Abstract
Phase sensitive in situ forming implants (ISFI) are a promising platform for the controlled release of therapeutic agents. The simple manufacturing, ease of placement, and diverse payload capacity make these implants an appealing delivery system for a wide range of applications. Tailoring the release profile is paramount for effective treatment of disease. In this study, three innovative formulation modifications were used to control drug release. Specifically, water, 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI), and bovine serum albumin (BSA) were incorporated into an ISFI solution containing the small molecular weight mock drug, sodium fluorescein. The effects of these additives on drug release, swelling, phase inversion, erosion, and implant microstructure were evaluated. Diagnostic ultrasound was used to monitor changes in swelling and phase inversion over time noninvasively. Water, DiI, and the combination of BSA/DiI functioned to reduce burst release 47.6%, 76.6%, and 59.0% respectively. Incorporation of water into the casting solution also enhanced the release of drug during the diffusion period of release by 165.2% relative to the excipient free control. Incorporation of BSA into the polymer solution did not significantly alter the burst release (P<0.05), however the onset of degradation facilitated release was delayed relative to the excipient free control by 5 d. This study demonstrates that the use of excipients provides a facile method to tailor the release profile and degradation rate of implants without changing the polymer or solvent used in the implant formulation, providing fine control of drug dissolution during distinct phases of release.
Keywords: phase inversion, in situ forming implant, PLGA, implant microstructure, excipient
1. Introduction
Phase sensitive in situ forming systems have been used for fabrication of numerous products ranging from asymmetric membranes for reverse osmosis filtration to drug eluting polymer depots for the treatment of prostate cancer1-7. These phase sensitive systems exist as liquid polymer solutions until introduced to a nonsolvent, such as water, where the polymer precipitates into a solid matrix through a process of phase inversion5,8-11. In medical applications, phase sensitive in situ forming implants (ISFI) are of interest because they provide a platform by which drug can be delivered through a minimally invasive injection, which facilitates accurate placement of the implant in a desired location for the controlled local delivery of drug over time12. These implants are manufactured by dissolving a biodegradable polymer into a biocompatible solvent, which can be mixed with a drug to form a suspension or solution13,14. During phase inversion the nonsolvent diffuses into the polymer solution, while the solvent diffuses out of the implant into the surrounding environment resulting in an unstable ternary system until sufficient nonsolvent can induce precipitation of the polymer5,6. A number of systems have been used to characterize the phase inversion process such as dark ground imaging, electron spin resonance, and ultrasound imaging10,15-18. Ultrasound imaging works by detecting the changes in acoustic impedance that occur as the polymer precipitates out of solution6,17. It is the only method that allows for the visualization of the process both in vitro and in vivo, and can be used to detect other processes such as implant swelling and erosion14,19.
Phase sensitive ISFIs have a release profile with three distinct phases20. Burst release occurs over the course of the first 24-48 h and is identified as the initial rapid release of drug from the implant. Diffusion facilitated release occurs as the drug moves through the polymer matrix and is controlled by the implant microstructure21-23. Degradation facilitated release occurs as a result of polymer degradation increasing the interconnectivity of the porous microstructure of the implant and results in an increase in the release of drug. A number of methods have been used to control the release profile of phase sensitive ISFIs; typically by altering the mass transfer kinetics of the solvent/nonsolvent in order to increase or decrease the porosity of the polymer matrix8,21,23-25. Factors such as changes in the polymer molecular weight (Mw), concentration of the casting solution, or the solvent composition have all been successfully used to change the release profile of the implant16,19,22,26-30. However, these changes often result in an increased solution viscosity that can limit the injectability of the solution31. The use of additives provides a means to alter distinct phases of the release profile of phase sensitive ISFIs, and tailor the release profile for a specific application, without increasing the solution viscosity.
In the present study the effect of three unconventional additives on the release profile from ISFIs was evaluated. These excipients function as agents added to the polymer formulation solution to enhance the function of the implants, without inducing a therapeutic effect32. Water was incorporated into the casting solution in order to change the solvent/nonsolvent mass transfer kinetics. 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) has been shown to increase the hydrophobicity of the polymer solution and consequently reduce the interconnectivity of the internal porous network14, and was selected to reduce both burst and diffusion facilitated release. Bovine serum albumin (BSA) has been shown to reduce the erosion of ISFIs, and was introduced to alter the onset of degradation facilitated release14. Finally, a combination of BSA/DiI was used to evaluate the combinatorial effect of additives on the release profile. Changes in drug release were evaluated by measuring the dissolution kinetics of the mock drug sodium fluorescein (Mw=376, Log P=−1.52, pKa=6.4)14,33. Sodium fluorescein was specifically chosen because it is a small molecular weight hydrophilic agent that has a release profile with distinct regions of release following a pattern of burst, diffusion, and degradation facilitated release. Furthermore, this mock drug also has a release profile similar to that observed with themotherapeutic agent doxorubicin14. Implant erosion and fluid uptake were measured by monitoring changes in the implant mass. Polymer phase inversion was evaluated using ultrasound imaging, and the implant microstructure was evaluated using scanning electron microscopy (SEM). Results from this study will provide insight into additional methods for controlling the release profile of ISFIs, providing control over the dissolution and degradation kinetics of drugs during each distinct phase of release.
2. Materials and Methods
2.1. Materials
Poly(DL-lactic-co-glycolic acid) (PLGA, 50:50, Mw 21,000 Da) was obtained from Evonik Industries (Rellinghauser Strasse, Germany) and used as received. N-methyl-2-pyrrolidinone (NMP), sodium fluorescein and BSA were used as received from Sigma Aldrich (St. Louis, MO). DiI was used as received from Advancing Assay and Test Technologies (Sunnyvale, CA). Agarose and phosphate buffered saline (PBS) were received from Fischer Scientific (Waltham, MA).
