Abstract
Although exposure to acute stress has been shown to reinstate extinguished responding for a wide variety of drugs, no studies have investigated stress-induced reinstatement in animals with a history of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) self-administration. Thus, rats were trained to press a lever for MDMA (0.50 mg/kg/infusion) in daily sessions, and lever pressing was subsequently extinguished in the absence of MDMA and conditioned cues (light and tone). We then tested the ability of acute yohimbine (2.0 mg/kg), a pharmacological stressor, to reinstate lever-pressing under extinction conditions. Additionally, to model chronic stress, some rats were injected daily with yohimbine (5.0 mg/kg × 10 days) prior to reinstatement tests. To assess dopaminergic involvement, chronic yohimbine injections were combined with injections of SCH-23390 (0.0 or 10.0 μg/kg), a dopamine D1-like receptor antagonist. In a separate experiment, rats with a history of food self-administration were treated and tested in the same way. Results showed that acute yohimbine injections reinstated extinguished MDMA and food seeking, but only in rats with a history of chronic yohimbine exposure. Co-administration of SCH-23390 with chronic yohimbine injections prevented the potentiation of subsequent food seeking, but not MDMA seeking. These results suggest that abstinent MDMA users who also are exposed to chronic stress may be at increased risk for future relapse, and also that the effects of chronic stress on relapse may be mediated by different mechanisms depending on one’s drug use history.
Keywords: DOPAMINE, MDMA, REINSTATEMENT, SCH-23390, STRESS, YOHIMBINE
Drug addiction is a chronic disorder characterized by high rates of relapse. Clinical data support a link between stress exposure and relapse [1]. Using the reinstatement model, an animal model of relapse [2], exposure to acute stress has been shown to reinstate extinguished responding for a wide variety of drugs of abuse [3]. More recently, the pharmacological stressor yohimbine has been used effectively to induce relapse to drug seeking in rats and monkeys [4, 5], as well as to induce drug craving in human drug addicts [6, 7]. Yohimbine is an α-2 adrenoceptor antagonist that increases brain norepinephrine release [8] and induces physiological and behavioral symptoms of stress and anxiety in both humans and laboratory animals [9, 10].
Although a large number of studies have shown that acute stress induces reinstatement of drug seeking in animals, to our knowledge, no studies have investigated stress-induced reinstatement following self-administration of the stimulant 3,4-methylenedioxymethamphetamine (MDMA; ecstasy). MDMA is an amphetamine derivative that is popular among adolescents and young adults [11] and causes increases in extracellular monoamine levels, particularly dopamine and serotonin [12]. Similar to amphetamine and cocaine, repeated exposure to MDMA causes enduring adaptations in the dendritic structure and electrophysiological characteristics of motive circuit neurons, including those in dorsal striatum, nucleus accumbens, and dorsomedial prefrontal cortex [13-15]. Moreover, it has been shown that MDMA-associated cues and MDMA priming injections reinstate extinguished MDMA seeking in rats [16-19]. However, there are very few data regarding the role of stress in addiction-like MDMA-associated behavior, including reinstatement.
Thus, a main goal of the present study was to determine whether acute injections of yohimbine would induce reinstatement of MDMA seeking in rats using the operant self-administration-extinction-reinstatement model. Given recent evidence from our laboratory that prior exposure to chronic yohimbine stress potentiates reinstatement of palatable food seeking under conditions that normally elicit little reinstatement [20], we also exposed some groups of rats to a chronic yohimbine regimen during the extinction phase.
Finally, given that chronic stress causes changes in dopamine D1-like receptor (D1R)-mediated transmission in prefrontal cortex [21, 22], and also that D1R activation in this region is critical for stress-induced reinstatement of both food and drug seeking [23, 24], we tested the hypothesis that chronic exposure to stress increases vulnerability to MDMA seeking via dopamine-mediated mechanisms. To do this, we combined repeated yohimbine injections with SCH-23390, a D1R antagonist.
