Abstract
This study examined the impact of motivational contingencies (reinforcement and punishment) on Go/No-Go (GNG) task performance in girls and boys with ADHD relative to typically developing (TD) children and associations with prefrontal anatomy. Children ages 8–12 with ADHD (n=107, 36 girls) and TD controls (n=95, 34 girls) completed a standard and a motivational GNG task and associations with prefrontal cortex (PFC) surface area were examined. Intrasubject variability (ISV) was lower during the motivational compared to the standard GNG among TD girls and boys, and boys with ADHD, but not among girls with ADHD. A greater reduction in ISV was associated with greater PFC surface area among children with ADHD. This novel demonstration of improvement in ISV with motivational contingencies for boys, but not girls, with ADHD and associations with PFC anatomy informs our understanding of sex differences and motivational factors contributing to ISV in children with ADHD.
Keywords: ADHD, motivation, cognition, response control, intrasubject variability, sex differences, prefrontal cortex
Introduction
Developmentally inappropriate levels of inattention, hyperactivity, and impulsivity are the defining features of Attention-Deficit/Hyperactivity Disorder (ADHD), the most common psychiatric disorder of childhood (American Psychiatric Association, 2013; Getahun et al., 2013). Multiple etiologic pathways involving cognitive dysfunction, atypical motivation, and inefficient state regulation are thought to contribute to these persistent and impairing symptoms (Luman, Tripp, & Scheres, 2010; Nigg & Casey, 2005; Sergeant, Geurts, Huijbregts, Scheres, & Oosterlaan, 2003; Sonuga-Barke, 2005). The multiple pathway models of ADHD implicate dissociable neural pathways involved in ‘cool’ executive functions (EF), referring to top-down relatively pure cognitive processes, and ‘hot’ EF, referring to cognitive processes that have a motivational or affective component (Kelly, Scheres, Sonuga-Barke, & Castellanos, 2007). At a neurobiological level, EF and motivation or reward processes involve closely related neuroanatomical circuits and neurochemistry, such that the interaction of cognitive and motivational processes may best characterize the behavioral dysregulation that defines ADHD (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006).
The interaction of cognition and motivation can be examined at a behavioral level by comparing cognitive performance during conditions with and without motivational contingencies (i.e., reinforcement and punishment). Reinforcement has been shown to improve various cognitive deficits associated with ADHD (see review by Luman, Oosterlaan, & Sergeant, 2005), including working memory (Shiels et al., 2008; Strand et al., 2012), sustained attention (Bubnik, Hawk, Pelham, Waxmonsky, & Rosch, 2015), and error processing (Rosch & Hawk, 2013). The impact of reinforcement and/or punishment on response inhibition in children with ADHD, relative to no contingency performance, has been somewhat inconsistent. These studies have reported improved response inhibition among children with ADHD when contingencies were in place for going and stopping (Michel, Kerns, & Mateer, 2005; Rosch et al., in press; Stevens, Quittner, Zuckerman, & Moore, 2002; Wodka et al., 2007) and when contingencies were in place only for stopping (Konrad, Gauggel, Manz, & Scholl, 2000; Scheres, Oosterlaan, & Sergeant, 2001). However, other studies have failed to show improved response inhibition with contingencies (e.g., Shanahan, Pennington, & Willcutt, 2008) or the effect has varied across inhibition tasks (Epstein et al., 2011). Traditionally, research has focused on the impact of motivational contingencies on error rates, as accuracy is often the target of the motivational contingencies (e.g., Bubnik et al., 2015; Shiels et al., 2008; Strand et al., 2012). However, motivational contingencies may also affect reaction time measures related to response efficiency, such as the speed and consistency of responses, as reflected in intrasubject variability (ISV) of reaction time (RT), even if RT speed and variability are not the contingency targets.