2.2. Polymer Solution Formulations
Control solutions with no additives were prepared using a 39:60:1 mass ratio of PLGA:NMP:fluorescein by first suspending or dissolving the fluorescein in NMP as previously described14,19. 1 h before injection into the bath solution, BSA was added to the caste solution (35:60:4:1 mass ratio of PLGA:NMP:BSA:fluorescein), and allowed to mix using a magnetic stirrer. The solutions were then warmed to 37°C in order to eliminate air bubbles in the solution. Solutions containing DiI (35:60:4:1 mass ratio of PLGA:NMP:DiI:fluorescein) were prepared by first dissolving the fluorescein and DiI in NMP, followed by addition of the PLGA, which was stirred overnight in a 37°C shaker table at 90 RPM. Solutions containing both DiI and BSA (BSA/DiI) (35:60:2:2:1 mass ratio of PLGA:NMP:BSA:DiI:fluorescein) were prepared by first dissolving the fluorescein and DiI in NMP, then adding PLGA and stirred overnight, with BSA added as described earlier. Solutions containing water (39:54:6:1 mass ratio of PLGA:NMP:water:fluorescein) were prepared similar to control solutions, but a 10% water in NMP solution was used instead of pure NMP. Polymer solutions with water were prepared the day before use, all other formulation were stored at 4°C and used within three days.
2.3. Cumulative Drug Release
Drug release profiles were evaluated as described previously14. First, polymer solution (42.9±5.2 mg) was injected into 10 ml of 37°C PBS (pH 7.4), and then placed in an incubated orbital shaker (37°C at 90 RPM). Over the course of the first 6 h, 1 ml of solution was sampled and then replaced with 1 ml of fresh warm PBS at predetermined time points (0, 0.5, 1, 2, 4, and 6 h after injection into PBS). After 24 h, a sample was taken, and then the bath solution was completely removed and replaced by 10 mL of fresh buffer daily for 14 days (d). After 14 d, implants were removed from the bath solution and degraded in 5 ml of 2M NaOH. Fluorescein mass was determined by measuring the fluorescence in the solution samples which was compared with a standard curve of known fluorescein concentrations using a multimode microplate reader (Tecan Ltd., Infinite 200 series) at excitation/emission wavelengths (Ex/Em) of 485/525 nm. Cumulative drug release was calculated from these measurements and normalized by the total mass of drug in the implant.
2.4. Implant Imaging
Implants were imaged using diagnostic ultrasound as previously described17. Briefly, implants were imaged through the Z-axis by immobilizing a 12 MHz transducer (Aplio XG, Toshiba Medical Systems) below an agarose mold containing the implant. Images were taken at predetermined time points over the course of the first 24 h (0, 0.5, 1, 2, 4, 6 and 24 h after the implants were added to PBS), and then imaged daily until the study was terminated. The buffer solution was replaced daily after the images were taken. Implants were maintained at 37°C at 90 RPM for the duration of the study. Image analysis was performed to monitor changes in the polymer shell thickness by using a custom MatLab code as previously described (MathWorks Inc., Natick, MA)17,34. First, a threshold was applied to the image in order to create a binary mask, which was normalized by the sum of the total number of pixels within the cross-sectional area of the implant17,34. Changes in the implant cross-sectional area over time were used as a metric of the implant swelling17,34.
2.5. Erosion and Bathside Uptake
Changes in implant mass with respect to time were monitored as describe previously14,19. First, the initial mass of implants added to 10 ml of warm PBS (pH 7.4), were recorded and then kept in an incubated shaker (37°C at 90 RPM). Then implants were removed from the bath solution at predefined time points (1, 3, 7, 10, 14, 17, and 21 d after the polymer solution was added to PBS). At each time point, implants were dried with a clean paper towel, and then weighed in order to obtain the wet mass. The implants were then frozen, followed by lyophilization for 4 days. After lyophilization the implant dry mass was recorded. Fluid uptake was calculated by subtracting the wet mass from the initial mass then normalizing by the initial implant mass. Polymer erosion was determined by normalizing the implant dry mass with the theoretical polymer mass of the implant. Sink conditions were maintained by replacing the total volume of the buffer solution daily.
2.6. Scanning Electron Microscope Imaging and Analysis
Implant microstructure was evaluated as previously described14. Briefly, the implants were frozen and then fractured over dry ice. After the implants were freeze fractured, the samples were lyophilized for 4 d. Next the implants were mounted on an aluminum stub using carbon tape, and sputter coated with 5 nm of Pd. The coated samples were then imaged using a Quanta 200 3D ESEM with an acceleration voltage of 3.5 kV and a hole size of 10.
2.7. Statistical Analysis
Statistical significance was performed using Minitab (Minitab inc., State College, PA). One-way analysis of variance (ANOVA, p<0.05) was used to determine statistical significance, and a Tukey multi-comparison test was used to compare differences between groups. All data were reported as mean ± standard deviation.
3. Results
3.1. Cumulative Drug Release
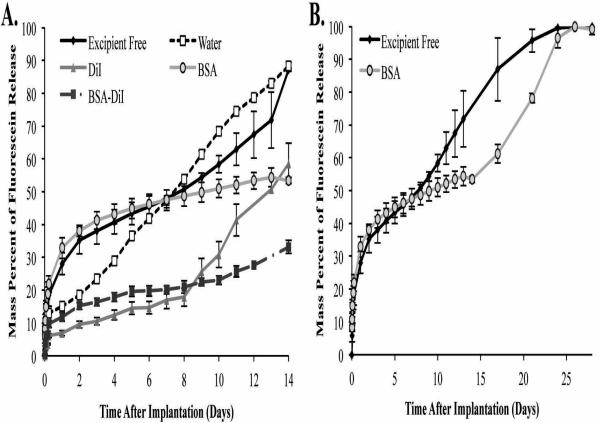
ISFIs have characteristic release kinetics consisting of three distinct phases of release: burst, diffusion, and degradation. Burst release occurred over the course of the first 24 h after injection into the bathside solution. No significant difference in burst release were observed between implants formulated without additives and those containing BSA (28.8±3.0% and 32.9±3.2% respectively), however these implant formulations did have a significantly greater burst release than all other depot formulations (Figure 1). The additives water, DiI, and BSA/DiI reduced the average burst release by 47.6%, 75.9%, and 58.8% respectively.