Data were collected from adult male, Sprague-Dawley rats (N = 67, 275-350 g at the commencement of experiments) that were obtained from Harlan Sprague-Dawley (Indianapolis, IN). Rats were housed individually under standard laboratory conditions (12-hr light cycle from 7:00 AM to 7:00 PM) with ad libitum access to water. Starting 1 week before operant training rats were placed on a restricted diet and maintained at ~85% of free-feeding body weight with the exception of a period before and after surgery during which animals were given free access to food. Food rations were adjusted for individual rats based on changes in body weight and were given immediately following each daily test session. All experiments were conducted during the light cycle, were in compliance with NIH guidelines, and were approved by the Bloomsburg University Institutional Animal Care and Use Committee.
(±)-MDMA was provided by the National Institute on Drug Abuse (Research Triangle Park, NC). Yohimbine hydrochloride and SCH-23390 hydrochloride were purchased from Sigma (St. Louis, MO). MDMA and SCH-23390 were dissolved in 0.9% sterile saline at concentrations of 5.0 mg/ml and 10.0 μg/ml, respectively. Yohimbine was dissolved in distilled water at concentrations of 5.0 and 2.0 mg/ml for chronic treatment and reinstatement testing, respectively. Doses of drugs were chosen based on our previous work [18, 20]. All doses refer to weight of salt.
Testing occurred in operant conditioning chambers (Coulbourn Instruments, Whitehall, PA) that were housed in sound-attenuating, ventilated cubicles and connected to a PC with the Graphic State software interface system (Coulbourn Instruments). Each chamber had two levers, but only one lever (active lever) activated the pellet dispenser or syringe pump. Chambers also included a house light, a row of multicolored stimuli lights (above active lever), and a tone generator. Each chamber was outfitted with a fluid swivel and spring leash assembly connected to a counterbalanced arm assembly. A motor-driven syringe pump (Coulbourn Instruments) for drug delivery was located outside of each cubicle. In addition to ventilation fans located within each cubicle, white noise (~75 dB[A]) was used to mask extraneous noises, and was delivered through a speaker located centrally in the testing room.
Rats first experienced 2 days of shaping sessions during which responses on the active lever were reinforced with 45-mg grain-based food pellets (Bio Serv, Frenchtown, NJ) contingent upon a fixed ratio (FR)-1 schedule of reinforcement. During shaping sessions, if 15 min elapsed without an active lever response a reinforcer was delivered non-contingently. Shaping sessions ended after 80 reinforcers were earned. Over the course of the next 7 days the reinforcement schedule was gradually increased to FR-5 in daily 30-min or 1-hr sessions for rats in Experiments 1 (n = 29) or 2 (n = 38), respectively (see below).
Five days following the conclusion of operant training, rats in Experiment 1 underwent catheterization surgery. After an injection of atropine sulfate (0.05 mg/kg; s.c.), animals were anesthetized with ketamine HCl (90 mg/kg; i.m.) and xylazine HCl (10 mg/kg; i.m.), with supplemental injections as needed. A catheter constructed from polyethylene tubing (PE10 and PE50; Fisher Scientific, Pittsburgh, PA) and a 22 gauge cannula-guide connector assembly (Plastics One, Roanoke, VA) was inserted into the right jugular vein. The catheter was routed subcutaneously and mounted to the skull with dental cement. During 1 week of recovery catheters were flushed with heparinized physiological saline (50 U/ml heparin) twice daily, and 0.1 ml (10 mg/ml; i.v.) of gentamycin (Lonza, Walkersville, MD) was administered once daily. During the period of MDMA self-administration, catheter patency was evaluated by injecting 0.1 ml Brevital (1%) as necessary. Loss of muscle tone within 5 s after injection indicates a patent catheter.