Increased ISV is among the most consistent findings in studies of cognitive function in ADHD (Karalunas, Geurts, Konrad, Bender, & Nigg, 2014; Kofler et al., 2013). At this time, it is unclear why ADHD is associated with greater ISV, which is likely the result of multiple processes including stimulus encoding, information processing speed, motor preparation, and response execution (Karalunas et al., 2014). Conceptually, ISV is thought to reflect attentional lapses (Leth-Steensen, Elbaz, & Douglas, 2000) or alterations in effortful control required to maintain consistent responding (Douglas, 1999), resulting in infrequent, significantly slower reaction times that contribute to positive skew in the RT distribution. It has also been suggested that ISV may reflect inconsistencies in maintaining instructional set or motivational state, such that both ‘cool’ and ‘hot’ EF may contribute to increased ISV in ADHD (Castellanos et al., 2006), making it a prime candidate for examining the impact of motivational contingencies. It is important to note that the majority of studies of ISV in ADHD have used standard deviation of reaction time (SDRT) to quantify RT variability, which is problematic because SDRT is multi-determined and highly correlated with mean RT (r between 0.7 and 0.9 in many studies) (Karalunas et al., 2014). In addition, standard statistics using SDRT assume that RTs fit a Gaussian (normal) distribution whereas RT distributions are nearly always positively skewed to some extent. Ex-Gaussian decomposition models RTs more accurately and provides estimates of the mean (mu) and standard deviation (sigma) of the normal (Gaussian) portion of the distribution and the mean and standard deviation of the exponential tail of the distribution (tau) (Leth-Steensen et al., 2000). Furthermore, it has been shown that increased SDRT in children with ADHD is largely explained by increased tau (Karalunas et al., 2014).
Studies that have examined the impact of contingencies on ISV in children with ADHD have produced inconsistent results, reporting reduced ISV (Andreou et al., 2007; Douglas & Parry, 1983; Gopin, Berwid, Marks, Mlodnicka, & Halperin, 2013; Uebel et al., 2010), no change in ISV (Scheres et al., 2001; Shanahan et al., 2008), and inconsistent effects on ISV (Epstein et al., 2011). These inconsistent findings may be due to a variety of factors, including differences in contingency structure, task parameters, methods of quantifying ISV, or characteristics of the sample (age, sex, or ADHD subtype). Meta-analytic results suggest increased ISV is consistently seen across ADHD subtypes and diagnostic group differences tend to decrease with age (Kofler et al., 2013). However, whether boys and girls with ADHD differ in the extent to which they show increased ISV or differential changes in ISV with motivational contingencies has not been adequately addressed due to the small number of girls in these studies (range 3–19), or a failure to test for or report sex differences in studies with larger samples of girls with ADHD (e.g., Epstein et al., 2011; Gopin et al., 2013, n=25 and n=21, respectively). Interestingly, Andreou et al (2007) found that children with ADHD showed a significantly greater reduction in ISV compared to the control group from a baseline to fast-incentive condition only after excluding girls with ADHD (n=14) from a predominantly (90%) male sample. This finding might suggest a differential response to incentives in girls compared to boys with ADHD, but further research is required with a larger sample of girls with ADHD.
Within the broader ADHD literature, significant differences between girls and boys with ADHD have been shown in symptom presentation, associated comorbidity, and psychosocial and cognitive functioning suggesting that sex differences may be important in understanding response control in ADHD (Gaub & Carlson, 1997; Gershon, 2002; Rucklidge, 2008a). Whether girls and boys with ADHD demonstrate similar cognitive profiles has received limited research attention, although some patterns have begun to emerge in this literature. For example, prior studies have shown that motor control deficits are more prominent in boys than in girls with ADHD (Cole, Mostofsky, Larson, Denckla, & Mahone, 2008), whereas impairments in working memory and related EFs are present in both boys and girls with the disorder (Rucklidge, 2010). Consistent with these earlier studies, recently published findings in a large sample of girls and boys with ADHD compared to typically developing (TD) children found evidence of impaired response control in boys with ADHD across tasks with varied cognitive demand, whereas girls with ADHD only performed more poorly when working memory was necessary to guide response selection (Seymour, Mostofsky, & Rosch, 2015). In addition, a recent structural neuroimaging study found evidence of reduced cortical surface area in premotor regions in boys, but not girls, with ADHD, and more widespread surface area reductions in prefrontal regions in girls compared to boys with ADHD (Dirlikov et al., 2015). These findings have begun to address whether the cognitive deficits associated with ADHD are similar for girls and boys, although the question of whether girls and boys with ADHD show similar improvements in cognitive task performance with motivational contingencies remains unknown.