Figure 1.
Cumulative release of sodium fluorescein over the course of 14 d (A) and 28 d (B). All error bars represent standard deviation with n=3.
The diffusion period of release occurred after 24 h, and the onset of degradation facilitated release was determined by evaluating the derivative of the release plots19. Implants that used water as an additive had a significantly greater release rate than all other implant formulations (6.1±0.1%/d). The addition of BSA or DiI into the polymer solution significantly reduced the daily release rate of mock drug from the implants (1.3±0.1%/d and 1.5±0.3 %/d for BSA and DiI respectively) compared with implants that contained no additives (2.3±0.2 %/d). The greatest decrease in diffusion facilitated release was observed using a combination of BSA/DiI in the depot, resulting in a release rate of 1.0±0.1 %/day.
Degradation facilitated release was not observed when water was used as the additive (Figure 1). However, a significant increase in the drug dissolution rate was observed for all other implant formulations. The use of DiI and BSA/DiI did not alter the onset of degradation facilitated release, but incorporation of BSA alone delayed degradation facilitated release for 5 d. The use of BSA or DiI as standalone additives did not significantly alter the daily dissolution kinetics of mock drug during degradation facilitated release. However, significantly lower dissolution kinetics were observed from implants that contained the combination of BSA/DiI. The release rate results are summarized in Table 1.
Table 1.
Average release of mock drug at each phase of release.
| Burst (%) | Diffusion (%/day) | Degradation (%/day) | |
|---|---|---|---|
|
| |||
| Excipient Free | 28.8±3.0 | 2.3±0.2 | 4.5±1.7 |
| Water | 15.1±0.3 | 6.1±0.1 | - |
| DiI | 6.9±0.8 | 1.5±0.3 | 6.5±1.0 |
| BSA | 32.9±3.2 | 1.3±0.1 | 4.4±0.3 |
| BSA-DiI | 11.8±1.3 | 1.0±0.1 | 2.0±0.3 |
3.2. Ultrasound Characterization
3.2.1. Implant Morphology
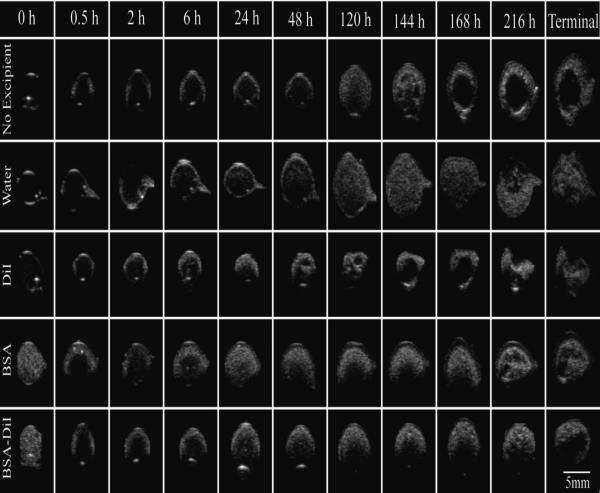
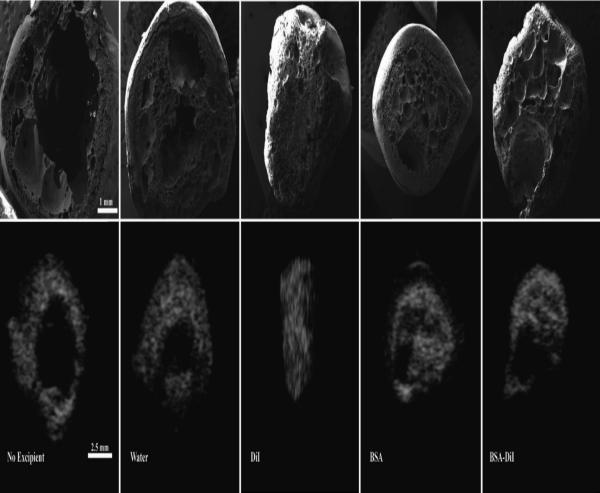
Representative gray-scale images of the implant cross-section over time are shown in Figure 2. Implants without an additive developed a thin shell immediately after injection into the bathside. Over time, an increase in hyperechoic regions were observed as the polymer precipitated. After 6 d in the nonsolvent, large pores began to form in the center of the implant, which increased in diameter over the duration of the study. When water was used as the additive, implant behavior followed a similar pattern of formation. However, the development of the central pore did not occur until after 9 d in the bathside, and the pore diameter did not expand (Figure 2). Implants formulated with DiI precipitated more rapidly than those without additives, additionally the formation of the central pore occurred after 5 d followed by the implant collapsing after 9 d (Figure 2). Implants that contained BSA had a high initial echogenic signal. This elevated echogenicity was lost within the first half hour, followed by the gradual increase in backscatter over the course of the first 24 h. Development of an interior pore did not occur until after 9 d in the nonsolvent, and the pore did not expand during the study. Implants with the combination of BSA/DiI had an initial elevated echogenic signal similar to implants with BSA alone, but the central pore did not occur until after 10 d. Image validation was performed by comparing the ultrasound images to SEM images of cryosectioned implants, demonstrating that pore formation observed using ultrasound imaging could be observed in the implants for all polymer solution formulations (Figure 3).
Figure 2.
Representative ultrasound images of the same implant over the course of the study. Polymer precipitation results in white while darker areas suggest a polymer lean domain. Row 1 is an excipient free control, row 2 contains water in the casting solution, row 3 contains DiI, row 4 contains BSA, and row 5 is a combination of BSA/DiI.
Figure 3.
Comparison of implant morphology obtained using SEM with those obtained from ultrasound.