For Experiment 1, rats were trained to respond for MDMA in 14 consecutive, daily, 2-hr sessions contingent on a modified FR-5 schedule: the first response on the active lever resulted in an intravenous infusion of 0.50 mg/kg of MDMA accompanied by conditioned stimuli (CS), which consisted of a tone + flashing cue light compound stimulus presented for 5 s. Drug concentration and pump delivery rate (10 μ l/s) were kept constant, and dose of drug was controlled by varying the duration of pump action (i.e., volume of injected solution) based on body weight. Delivery of the drug and CS was followed by a 15 s time-out period signaled by illumination of the house light. During MDMA infusions and time-outs responses were recorded but had no programmed consequences. After the first infusion, MDMA infusions and presentations of the CS were contingent upon an FR-5 schedule. Rats in Experiment 2 did not undergo surgery or self-administer MDMA, but instead continued to respond for food reinforcers on an FR-5 schedule in daily 1-hr sessions for 5 additional days.
After self-administration, rats in both Experiments 1 and 2 began daily 1-hr extinction training, which continued for 7 days (Extinction 1). During the extinction sessions, responses were recorded but had no programmed consequences (i.e., no CS, or primary reinforcer). On the day following the 7th extinction session, extinction training stopped and daily treatments began. To model chronic stress, rats were injected daily with yohimbine (5.0 mg/kg × 10 days; i.p.) or vehicle. To assess dopaminergic involvement in chronic stress effects, chronic yohimbine and vehicle injections were combined with injections of SCH-23390 (10.0 μg/kg; i.p.), a D1R antagonist, or vehicle. Thus, rats were randomly assigned to one of four treatment conditions: saline + water, saline + yohimbine, SCH-23390 + water, or SCH-23390 + yohimbine (n = 7-10/treatment condition). Rats were returned to their home cages immediately following injections. Beginning the day after the last treatment, daily 1-hr extinction sessions resumed for 4 days (Extinction 2).
Rats in both Experiments 1 and 2 continued to be tested under extinction conditions for 3 more days, during which yohimbine-induced reinstatement testing occurred using a repeated-measures design. Thus, each rat received injections of both yohimbine (2.0 mg/kg; i.p.) and vehicle 30 min prior to the commencement of sessions on 2 different days during reinstatement testing (Days 1 and 3). Doses were given in a counterbalanced order based on active lever responding during the last three days of self-administration. On Day 2, no injections were administered.
Total number of MDMA infusions/session for Experiment 1 were analyzed by means of a repeated-measures ANOVA. Total number of active lever responses/session were analyzed separately for Experiments 1 and 2 using mixed factorial ANOVAs. The between factors were chronic yohimbine dose and chronic SCH-23390 dose. The within factors were day (for extinction training) and acute yohimbine dose (for yohimbine-induced reinstatement testing). In addition, for yohimbine-induced reinstatement data, lever (active and inactive) was included as a within-subjects factor. To verify the chronic stress manipulation, amount of daily food ration required to maintain weight during treatment was determined for each chronic treatment group and was calculated as g/100 g body weight. Food ration data were analyzed with a mixed factorial ANOVA (chronic yohimbine dose X chronic SCH-23390 dose X day). All ANOVAs were followed by Bonferroni post-tests.