Examining the neuroanatomical correlates of cognitive task performance among children with ADHD is also an important question that has only been addressed in a few published studies (e.g., Batty et al., 2010; Lin et al., 2014; Mahone et al., 2011). Furthermore, identifying the neuroanatomical correlates of change in performance in response to motivational contingencies has important implications for characterizing individuals who may be most (or least) responsive to evidence-based behavioral treatments for ADHD, which are based on the principles of contingency management (Evans, Owens, & Bunford, 2013; Pelham & Fabiano, 2008). Studies of non-clinical populations suggest brain regions implicated in ADHD contribute to ISV, including the dlPFC, OFC, and ACC (Bellgrove, Hester, & Garavan, 2004; Kofler et al., 2013; MacDonald, Li, & Backman, 2009). The neuroanatomical correlates of increased ISV in children with ADHD have also been directly investigated, with evidence of associations with reduced white matter integrity in frontostriatal networks and the cingulum (Lin et al., 2014), and reduced lateral premotor gray matter volumes among girls with ADHD (Mahone et al., 2011). Functional MRI studies of ISV in children with ADHD have reported increased pre-supplementary motor area activation (Simmonds et al., 2007) and greater prefrontal activation (Suskauer et al., 2008) was associated with less ISV. However, none of these studies examined ISV within a motivational context or examined the neural correlates of change in ISV with motivational contingencies. If increased ISV in children with ADHD involves both ‘cool’ and ‘hot’ EF, we might expect several regions of the prefrontal cortex (PFC) to be associated with ISV. In particular, those PFC regions with strong connections to the ventral striatum (VS), a central component of the brain reward circuit (Haber & Knutson, 2010; Sallet et al., 2011), including the orbitofrontal (OFC), ventromedial (vmPFC), and anterior cingulate (ACC), are thought to be involved in relatively ‘hot’ aspects of EF, whereas the dorsolateral PFC (dlPFC) is thought to be involved in more purely cognitive ‘cool’ aspects of EF (Castellanos et al., 2006; Haber & Knutson, 2010). This theoretical framework is supported by evidence of involvement of the ACC in motivating effortful behavior (Holroyd & Yeung, 2011), the OFC in control of voluntary, goal-directed behavior (Tremblay & Schultz, 2000), and the vmPFC in reward processing (Haber & Knutson, 2010), whereas the dlPFC is consistently implicated in EF and cognitive control (Arnsten & Rubia, 2012).
The current study builds on the existing literature by examining the neuranatomical correlates of improved response control in response to motivational contingencies among children with ADHD. Specifically, Go/No-Go (GNG) task performance was compared during conditions with and without performance-based feedback paired with monetary gain and loss (i.e., reinforcement and punishment) in a large sample of girls and boys with ADHD relative to TD children. Given the lack of studies examining ADHD-related sex differences in cognitive deficits and response to motivational contingencies, we examined whether response control, or consistent and accurate execution of a motor response as reflected in measures of response inhibition and consistency (i.e., reaction time variability), was similar among boys and girls. The specific hypotheses include: (1) boys, but not girls, with ADHD will show poorer response control relative to TD children during a standard GNG task (see Seymour et al., 2015), (2) response control will improve during a motivational GNG task for all participants with the greatest improvement in the ADHD group, possibly eliminating diagnostic differences present during the standard condition, and (3) the extent to which response control is improved during the motivational GNG will be correlated with prefrontal cortex surface area.
Method
Participants
A total of 202 8–12 year-old children (107 ADHD, 36 girls; 95 TD, 34 girls) completed the standard and motivational GNG tasks (demographic information presented in Table 1). Of this sample, a 3T MPRAGE was also obtained on 153 children (76 ADHD, 29 girls; 77 TD, 29 girls; demographic information presented in Supplementary Table S1). Participants were primarily recruited through local schools, with additional resources including community-wide advertisement, volunteer organizations, medical institutions, and word of mouth. This study was approved by the Johns Hopkins Institutional Review Board. After complete description of the study to the participants, written informed consent was obtained from a parent/guardian and assent was obtained from the child.