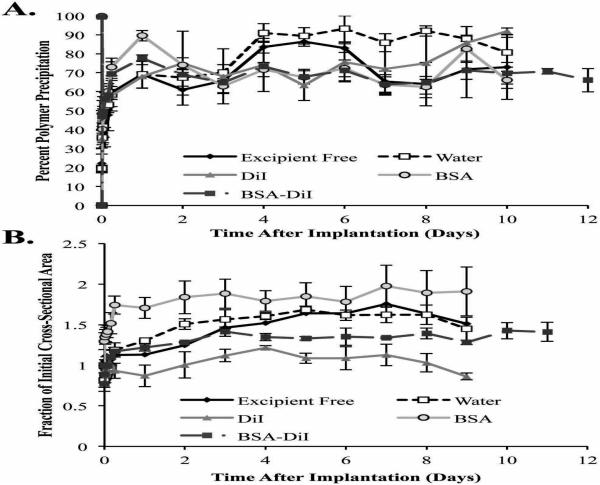
3.2.2. Phase Inversion
Initial polymer precipitation occured rapidly, reaching 69.1±7.3% after 1 d for implants with no additives. These implants then continued to phase invert more slowly, with a peak polymer precipitation occurring after 4 d lasting through 6 d. After 6 d, a loss of signal was observed due to the formation of the central pore, which resulted in a decrease in the precipitated area. When water was used as the excipient, peak polymer precipitation occurred after 4 d, with no significant loss in signal occurring throughout the remainder of the study (Figure 4A). Implants containing DiI rapidly precipitated over the course of the first 2 h, with no significant changes occurring until after 6 h, where more gradual precipitation occurred until reaching a peak after 2 d. After 5 d, a loss of signal was observed as a result of the formation of the pore. Implants with BSA and BSA/DiI both reached a maximum polymer precipitation after 1 d. A reduction in backscatter occurred after 1 d, but was not the result of a pore.
Figure 4.
Quantitative formation data of ISFIs (A) and the change in the implant cross-sectional area over time (B). All error bars represent standard deviation with n=3.
3.2.3. Implant Swelling
Implants without excipient initially shrink, and returned to the initial cross-sectional area after 4 h. The implants then continued to swell, reaching a maximum of 1.64 fold the initial cross-sectional area after 6 d (Figure 4B). Implants using water as the excipient followed a similar pattern of swelling. Peak swelling occurred after 6 d, and reached a maximum of 1.69±0.04 fold more than the initial cross-sectional area. Implants that contained DiI initially shrink and then reached a maximum cross-sectional area after 2 d in PBS (1.22±0.03 fold the initial area); however no significant changes in cross-sectional area occurred after 24 h (Figure 4B). BSA loaded implants did not undergo an initial loss in area, increasing to 1.74±0.11 fold the initial cross-sectional area after 1 d, with no significant changes occurring throughout the remainder of the study. Implants that contained the combination of BSA/DiI initially shrink, returning to the initial area after 4 h. The implants then continued to swell through 4 d, and achieved a maximum area of 1.41±0.06 fold the initial cross-sectional area, with no significant changes occurring through the remainder of the study.
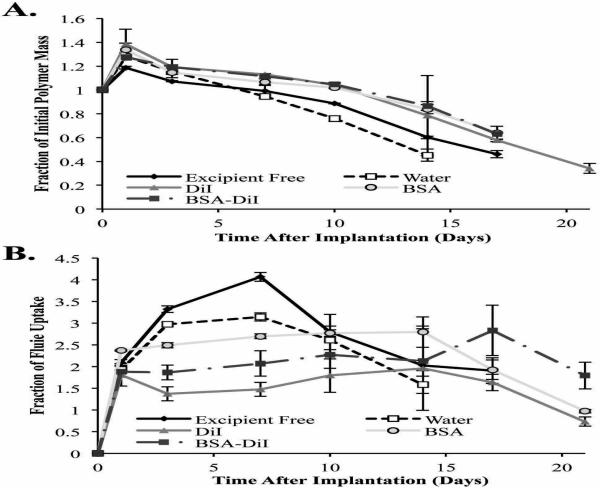
3.3. Erosion and Bathside Uptake
Initially, all implant formulations had masses greater than the maximum theoretical polymer mass, indicating the presence of residual solvent (Figure 5A). Implants without excipients and implants incorporating water as the additive had significantly lower masses than all other implant formulations after 7 d (99.1±5.1% and 94.5±1.8 of the initial polymer mass respectively), and were below the theoretical polymer mass, indicating the onset of erosion. Within 10 d, implants that incorporated water as the excipient had a significantly lower mass than all other implant formulations. Implants with DiI, BSA, and BSA/DiI still contained residual solvent on day 10 (104.1±2.1%, 101.9±0.7%, and 104.8±0.8% of the theoretical polymer mass respectively), but by 14 d all implant formulations had begun eroding. No statistical differences were observed in the erosion profile of implants containing DiI, BSA, or BSA/DiI (Figure 5A).
Figure 5.
Implant erosion over time (A) and the change in fluid uptake over time (B). All error bars represent standard deviation with n=3.
Significant differences in fluid uptake occurred between all groups after 3 d in solution and these differences were maintained until after 10 d in the bathside solution (Figure 5B). The excipient free control demonstrated the greatest fluid uptake, followed by implants containing water, then those with BSA, which was followed by those with BSA/DiI, and finally implants with DiI alone. Implants without an excipient had a 4.1±0.1 fold increase in the initial implant mass after 7 d in PBS. The initial 7 d period of fluid uptake was followed by a decrease in the wet mass of the implants over the remainder of the study (Figure 5B). The implant with water incorporated into the casting solution followed a similar pattern, but had a significantly lower fluid uptake than the excipient free control, reaching a maximum 3.1±0.1 fold increase in implant mass after 7 d. Implants containing BSA rapidly reached a steady state, and were 2.4±0.01 fold the initial implant mass after 24 h in the nonsolvent, with no significant changes in the wet mass of the implants occurring until after 14 d (Figure 5B). After 14 d, a decrease in the wet mass was observed throughout the remainder of the study. Implants that contained a combination of BSA/DiI had a 1.9±0.03 fold increase in mass after 1 d in the bathside solution, and no significant changes occurred in the implant mass afterward. Implants the contained DiI had an initial period of fluid uptake resulting in a 1.8±0.3 fold increase in the implant mass, with no significant changes observed until after 17 d in the bathside solution. On the final day of the study, a significant decrease in the implant mass was observed, resulting in an implant with a wet mass 0.7±0.1 fold the initial mass of the implant.