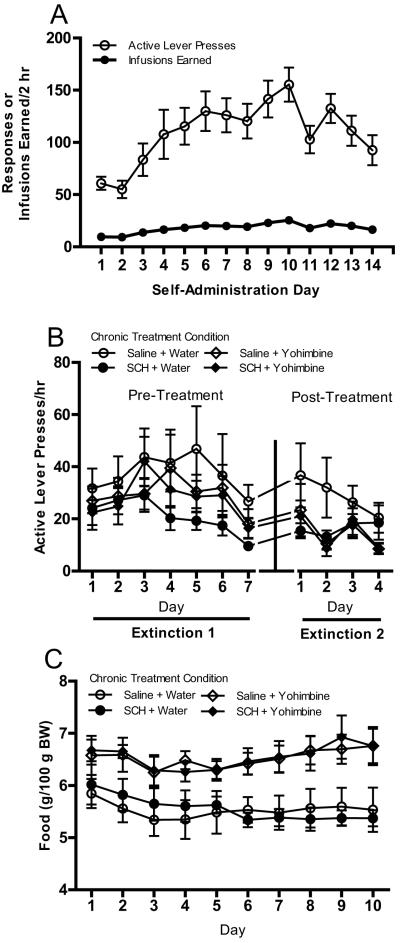
Results showed that all rats demonstrated reliable MDMA self-administration (see Fig. 1a). Overall, there was an escalation of intake over days that peaked on Day 10, F(13, 364) = 6.39, p < .001. During subsequent extinction training, lever pressing decreased over days [main effect of day; F(6, 150) = 4.09, p < .01 and F(3, 75) = 4.59, p < .01 for Extinction 1 and 2, respectively; see Fig. 1b]. As shown in Fig. 1c, more food/100 g body weight was necessary during the chronic treatment period in rats receiving yohimbine injections [main effect of chronic yohimbine dose; F(1, 25) = 21.00, p < .001].
Fig. 1.
(a) Active lever presses and MDMA infusions earned/2 hr in all animals across 14 days of self-administration. Rats were trained on a modified FR-5 schedule of reinforcement. Delivery of MDMA (0.50 mg/kg) was accompanied by a tone + flashing cue light conditioned stimulus (CS) presented for 5 s, which was followed by a 15-s time-out period signaled by illumination of the house light. (b) Active lever responses/hr across 7 days of extinction before chronic treatment and 4 days of extinction after chronic treatment. Responses had no programmed consequences (i.e., no CS or MDMA). Rats received one of four treatments (saline + water, saline + yohimbine, SCH-23390 + water, or SCH-23390 + yohimbine) once each day for 10 days between the two extinction periods (n = 7-10/treatment condition). (c) Amount of food (g/100 g body weight) provided in home cage to maintain body weight during the chronic treatment phase in the four chronic treatment groups. All data in figure are represented as mean ± SEM.
As shown in Fig. 2, acute yohimbine injections increased responding [main effect of acute yohimbine dose; F(1, 25) = 28.15, p < .001]. There also was a main effect of lever [F(1, 25) = 38.30, p < .001] and an acute yohimbine dose X lever interaction [F(1, 25) = 8.01, p < .01], in that increases in responding as a function of acute yohimbine dose were greater on the active lever relative to the inactive lever. Although baseline responding (0.0 mg/kg yohimbine prime) was similar across all chronic treatment groups, Bonferroni post-tests indicated that responding increased significantly with acute yohimbine injections only in rats treated previously with chronic yohimbine.
Fig. 2.
Effect of repeated yohimbine (5.0 mg/kg × 10 days) and repeated SCH-23390 (10 μg/kg × 10 days) on subsequent active (a) and inactive (b) lever pressing during acute yohimbine-induced reinstatement testing in rats with a history of MDMA self-administration. Each rat received injections of both yohimbine (2.0 mg/kg; i.p.) and vehicle (counterbalanced), on separate days, 30 min prior to the commencement of sessions that were identical to extinction sessions. *p < .05 compared to 0.0 mg/kg prime in same chronic treatment group, Bonferroni post-test. All data in figure are represented as mean + SEM.
