |
| lis regression | 0.133 ± 0.026*‡ | 0.93 ± 0.02 |
Values are mean ± SD. rPmax−V Range, range of regression coefficient of the linear Pmax−V relations. For statistical purposes all age-matched control groups were combined into one group.
= p<0.05 compared to control;
= p<0.5 compared to 21 wk Fist+lis.
DISCUSSION
ACE inhibitors are recommended for the treatment of heart failure largely because of their ability to regress LV dilatation and improve LV function [2;11-14]. In a clinical setting ACE inhibitor therapy is often initiated only after symptoms of heart failure have presented, at which point the foundations of the disease are already in place. Several clinical trials have demonstrated the ability of ACE inhibitors to improve LV remodelling and function [2;3], however, these trials vary in aspects of patient composition, such as time of onset of treatment, use of other medications, stage of heart failure and gender composition of the study cohorts. These differences may be important factors in determining the effectiveness of ACE inhibitor therapy on an individual basis. Therefore, it is important to evaluate in a more controlled environment whether the timing of lisinopril administration elicits different structural and functional responses. The major findings of this study are that lisinopril treatment initiated at the time of creation of volume overload and lisinopril initiated six weeks after the creation of volume overload were both able to reduce LV mass and dilatation. Conversely, only early intervention with lisinopril treatment maintained throughout the entire 21 weeks was able to preserve normal LV compliance and intrinsic contractile function.
Significant LV dilatation was observed at 21 weeks post-fistula in untreated AV fistula rats, as determined by the LV volume at an EDP of 0 mmHg (V0). However, this change was absent at the study endpoint in both fistula groups treated with lisinopril. Therefore, lisinopril appears to be equally capable of reversing LV structural dilatation, as well as preventing its development. This also manifested as a comparable reduction in LV weight observed in both lisinopril groups. One mechanism by which lisinopril improves LV chamber size appears to be related to altering the breakdown of the extracellular matrix (ECM) by MMPs. We previously demonstrated that lisinopril, as well as other ACE inhibitors, can directly inhibit the activation of myocardial MMP-2 [4]. In fact, the effect of lisinopril treatment on LV chamber size, closely mimics the results achieved with the MMP inhibitor, PD 166793 [8]. Thus, MMP activation seems to be a critical factor in the structural dilatation of the ventricle in response to volume overload. Another contributing factor may be that lisinopril has effects on integrin function. We recently showed that infusion of a neutralising antibody against β1-integrin, but not β3-integrin, into isolated hearts caused an increase in V0 without inducing a change in compliance [15]. Thus, a loss of β1-integrin function appears to play a causative role in producing LV dilatation, but not LV compliance. In fact, there is a reduction in cardiomyocyte adhesion to several substrates, including laminin, collagen IV and fibronectin from eight weeks post-fistula onwards; including cardiomyocytes from failing hearts, indicative of a loss of integrin function potentially mediating ventricular dilatation. Lisinopril may restore integrin function to prevent dilatation. Although this is not likely via effects on angiotensin II, since angiotensin II typically augments β1-integrin effects [16]. Instead, the effects of lisinopril on integrin function may again involve inhibition of MMPs given that MMP-2 is capable of cleaving β1-integrins [17] and we have shown direct inhibitory effects of ACE inhibition on MMP activity [4].
Similar to the findings for LV dilatation, lisinopril, when administered at the time of creation of an AV fistula, maintained normal LV compliance. However, administration of lisinopril beginning six weeks post-fistula, when ventricular compliance has already increased [4], was not able to restore normal LV compliance. These disparate findings emphasise that LV dilatation and compliance are independent variables. This is consistent with our previous findings using the MMP inhibitor, PD 166793, which was also unable to prevent increased compliance despite normalising V0 [8]. These observations suggest that MMP activation and ECM degradation are not critical to alterations in LV compliance seen in chronic volume overload, and that other factors may be important to this aspect of the remodelling process. Myocyte lengthening, without transverse growth, is postulated to represent an early cellular event in the transition to heart failure [18] and may contribute to LV compliance due to increases in the giant protein titin. Titin has been estimated to have an elastic modulus (3.5 × 106 dyne/cm2) similar to that of elastin and acts as a spring to return cardiomyocytes to the appropriate resting length after relaxation [19]. However, Makarenko et al. [20] demonstrated that failing hearts from idiopathic dilated cardiomyopathy patients have an increased expression of the more compliant, higher molecular-weight titin isoform, which has the effect of reducing the contribution of titin to passive tension. Similarly, Neagoe et al. [21] found that there was a shift towards the more compliant N2BA isoform of titin in cardiomyocytes from patients with coronary artery disease concomitant with decreased myofibrillar stiffness. Accordingly, as the dilated heart undergoes an in-series addition of sarcomeres in response to chronic volume overload [22], the incorporation of the more compliant titin isoform may be increased, thereby, rendering the LV more compliant overall. Further work is required to confirm whether ACE inhibitors have effects on titin composition in the remodelled heart.