3.4. Implant Microstructure Analysis
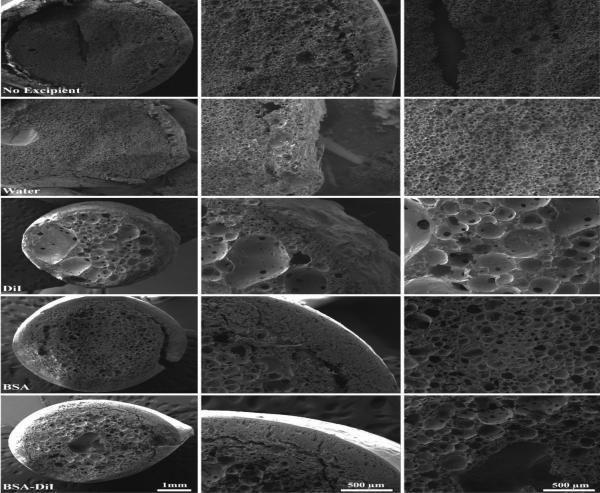
Within 3 d, the microstructure of implants without an excipient had a shell and an interior spongy domain, with a central region that still had residual solvent (Figure 6). This interior spongy domain consisted of small interconnected porous network, of polymer rich and polymer lean domains. The incorporation of water into the casting solution resulted in a decrease in the implant shell thickness, and resulted in the formation of continuous porous network throughout the implant structure, including the shell. Incorporation of DiI into the polymer solution resulted in an implant with a thinner shell than the excipient free control, but with larger less interconnected pores in the interior of the implant. Incorporation of BSA resulted in a thick outer shell similar to what is observed in the excipient free control. The interior domain had smaller pores than what was observed when DiI was included in the casting solution, but the pores were less interconnected than the excipient free control. The combination of BSA/DiI resulted in an implant with a microstructure similar to what was observed with implants containing BSA alone. The implants containing BSA/DiI also had a few larger pores in the interior domain of the implants similar to what was observed with implants containing only DiI (Figure 6).
Figure 6.
SEM images of implants in the bath solution after 3 days. Each†row†is representative of implants loaded with different excipients. Column 1 is a low magnification image of the entire implant, column 2 is an image of the shell, and column 3 is an image of the center of the implant.
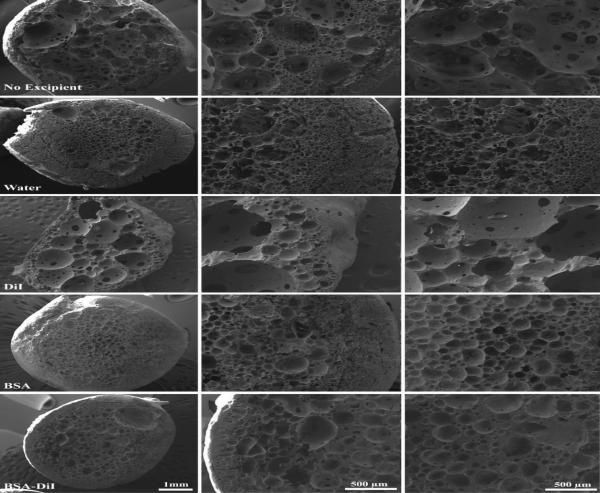
After 7 d, the shell of the excipient free control became thinner and the interior domain became more porous and interconnected (Figure 7). The microstructure of implants that incorporated water into the casting solution had a less dense shell and interior domain, with greater interconnectivity of the porous interior domain. Implants containing DiI developed larger pores just beginning to become interconnected (Figure 7). While the shell remained dense, polymer lean domains were also beginning to form. Implants that contained BSA did not significantly change between day 3 and day 7. The microstructure of implants with the combination of BSA/DiI appeared denser, with a less interconnected porous network than the excipient free control, and an increase in the size of the polymer lean domains (Figure 7).
Figure 7.
SEM images of implants in the bath solution after 7 days. Each†row†is representative of implants loaded with different excipients. Column 1 is a low magnification image of the entire implant, column 2 is an image of the shell, and column 3 is an image of the center of the implant.
4. Discussion
Additives provide a means by which the drug release profile of ISFIs can be specifically tailored at unique phases of the release profile, without making changes to the polymer or the solvent, which can have adverse affects on the solution viscosity. The effect that the additive has on drug release is dependent on the way in which the excipient interacts with the surrounding matrix, payload, or how it alters the mass transfer kinetics of the solvent/nonsolvent5,8,35. For highly miscible solvents, the polymer precipitates instantaneously resulting in a dense polymer shell5. Within the implant interior, polymer lean droplets consisting of a mixture of solvent and nonsolvent form. These droplets will aggregate into larger domains unless a sufficient concentration of nonsolvent is present, which stabilizes the structure by causing the polymer to precipitate5,6. This process results in the formation of a highly interconnected porous network, observed in the implants without excipients. The interconnectivity of the pores was hypothesized by McHugh et al. to provide a network for drug diffusion ultimately leading to elevated burst release9,21-23, which occurs as drug is depleted from these polymer lean regions. As a consequence of the structure, diffusion is limited resulting in the characteristic release profile.
We hypothesized that the incorporation of DiI into the polymer solution would function to increase the hydrophobicity of the polymer solution and reduce water uptake. The increased hydrophobicity and decrease in water uptake resulted in delayed demixing to occur after the initial shell formation. As a consequence of the delayed demixing, the polymer lean domains could not be stabilized by the nonsolvent and aggregated, resulting in the observed microstructure that consisted of large pores with little interconnectivity (Figure 6). The limited interconnectivity of the pores reduced burst release and limited diffusion of drug through the implant.