Table 1.
Demographic characteristics for TD and ADHD girls and boys.
| TD | ADHD | Group comparisons | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Girls (n=34) | Boys (n=61) | All (n=95) | Girls (n=36) | Boys (n=71) | All (n=107) | TD vs. ADHD | TD boys vs. girls | ADHD boys vs. girls | Boys TD vs. ADHD | Girls TD vs. ADHD | |||||||
|
| |||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p-values | |||||
| Age (years) | 10.1 | 1.0 | 10.1 | 1.2 | 10.1 | 1.1 | 10.0 | 1.3 | 9.7 | 1.3 | 9.8 | 1.3 | .083 | .998 | .362 | .086 | .613 |
| Ethnicity (% caucasian) | 88% | 71% | 77% | 81% | 68% | 72% | .429 | .071 | .400 | .721 | .378 | ||||||
| SES | 53.3 | 9.1 | 52.0 | 9.8 | 52.5 | 9.6 | 51.7 | 10.0 | 52.2 | 9.9 | 52.0 | 9.9 | .734 | .549 | .812 | .937 | .493 |
| Handedness | 0.74 | 0.39 | 0.56 | 0.64 | 0.63 | 0.57 | 0.69 | 0.40 | 0.70 | 0.45 | 0.70 | 0.43 | .333 | .147 | .966 | .168 | .607 |
| Left:Mixed:Right | 2:2:30 | 8:5:48 | 10:7:78 | 2:3:31 | 4:5:62 | 6:8:93 | .433 | .476 | .972 | .306 | .923 | ||||||
| WISC-IV FSIQ | 113.0 | 10.3 | 115.5 | 11.2 | 114.6 | 10.9 | 111.0 | 12.5 | 107.6 | 12.8 | 108.8 | 12.7 | .001 | .283 | .198 | <.001 | .462 |
| WISC-IV GAI | 113.8 | 10.4 | 118.8 | 12.1 | 117.0 | 11.7 | 112.3 | 13.0 | 113.1 | 14.0 | 112.8 | 13.6 | .021 | .045 | .762 | .014 | .585 |
| WISC-IV VCI | 114.9 | 11.3 | 120.7 | 12.8 | 118.6 | 12.5 | 113.1 | 14.4 | 113.2 | 14.0 | 113.1 | 14.1 | .004 | .028 | .980 | .002 | .570 |
| WISC-IV PRI | 108.5 | 11.0 | 111.2 | 12.1 | 110.3 | 11.7 | 109.9 | 12.6 | 109.7 | 13.3 | 109.8 | 13.0 | .785 | .281 | .945 | .498 | .627 |
| WISC-IV WMI | 108.0 | 13.1 | 109.3 | 13.2 | 108.9 | 13.1 | 105.4 | 14.4 | 102.2 | 12.3 | 103.3 | 95.7 | .003 | .626 | .230 | .002 | .447 |
| WISC-IV PSI | 106.6 | 12.7 | 101.4 | 13.0 | 103.3 | 13.1 | 102.4 | 11.9 | 92.3 | 11.4 | 95.7 | 12.4 | <.001 | .066 | <.001 | <.001 | .161 |
| ADHD Subtype (count) | n/a | n/a | n/a | 26:9:1 | 53:17:1 | 79:26:2 | n/a | n/a | .874 | n/a | n/a | ||||||
| Combined:Inatt:HypImp | |||||||||||||||||
| ADHD-RS Inatt Raw | 1.7 | 2.2 | 3.2 | 3.0 | 2.7 | 2.8 | 19.4 | 4.4 | 18.7 | 4.5 | 19.0 | 4.5 | <.001 | .012 | .455 | <.001 | <.001 |
| ADHD-RS HypImp Raw | 1.2 | 1.4 | 2.1 | 2.3 | 1.8 | 2.1 | 12.9 | 6.8 | 13.4 | 6.2 | 13.3 | 6.4 | <.001 | .046 | .664 | <.001 | <.001 |
| CPRS Inatt T | 44.9 | 4.5 | 44.8 | 5.4 | 44.8 | 5.1 | 79.0 | 10.3 | 71.1 | 8.9 | 73.7 | 10.1 | <.001 | .964 | <.001 | <.001 | <.001 |
| CPRS HypImp T | 46.4 | 4.4 | 46.1 | 4.4 | 46.2 | 4.4 | 73.5 | 14.7 | 69.1 | 12.9 | 70.6 | 13.6 | <.001 | .742 | .115 | <.001 | <.001 |
| Comorbid ODD % | n/a | n/a | n/a | 47% | 37% | 40% | n/a | n/a | .291 | n/a | n/a | ||||||
| Stimulant medication % | n/a | n/a | n/a | 67% | 54% | 59% | n/a | n/a | .221 | n/a | n/a | ||||||
Procedures
An initial screening was conducted through a telephone interview with a parent. Children with a history of intellectual disability, seizures, traumatic brain injury or other neurological illnesses were excluded from participation. Eligible participants attended two laboratory sessions from 8:30 AM to 3:30 PM with a one-hour lunch break. During the first session, intellectual ability was assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) and participants with full scale intelligence quotient (FSIQ) scores below 80 were excluded. Children were also administered the Word Reading subtest from the Wechsler Individual Achievement Test, Second Edition (WIAT-II; Wechsler, 2002) to screen for a reading disorder and were excluded for a significant discrepancy between FSIQ and WIAT-II.