In addition to improving LV chamber size and compliance, lisinopril was also able to maintain intrinsic contractility at normal levels when administered for the full 21 weeks of volume overload. However, in animals treated from six weeks post-fistula, no recovery in contractility was observed. It would therefore appear that the depressed intrinsic systolic contractility may be a product of increased myocardial compliance rather than LV dilatation. Again this demonstrates the importance of early administration of lisinopril in order to prevent depressed myocardial contractility.
Our findings for the most part, agree with a previous study that used the same model of volume overload also in rats, however, they administered a different ACE inhibitor, enalipril (250 mg/L of drinking water) [23]. In that study, enalipril treatment was initiated five weeks after creation of volume overload and was continued for another five weeks. They also found that ACE inhibition reduced LV mass including attenuating ventricular dilatation. However, in their study, cardiac index was still increased following 10 weeks of fistula (untreated), since it is a model of high cardiac output, but indicates that those rats were not yet in heart failure. A lack of increased body weight supports this conclusion. This is where our study differs from this previous study. We followed volume overload rats until failure and showed that only preventative lisinopril treatment and not regression treatment protected from heart failure. Thus, despite attenuation of structural remodelling by regression treatment, late administration of an ACE inhibitor could not rescue the loss of systolic function. This is a critical observation. Interestingly, Ruzicka et al.[23] found similar results for the angiotensin receptor antagonist losartan, which would argue that these effects were mediated by angiotensin II acting on the AT1 receptor. Ruzicka et al. [23] found similar effects in a minoxidil-induced model of volume overload, although those animals also did not appear to be in failure at the time of experimentation. It does, however, suggest that these findings are applicable to volume overload in general.
In summary, we have shown that lisinopril treatment initiated at the time of creating volume overload prevents LV dilatation and increased myocardial compliance. In contrast, lisinopril treatment initiated only after established remodelling, was able to reverse LV dilatation, but could not alter the increase in LV compliance. Together, these results raise the possibility that the effective uncoupling of cardiomyocytes from the ECM may be the stimulus for in series sarcomeric addition in the volume-overloaded heart. This finding may have important clinical repercussions for the future treatment of heart failure with the take home message being that the earlier ACE inhibitor therapy is initiated the better.