Incorporation of nonsolvent into the casting solution has been used to control the microstructure of asymmetric and symmetric membranes fabricated using phase inversion1,5,10,11,18,35. Therefore, water was added to the casting solution in order to reduce the solvent/nonsolvent gradient. As a consequence of the reduced solvent exchange, lower fluid uptake was observed, as well as a decrease in burst release which we hypothesize to be a result of a decrease in solvent transport. SEM analysis of the microstructure shows that incorporating water into the polymer solution also reduced the shell thickness of the implants, which we hypothesize resulted in the enhanced release of drug during the diffusion phase of release.
Through ultrasound imaging, all of the implants were observed to become hollow if given a large enough timescale (Figure 1 and 3). A similar observation has been described in previous studies14,19,28,36. We hypothesize that the development of this macro-scale feature occurs as a result of autocatalytic degradation and can be explained with percolation theory19,37-39. Due to removal of the acidic degradation bi-products from the surface of the implant by the nonsolvent, and diffusion limitations of the acidic bi-products imparted by the shell, a pH gradient develops within the implant19,37,40-42. The buildup of acidic bi-products continues until the matrix that makes up the shell erodes below the percolation threshold, resulting in a continuous path through which the oligomers can diffuse out of the implant, and consequently causes the formation of a pore in the center of the implant19. Recent studies by Schädlich et al. have demonstrated that the internal pH of an ISFI decreases below 3 within the first 6 d in the bathside solution, and is followed by an increase in pH indicating loss of the acidic bi-products43.
We also hypothesize that the increased diffusion of the implants that contained water in the casting solution reduced the buildup of degradation bi-products and resulted in the prolonged formation of the central pore relative to the excipient free control. In previous studies, DiI was shown to enhance the degradation of ISFIs14. Therefore, we postulate that the reduced interconnectivity, dense polymer regions, and elevated hydrophobicity within the implants containing DiI result in a greater decrease in pH within the macropores enhancing the autocatalysis effect. Incorporation of BSA into ISFIs has been shown to reduce polymer erosion and degradation, and was not anticipated to alter burst release14. BSA is not soluble in the polymer solution, and was suspended before injection into the bathside solution. The BSA suspension provided points of acoustic impedance mismatch within the polymer solution resulting in an initial elevated backscatter. This increase in echogenicity was lost within a half hour due to diffusion of water into the polymer solution. Interestingly a decrease in signal was observed after 48 h, which we hypothesize to be a result of increased polymer density attenuating the ultrasound signal transmitted through the implant. SEM images showed that the implants had a denser, less interconnected porous microstructure than the excipient free control, which we hypothesize functioned to reduce drug release during the diffusion period of release. Furthermore, due to the isoelectric point (IEP) of the BSA, the pH gradient that develops in the implant would serve to facilitate polyionic complexation of the degraded oligomers which function to maintain the matrix architecture ultimately reducing polymer erosion and delaying the onset of degradation facilitated release. The reduction in release may also be effected by interaction of fluorescein with BSA, which would also serve to reduce release during the diffusion and degradation phases of drug dissolution.
When a combination of DiI/BSA was incorporated into the casting solution, synergistic effects of both excipients were observed. A dense polymer shell was formed similar to what was observed with implants containing BSA alone (Figure 6). Due to the reduced fluid uptake, a period of delayed demixing occurred, and similar to what was observed with implants containing DiI, the polymer lean domains aggregated resulting in the observed increase in size and decrease in interconnectivity of the porous network between days 3 and 7 (Figure 6 and 7). The subsequent changes in microstructure most likely resulted in a decrease in the dissolution of drug during the diffusion period of release.
5. Conclusions
Medical imaging systems provide a powerful tool by which the long term in situ behavior of implants can be evaluated nondestructively and aid in the rational design of implant formulations. Excipients provide an ideal method by which the release profile can be tailored for specific applications. The findings in this study have demonstrated that excipients can be used to reduce burst release, alter diffusivity, as well as delay the onset of degradation facilitated release. Understanding how the additives alter microstructure and interact with the matrix can be used to tailor the release profile of ISFIs for specific applications. It has been demonstrated that changes in the microstructure can significantly alter the release profile14,22,23,44. Currently only a handful of additives have been evaluated30,31,45, with a large focus of research evaluating the role of solvents and the polymer21,24,25,29,46-51. These findings demonstrate that the use of additives can significantly alter the implant release profile, within the different periods of release.
6. Acknowledgements
This work was supported by the NIH grant under award number R01CA118399 to AAE and DOD Breast Cancer Research Fellowship under award number W81XWH-10-1-0582 to LS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or DOD.