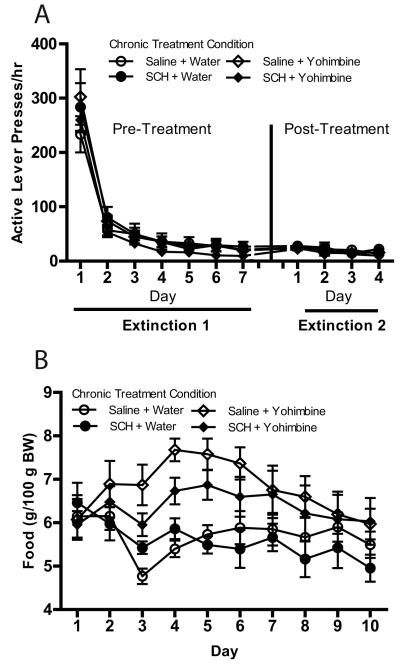
In Experiment 2, all rats demonstrated reliable pellet self-administration. Mean (±SEM) active lever pressing on the last day of self-administration was 1,632.32 (±84.18) presses/hr. Lever pressing decreased over days during subsequent extinction training [main effect of day; F(6, 204) = 131.07, p < .001 and F(3, 102) = 5.21, p < .01 for Extinction 1 and 2, respectively; see Fig. 3a]. As in Experiment 1, more food/100 g body weight was necessary during the chronic treatment period in rats receiving yohimbine injections [main effect of chronic yohimbine dose; F(1, 34) = 10.71, p < .01; see Fig. 3b]. There also was a significant main effect of treatment day and a significant treatment day X chronic yohimbine dose interaction [F(9, 306) = 3.90, p < .001 and F(9, 306) = 3.79, p < .001, respectively] with regard to daily food ration.
Fig. 3.
(a) Active lever responses/hr across 7 days of extinction before chronic treatment and 4 days of extinction after chronic treatment in rats with a history of food self-administration. Responses had no programmed consequences (i.e., no CS or pellets). Rats received one of four treatments (saline + water, saline + yohimbine, SCH-23390 + water, or SCH-23390 + yohimbine) once each day for 10 days between the two extinction periods (n = 7-10/treatment condition). (c) Amount of food (g/100 g body weight) provided in home cage to maintain body weight during the chronic treatment phase in the four chronic treatment groups. All data in figure are represented as mean ± SEM.
As shown in Fig. 4, acute yohimbine injections increased responding [main effect of acute yohimbine dose; F(1, 34) = 16.84, p < .001]. There also was an acute yohimbine dose X chronic yohimbine dose interaction [F(1, 34) = 5.15, p < .05], indicating that yohimbine-induced reinstatement was greater in rats treated previously with chronic yohimbine. However, Bonferroni post-tests indicated that responding increased significantly with acute yohimbine injections only in rats treated previously with chronic saline + yohimbine; active-lever responding after acute yohimbine priming in the SCH-23390 + yohimbine chronic treatment group was low and comparable to animals treated previously with water, indicating that SCH-23390 prevented the effects of chronic yohimbine on later reinstatement. Finally, there was a main effect of lever [F(1, 34) = 12.27, p < .01], and an acute yohimbine dose X lever interaction [F(1, 34) = 6.59, p < .05].
Fig. 4.
Effect of repeated yohimbine (5.0 mg/kg × 10 days) and repeated SCH-23390 (10 μg/kg × 10 days) on subsequent active (a) and inactive (b) lever pressing during acute yohimbine-induced reinstatement testing in rats with a history of food self-administration. Each rat received injections of both yohimbine (2.0 mg/kg; i.p.) and vehicle (counterbalanced), on separate days, 30 min prior to the commencement of sessions that were identical to extinction sessions. **p < .01 compared to 0.0 mg/kg prime in same chronic treatment group, Bonferroni post-test. All data in figure are represented as mean + SEM.
Although studies with other drugs of abuse have shown that animals tend to display escalation only when given extended access (≥ 6 hr/day) [25], the present results support our previous findings, which showed that the majority of rats self-administering MDMA displayed escalation of intake under our short-access conditions [17]. Thus, at least with regard to MDMA, even limited access conditions can promote escalation of self-administration under some circumstances. Given that escalation of self-administration is a pattern of behavior suggested to model the transition from controlled to compulsive drug use in humans [25], this finding adds to the accumulating evidence that MDMA shares many features with highly addictive drugs.