Acknowledgments
This study was supported by National Institute of Health grants R01-HL-62228 and R01-HL-073990 (JSJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.HFSA Heart Failure Society of America (HFSA) practice guidelines. J Cardiac Fail. 1999;5:357–382. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Konstam MA, Rousseau MF, Kronenberg MW, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D, SOLVD investigators Effects of angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. Circ. 1992;86:431–438. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. The SAVE investigators Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Eng J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 4.Brower GL, Levick SP, Janicki JS. Inhibition of matrix metalloproteinase activity by ACE inhibitors prevents left ventricular remodeling in a rat model of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H3057–H3064. doi: 10.1152/ajpheart.00447.2006. [DOI] [PubMed] [Google Scholar]
- 5.Brower GL, Henegar JR, Janicki JS. Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. Am J Physiol. 1996;271:H2071–H2078. doi: 10.1152/ajpheart.1996.271.5.H2071. [DOI] [PubMed] [Google Scholar]
- 6.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Cardiac Fail. 2005;11:548–556. doi: 10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Chancey AL, Brower GL, Janicki JS. Cardiac mast cell-mediated activation of gelatinase and alteration of ventricular diastolic function. Am J Physiol. 2002;282:H2152–H2158. doi: 10.1152/ajpheart.00777.2001. [DOI] [PubMed] [Google Scholar]
- 8.Chancey AL, Brower GL, Peterson JT, Janicki JS. Effects of matrix metalloproteinase inhibition on ventricular remodeling due to volume overload. Circ. 2002;105:1983–1988. doi: 10.1161/01.cir.0000014686.73212.da. [DOI] [PubMed] [Google Scholar]
- 9.Jobe LJ, Melendez GC, Levick SP, Du Y, Brower GL, Janicki JS. TNF-α inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol. 2009;297:H1462–H1468. doi: 10.1152/ajpheart.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakata Y, Yamamoto K, Mano T, Nishikawa N, Yoshida J, Hori M, Miwa T, Masuyama T. Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of angiotensin-converting enzyme inhibitor. Circ. 2004;109:2143–2149. doi: 10.1161/01.CIR.0000125741.88712.77. [DOI] [PubMed] [Google Scholar]
- 12.Ruzicka M, Skarda V, Leenen FH. Effects of ACE inhibitors on circulating versus cardiac angiotensin II in volume overload induced cardiac hypertrophy in rats. Circ. 1995;92:3568–3573. doi: 10.1161/01.cir.92.12.3568. [DOI] [PubMed] [Google Scholar]
- 13.McElmurray JH, Mukherjee R, New RB, Sampson AC, King MK, Hendrick JW, Goldberg A, Peterson TJ, Hallak H, Zile MR, Spinale FG. Angiotensin-converting enzyme and matrix metalloproteinase inhibition with developing heart failure: comparative effects on left ventricular function and geometry. JPET. 1999;291:799–811. [PubMed] [Google Scholar]
- 14.Spinale FG, de Gasparo M, Whitebread S, Hebbar L, Clair MJ, Melton DM, Krombach RS, Mukherjee R, Iannini JP, O SJ. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure. Circ. 1997;96:2385–2396. doi: 10.1161/01.cir.96.7.2385. [DOI] [PubMed] [Google Scholar]
- 15.Stewart JA, Jr, Gardner JD, Brower GL, Janicki JS. Temporal changes in integrin-mediated cardiomyocyte adhesion secondary to chronic cardiac volume overload in rats. Am. J. Physiol Heart Circ. Physiol. 2014;306:H101–H108. doi: 10.1152/ajpheart.00541.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess ML, Carver WE, Terracio L, Wilson SP, Wilson MA, Borg TK. Integrin-mediated collagen gel contraction by cardiac fibroblasts. Effects of angiotensin II. Circ Res. 1994;74:291–298. doi: 10.1161/01.res.74.2.291. [DOI] [PubMed] [Google Scholar]
- 17.Kryczka J, Stasiak M, Dziki L, Mik M, Dziki A, Cierniewski C. Matrix metalloproteinase-2 cleavage of the beta1 integrin ectodomain facilitates colon cancer cell motility. J. Biol. Chem. 2012;287:36556–36566. doi: 10.1074/jbc.M112.384909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerdes AM. Cardiac myocyte remodeling in hypertrophy and progression to failure. Journal of Cardiac Failure. 2002;8:S264–S268. doi: 10.1054/jcaf.2002.129280. [DOI] [PubMed] [Google Scholar]
- 19.Linke WA, Popov VI, Pollack GH. Passive and active tension in single cardiac myofibrils. Biophysical J. 1994;67:782–792. doi: 10.1016/S0006-3495(94)80538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titan isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 21.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin Isoform Switch in Ischemic Human Heart Disease. Circulation. 2002;106:1333–1341. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 22.Du Y, Plante E, Janicki JS, Brower GL. Temporal evaluation of cardiac myocyte hypertrophy and hyperplasia in male rats secondary to chronic volume overload. Am. J. Pathol. 2010;177:1155–1163. doi: 10.2353/ajpath.2010.090587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruzicka M, Yuan B, Leenen FH. Effects of enalapril versus losartan on regression of volume overload-induced cardiac hypertrophy in rats. Circulation. 1994;90:484–491. doi: 10.1161/01.cir.90.1.484. [DOI] [PubMed] [Google Scholar]