Diagnostic status was established through administration of the Diagnostic Interview for Children and Adolescents, Fourth Edition (DICA-IV; Reich, Welner, & Herjanic, 1997). Children meeting criteria for diagnosis of conduct, mood, generalized anxiety, separation anxiety or obsessive–compulsive disorders on DICA-IV interview were excluded. A comorbid diagnosis of oppositional defiant disorder (ODD) was permitted. Parents and teachers (when available) also completed the Conners’ Parent and Teacher Rating Scales-Revised Long Version or the Conners-3 (CPRS and CTRS) (Conners, 2002, 2008), and the ADHD Rating Scale-IV, home and school versions (ADHD-RS) (DuPaul, Power, Anastopoulos, & Reid, 1998). For the vast majority of participants with ADHD, the diagnostic procedures confirmed a diagnosis of ADHD that they had already received prior to enrolling in the study.
An ADHD diagnosis was confirmed or established based on the following criteria: (1) T-score of 60 or higher on scale L (DSM-IV: inattentive) or M (DSM-IV: hyperactive-impulsive) on the CPRS or CTRS, when available, or a score of 2 or 3 on at least 6/9 items on the Inattentive or Hyperactivity/Impulsivity scales of the ADHD-RS and (2) an ADHD diagnosis on the DICA-IV. This information was then reviewed and the diagnosis was confirmed by a child neurologist (S.H.M.). Children taking psychotropic medications other than stimulants were excluded from participation and all children taking stimulants were asked to withhold medication the day prior to and day of testing.
Inclusion in the TD group required scores below clinical cutoffs on the parent and teacher (when available) rating scales (CPRS, CTRS, and ADHD-RS). Control participants could not meet diagnostic criteria for any psychiatric disorder based on DICA-IV nor could they have history of neurological disorder, be taking psychotropic medication or meet criteria for diagnosis of learning disability based on WIAT-II word reading scores being significantly discrepant from IQ, and were required to have an FSIQ above 80. Children included in the TD group also could not have an immediate family member diagnosed with ADHD.
Go/No-Go Tasks
Standard Go/No-Go
Participants completed a computer-based Go/No-Go (GNG) task (e.g., Shiels Rosch, Dirlikov, & Mostofsky, 2013). Task stimuli consisted of green spaceships for “go” trials and red spaceships for “no-go” trials (20% of trials) presented for 300 ms with an interstimulus interval of 2000 ms. Participants were instructed to push the spacebar with their index finger as quickly as possible in response to green spaceships only. There were 11 practice trials followed by 217 experimental trials presented in a pseudorandom order. Reaction times (RT) were recorded during the entire trial length (2300 ms). This task was typically administered on the first day of testing.