References
- 1.Boom RM, Wienk IM, Vandenboomgaard T, Smolders CA. Microstructures in Phase Inversion Membranes .2. The Role of a Polymeric Additive. Journal of Membrane Science. 1992;73(2-3):277–292. [Google Scholar]
- 2.Dunn RL, English JP, Cowsar DR, Vanderbilt DP. U.S: 1990. Biodegradable in situ forming implants and methods of producing the same. [Google Scholar]
- 3.Dunn RL, Tipton AJ. U.S.: 1997. Polymeric compositions useful as controlled release implants. [Google Scholar]
- 4.Ravivarapu HB, Moyer KL, Dunn RL. Sustained suppression of pituitary-gonadal axis with an injectable, in situ forming implant of leuprolide acetate. J Pharm Sci. 2000;89(6):732–741. doi: 10.1002/(SICI)1520-6017(200006)89:6<732::AID-JPS4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Smolders CA, Reuvers AJ, Boom RM, Wienk IM. Microstructures in Phase-Inversion Membranes .1. Formation of Macrovoids. Journal of Membrane Science. 1992;73(2-3):259–275. [Google Scholar]
- 6.Solorio L, Solorio LD, Gleeson S, Olear AM, Carlson AC, Exner AA, Ehlers TP, Wilhelm JK. Polymer Phase Behavior. Nova Science Publishers, Inc; 2011. Phase Inverting Polymer Systems In Drug Delivery And Medicine. [Google Scholar]
- 7.Vogrin N, Stropnik C, Musil V, Brumen M. The wet phase separation: the effect of cast solution thickness on the appearance of macrovoids in the membrane forming ternary cellulose acetate/acetone/water system. Journal of Membrane Science. 2002;207(1):139–141. [Google Scholar]
- 8.McHugh AJ. The role of polymer membrane formation in sustained release drug delivery systems. Journal of Controlled Release. 2005;109(1-3):211–221. doi: 10.1016/j.jconrel.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 9.McHugh AJ, Graham PD, Brodbeck KJ. Phase inversion dynamics of PLGA solutions related to drug delivery. Biomedical Materials-Drug Delivery, Implants and Tissue Engineering. 1999;550:41–46. 376. doi: 10.1016/s0168-3659(98)00158-8. [DOI] [PubMed] [Google Scholar]
- 10.Mchugh AJ, Miller DC. The Dynamics of Diffusion and Gel Growth during Nonsolvent-Induced Phase Inversion of Polyethersulfone. Journal of Membrane Science. 1995;105(1-2):121–136. [Google Scholar]
- 11.Mchugh AJ, Tsay CS. Dynamics of the Phase Inversion Process. Journal of Applied Polymer Science. 1992;46(11):2011–2021. [Google Scholar]
- 12.Exner AA, Saidel GM. Drug-eluting polymer implants in cancer therapy. Expert Opin Drug Deliv. 2008;5(7):775–788. doi: 10.1517/17425247.5.7.775. [DOI] [PubMed] [Google Scholar]
- 13.Dunn RL, Garrett S. The drug delivery and biomaterial attributes of the ATRIGEL technology in the treatment of periodontal disease. Expert opinion on investigational drugs. 1998;7(9):1483–1491. doi: 10.1517/13543784.7.9.1483. [DOI] [PubMed] [Google Scholar]
- 14.Solorio L, Olear AM, Zhou H, Beiswenger AC, Exner AA. Effect of cargo properties on in situ forming implant behavior determined by noninvasive ultrasound imaging. Drug Deliv Transl Res. 2012;2(1):45–55. doi: 10.1007/s13346-011-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempe S, Metz H, Mader K. Do in situ forming PLG/NMP implants behave similar in vitro and in vivo? A non-invasive and quantitative EPR investigation on the mechanisms of the implant formation process. J Control Release. 2008;130(3):220–225. doi: 10.1016/j.jconrel.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Kempe S, Metz H, Pereira PGC, Mader K. Non-invasive in vivo evaluation of in situ forming PLGA implants by benchtop magnetic resonance imaging (BT-MRI) and EPR spectroscopy. European Journal of Pharmaceutics and Biopharmaceutics. 2010;74(1):102–108. doi: 10.1016/j.ejpb.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Solorio L, Babin BM, Patel RB, Mach J, Azar N, Exner AA. Noninvasive characterization of in situ forming implants using diagnostic ultrasound. Journal of Controlled Release. 2010;143(2):183–190. doi: 10.1016/j.jconrel.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsay CS, Mchugh AJ. A Technique for Rapid Measurement of Diffusion-Coefficients. Industrial & Engineering Chemistry Research. 1992;31(1):449–452. [Google Scholar]
- 19.Solorio L, Olear AM, Hamilton JI, Patel RB, Beiswenger AC, Wallace JE, Zhou H, Exner AA. Noninvasive characterization of the effect of varying PLGA molecular weight blends on in situ forming implant behavior using ultrasound imaging. Theranostics. 2012;2(11):1064–1077. doi: 10.7150/thno.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Astaneh R, Erfan M, Moghimi H, Mobedi H. Changes in morphology of in situ forming PLGA implant prepared by different polymer molecular weight and its effect on release behavior. J Pharm Sci. 2009;98(1):135–145. doi: 10.1002/jps.21415. [DOI] [PubMed] [Google Scholar]
- 21.Brodbeck KJ, DesNoyer JR, McHugh AJ. Phase inversion dynamics of PLGA solutions related to drug delivery. Part II. The role of solution thermodynamics and bath-side mass transfer. J Control Release. 1999;62(3):333–344. doi: 10.1016/s0168-3659(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 22.DesNoyer JR, McHugh AJ. Role of crystallization in the phase inversion dynamics and protein release kinetics of injectable drug delivery systems. J Control Release. 2001;70(3):285–294. doi: 10.1016/s0168-3659(00)00354-0. [DOI] [PubMed] [Google Scholar]
- 23.Graham PD, Brodbeck KJ, McHugh AJ. Phase inversion dynamics of PLGA solutions related to drug delivery. Journal of Controlled Release. 1999;58(2):233–245. doi: 10.1016/s0168-3659(98)00158-8. [DOI] [PubMed] [Google Scholar]
- 24.Astaneh R, Erfan M, Barzin J, Mobedi H, Moghimi H. Effects of Ethyl Benzoate on Performance, Morphology, and Erosion of PLGA Implants Formed In Situ. Advances in Polymer Technology. 2008;27(1):17–26. [Google Scholar]