A novel finding in the present study was that acute yohimbine injections reinstated extinguished MDMA-seeking behavior. To our knowledge, this is the first report of stress-induced reinstatement of operant responding for MDMA. Importantly, only rats treated previously with chronic yohimbine displayed significant reinstatement responding following acute yohimbine priming. In fact, rats that were not exposed to yohimbine prior to reinstatement tests displayed little reinstatement of MDMA seeking. This is consistent with previous studies of stress-induced reinstatement of both drug (cocaine, heroin, and ethanol) and palatable food seeking under conditions in which discrete CSs were not available during extinction and reinstatement testing. Indeed, reinstatement induced by both yohimbine and footshock stress is weaker or completely absent when drug- or food-paired discrete cues are omitted [20, 26-29]. Consistent with our recent report of yohimbine-induced reinstatement of palatable food seeking [20], significant reinstatement of MDMA seeking was observed under these conditions (CS absent) only in rats with a history of chronic yohimbine exposure. Thus, it is possible that abstinent MDMA users who also are exposed to chronic stress may be at increased risk for future relapse under conditions that would not otherwise promote robust drug seeking.
Another main finding was that co-administration of SCH-23390 with chronic yohimbine injections did not attenuate the potentiation of subsequent MDMA-seeking behavior induced by acute yohimbine. This was surprising given that the same dose of SCH-23390 reversed or attenuated all effects of repeated yohimbine (5.0 mg/kg) in our previous study [20], including effects on yohimbine- and food-primed reinstatement of palatable food seeking, extinction responding, and maintenance of body weight. The results of Experiment 2 in the present study support our previous findings by showing that SCH-23390 blocked the effects of chronic yohimbine on reinstatement of food seeking and attenuated its effects on body weight. The reason for the lack of effect of SCH-23390 in rats with a history of MDMA self-administration is not known, but it appears that D1R blockade did not reduce the “stressfulness” of yohimbine injections in rats that had previously self-administered MDMA. Indeed, both the saline + yohimbine and the SCH-23390 + yohimbine groups required significantly larger daily food rations to maintain body weight across the entire 10 days of chronic stress compared to unstressed groups (see Fig. 1c). These results suggest that the same behavioral outcome of chronic stress (i.e., enhanced relapse vulnerability) may be mediated by different mechanisms depending on one’s drug use history. Therefore, a pharmacotherapy targeting a single neurotransmitter system is unlikely to prevent the effects of chronic stress on relapse under all circumstances.
In sum, we report that a history of chronic yohimbine stress predisposes rats to reinstatement of MDMA seeking under conditions that induce little reinstatement in unstressed rats. Also, as opposed to rats with a history of food self-administration, antagonism of dopamine D1Rs during chronic stress did not attenuate the effects of the stress on subsequent reinstatement. Given the scarcity of data on the relationship between chronic stress and relapse, more research in this area is warranted.
Highlights.
▶We tested the effects of acute and chronic yohimbine stress on MDMA seeking.
▶ Yohimbine reinstated MDMA seeking in rats with prior chronic yohimbine exposure.
▶ D1 blockade during chronic stress blocked potentiated food, but not MDMA, seeking.
▶ Thus, chronic stress exposure may increase risk for future relapse to MDMA seeking.
▶ Drug use history may alter the mechanisms by which chronic stress exerts its effects.
Acknowledgements
This work was supported by the National Institutes of Health (NIDA R15 DA035432 to KTB). MDMA was generously provided by the National Institute on Drug Abuse.
Abbreviations
- CS
conditioned stimulus
- D1R
D1-like receptor
- FR
fixed ratio
- MDMA
3,4-methylenedioxymethamphetamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest.
References
- [1].Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Current Psychiatry Reports. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- [3].Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2015;15:142. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–93. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- [5].Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–9. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- [6].Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–51. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- [7].Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, et al. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36:1178–86. doi: 10.1038/npp.2010.253. doi: 10.038/npp.2010.253. Epub 1 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abercrombie ED, Keller RWJ, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- [9].Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [10].Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- [11].Strote J, Lee JE, Wechsler H. Increasing MDMA use among college students: results of a national survey. J Adolescent Health. 2002;30:64–72. doi: 10.1016/s1054-139x(01)00315-9. [DOI] [PubMed] [Google Scholar]
- [12].Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or "Ecstasy") Psychopharmacology. 1995;119:247–60. doi: 10.1007/BF02246288. [DOI] [PubMed] [Google Scholar]
- [13].Ball KT, Budreau D, Rebec GV. Context-dependent behavioural and neuronal sensitization in striatum to MDMA (ecstasy) administration in rats. Eur J Neurosci. 2006;24:217–28. doi: 10.1111/j.1460-9568.2006.04885.x. [DOI] [PubMed] [Google Scholar]
- [14].Ball KT, Wellman CL, Fortenberry E, Rebec GV. Sensitizing regimens of (±)3,4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience. 2009;160:264–74. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ball KT, Wellman CL, Miller BR, Rebec GV. Electrophysiological and structural alterations in striatum associated with behavioral sensitization to (+/-)3,4-methylenedioxymethamphetamine (ecstasy) in rats: role of drug context. Neuroscience. 2010;171:794–811. doi: 10.1016/j.neuroscience.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ball KT, Slane M. Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psychopharmacology. 2012;224:377–85. doi: 10.1007/s00213-012-2762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ball KT, Slane M. Tolerance to the locomotor-activating effects of 3,4-methylenedioxymethamphetamine (MDMA) predicts escalation of MDMA self-administration and cue-induced reinstatement of MDMA seeking in rats. Behav Brain Res. 2014;274:143–8. doi: 10.1016/j.bbr.2014.08.010. 10.1016/j.bbr.2014.08.010. Epub Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ball KT, Walsh KM, Rebec GV. Reinstatement of MDMA (ecstasy) seeking by exposure to discrete drug-conditioned cues. Pharmacol Biochem Behav. 2007;87:420–5. doi: 10.1016/j.pbb.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Colussi-Mas J, Wise RJ, Howard A, Schenk S. Drug seeking in response to a priming injection of MDMA in rats: relationship to initial sensitivity to self-administered MDMA and dorsal striatal dopamine. International Journal of Neuropsychopharmacology. 2010;13:1315–27. doi: 10.1017/S1461145710000283. doi: 10.017/S1461145710000283. Epub 2010 Mar 25. [DOI] [PubMed] [Google Scholar]
- [20].Ball KT, Miller L, Sullivan C, Wells A, Best O, Cavanaugh B, et al. Effects of repeated yohimbine administration on reinstatement of palatable food seeking: involvement of dopamine D1-like receptors and food-associated cues. Addict Biol. doi: 10.1111/adb.12287. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui D-H, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–74. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mizoguchi K, Shoji H, Ikeda R, Tanaka Y, Tabira T. Persistent depressive state after chronic stress in rats is accompanied by HPA axis dysregulation and reduced prefrontal dopaminergic neurotransmission. Pharmacol Biochem Behav. 2008;91:170–5. doi: 10.1016/j.pbb.2008.07.002. Epub 2008 Jul 13. [DOI] [PubMed] [Google Scholar]
- [23].Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. Epub 2002 Nov 20. [DOI] [PubMed] [Google Scholar]
- [24].Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology. 2011;36:497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- [26].Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–61. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–8. doi: 10.1016/j.bbr.2009.11.030. doi: 10.1016/j.bbr.2009.11.030. Epub Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shelton KL, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. Int J Comp Psych. 2005;18:154–66. [Google Scholar]
- [29].Lin P, Pratt WE. Inactivation of the nucleus accumbens core or medial shell attenuates reinstatement of sugar-seeking behavior following sugar priming or exposure to food-associated cues. PLoS One. 2014;9:e99301. doi: 10.1371/journal.pone.0099301. doi: 10.1371/journal.pone.0099301. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]