- 25.Liu QF, Zhang H, Zhou GC, Xie SB, Zou H, Yu YA, Li GD, Sun DX, Zhang GQ, Lu Y, Zhong YQ. In vitro and in vivo study of thymosin alpha1 biodegradable in situ forming poly(lactide-co-glycolide) implants. International Journal of Pharmaceutics. 2010;397(1-2):122–129. doi: 10.1016/j.ijpharm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Astaneh R, Erfan M, Mobedi H, Moghimi HR. Formulation of an Injectable Implant for Peptide Delivery and Studying the Effect of Polymer Molecular Weight on Its Release Behavior. Journal of Peptide Science. 2004;10:142–142. [Google Scholar]
- 27.Liu H, Venkatraman SS. Effect of Polymer Type on the Dynamics of Phase Inversion and Drug Release in Injectable In Situ Gelling Systems. J Biomater Sci Polym Ed. 2012 doi: 10.1163/092050610X549171. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Venkatraman SS. Solid/Hollow depots for drug delivery, part 1: effect of drug characteristics and polymer molecular weight on the phase-inversion dynamics, depot morphology, and drug release. J Pharm Sci. 2014;103(2):485–495. doi: 10.1002/jps.23790. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Kemmer A, Keim K, Curdy C, Petersen H, Kissel T. Poly(ethylene carbonate) as a surface-eroding biomaterial for in situ forming parenteral drug delivery systems: A feasibility study. European Journal of Pharmaceutics and Biopharmaceutics. 2010;76(2):222–229. doi: 10.1016/j.ejpb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Patel RB, Carlson AN, Solorio L, Exner AA. Characterization of formulation parameters affecting low molecular weight drug release from in situ forming drug delivery systems. Journal of Biomedical Materials Research Part A. 2010;94A(2):476–484. doi: 10.1002/jbm.a.32724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DesNoyer JR, McHugh AJ. The effect of Pluronic on the protein release kinetics of an injectable drug delivery system. J Control Release. 2003;86(1):15–24. doi: 10.1016/s0168-3659(02)00293-6. [DOI] [PubMed] [Google Scholar]
- 32.Martindale: The Complete Drug Reference. 37 Pharmaceutical Press; London, England, UK: 2011. p. 4142. [Google Scholar]
- 33.Sakai M, Imai T, Ohtake H, Azuma H, Otagiri M. Effects of absorption enhancers on the transport of model compounds in Caco-2 cell monolayers: assessment by confocal laser scanning microscopy. J Pharm Sci. 1997;86(7):779–785. doi: 10.1021/js960529n. [DOI] [PubMed] [Google Scholar]
- 34.Patel RB, Solorio L, Wu HP, Krupka T, Exner AA. Effect of injection site on in situ implant formation and drug release in vivo. Journal of Controlled Release. 2010;147(3):350–358. doi: 10.1016/j.jconrel.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barton BF, Reeve JL, McHugh AJ. Observations on the dynamics of nonsolvent-induced phase inversion. Journal of Polymer Science Part B-Polymer Physics. 1997;35(4):569–585. [Google Scholar]
- 36.Tang Y, Singh J. Controlled delivery of aspirin: effect of aspirin on polymer degradation and in vitro release from PLGA based phase sensitive systems. Int J Pharm. 2008;357(1-2):119–125. doi: 10.1016/j.ijpharm.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Gopferich A. Polymer bulk erosion. Macromolecules. 1997;30(9):2598–2604. [Google Scholar]
- 38.Gopferich A, Langer R. Modeling of Polymer Erosion. Macromolecules. 1993;26(16):4105–4112. [Google Scholar]
- 39.Leuenberger H, Rohera BD, Haas C. Percolation Theory - a Novel-Approach to Solid Dosage Form Design. International Journal of Pharmaceutics. 1987;38(1-3):109–115. [Google Scholar]
- 40.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polymer International. 2005;54(1):36–46. [Google Scholar]
- 41.Li S, Vert M. Hydrolytic degradation of the coral/poly(DL-lactic acid) bioresorbable material. J Biomater Sci Polym Ed. 1996;7(9):817–827. doi: 10.1163/156856296x00156. [DOI] [PubMed] [Google Scholar]
- 42.von Burkersroda F, Schedl L, Gopferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23(21):4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 43.Schadlich A, Kempe S, Mader K. Non-invasive in vivo characterization of microclimate pH inside in situ forming PLGA implants using multispectral fluorescence imaging. J Control Release. 2014;179:52–62. doi: 10.1016/j.jconrel.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Brodbeck KJ, DesNoyer JR, McHugh AJ. Phase inversion dynamics of PLGA solutions related to drug delivery - Part II. The role of solution thermodynamics and bath-side mass transfer. Journal of Controlled Release. 1999;62(3):333–344. doi: 10.1016/s0168-3659(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 45.Zare M, Mobedi H, Barzin J, Mivehchi H, Jamshidi A, Mashayekhi R. Effect of additives on release profile of leuprolide acetate in an in situ forming controlled-release system: In vitro study. Journal of Applied Polymer Science. 2008;107(6):3781–3787. [Google Scholar]
- 46.Wang LW, Kleiner L, Venkatraman S. Structure formation in injectable poly(lactide-co-glycolide) depots. Journal of Controlled Release. 2003;90(3):345–354. doi: 10.1016/s0168-3659(03)00198-6. [DOI] [PubMed] [Google Scholar]
- 47.Dhawan S, Kapil R, Kapoor DN, Kumar M. Development and evaluation of in situ gel forming system for sustained delivery of cyclosporine. Curr Drug Deliv. 2009;6(5):495–504. doi: 10.2174/156720109789941669. [DOI] [PubMed] [Google Scholar]
- 48.Kang FR, Singh J. In vitro release of insulin and biocompatibility of in situ forming gel systems. International Journal of Pharmaceutics. 2005;304(1-2):83–90. doi: 10.1016/j.ijpharm.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Prabhu S, Tran LP, Betageri GV. Effect of co-solvents on the controlled release of calcitonin polypeptide from in situ biodegradable polymer implants. Drug Delivery. 2005;12(6):393–398. doi: 10.1080/10717540590968873. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Venkatraman S, Kleiner L. Drug release from injectable depots: two different in vitro mechanisms. J Control Release. 2004;99(2):207–216. doi: 10.1016/j.jconrel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Yapar A, Baykara T. Effects of Solvent Combinations on Drug Release from Injectable Phase Sensitive Liquid Implants. Turk J Pharm Sci. 2010;7(1):49–56. [Google Scholar]