Motivational Go/No-Go
Participants also completed a task similar to the standard GNG task with the addition of immediate trial-by-trial feedback paired with monetary gain and loss (i.e., reinforcement and punishment). The stimuli were identical to those in the standard GNG task, consisting of red or green spaceships presented for 300 ms, followed by a blank screen for 1000 ms, the presentation of visual feedback for 1700 ms, and another blank screen for 500 ms. Responses were recorded during the entire trial length (3500ms). Contingencies were structured to reinforce fast, accurate responses to go stimuli and to punish failures to inhibit responses to no-go stimuli (commission errors). For correct go responses that were faster than an individualized response deadline (mean + 1 standard deviation of go RT during the 217 trials of the standard GNG task), participants earned 10 cents and feedback consisting of three yellow happy faces and picture of a dime was presented (Figure 1). Responses were required to be faster than this individualized deadline to reduce slowing of responses to go stimuli, which would make it easier to inhibit to no-go stimuli. For responses to no-go stimuli (i.e., commission errors), participants lost 50 cents and feedback consisting of 3 purple frowning faces and 50 cents crossed out with a red “X”) was presented (Figure 1). Immediate feedback regarding the accuracy of the response and the amount of money earned or lost was intended to maximize the impact of the contingencies on task performance relative to the standard GNG, similar to previous studies in the literature (e.g., Bubnik et al., 2015; Shiels Rosch et al., 2013; Strand et al., 2012). This task was typically administered on the second day of testing.
Figure 1.
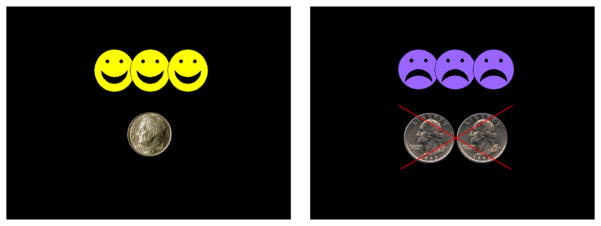
Feedback stimuli presented for fast, correct responses to “go” stimuli (left) and failed inhibitions to “no-go” stimuli (right).
To balance the contingency magnitude according to the frequency of go (80%) and no-go (20%) trials, no-go trials were worth more than go trials. If an equivalent contingency magnitude was given for go and no-go trials, this likely would have resulted in a greater number of commission errors as it would be more advantageous to respond quickly to go stimuli than to inhibit to no-go stimuli. Correct responses that exceeded the individualized response deadline and omission errors did not result in any money earned/lost and no feedback was presented. Based on this contingency structure, we predicted to see fewer commission errors and faster and less variable RTs during the motivational compared to the standard GNG task. Participants received the money they earned during the task in a check mailed upon completion of the study.
Structural MRI
MRI Acquisition and Processing
Before each scanning session the participants completed a practice scanning session to acquaint themselves with the scanning environment. Participants entered the mock scanner room with an instructor and were guided through the sequence of events that occur on the day of their actual scan, including sliding into the scanner, wearing ear plugs, hearing loud MRI scanner noises, and being alone in the scanner for 10 minutes.
All scanning acquisition was completed using a 3.0T Philips Achieva scanner (Best, The Netherlands). MPRAGE images (Slice thickness=1.0 mm; FOV=26 cm; Matrix size: 256x256) were checked for motion and only images with minimal motion were used for FreeSurfer processing. Atlas based regions of interest (ROIs) and total cerebral volume measurements were obtained using FreeSurfer (Fischl et al., 2004). Within FreeSurfer, ROIs were delineated using a novel automated frontal lobe atlas, the Ranta atlas (Ranta et al., 2014). Compared to the Desikan atlas, the Ranta atlas is based on functionally distinct regions of interest that were manually delineated using a pediatric population (8–12 year olds). The Ranta frontal lobe atlas includes left and right hemisphere anterior cingulate cortex (ACC), dorsal lateral prefrontal cortex (dlPFC), medial prefrontal cortex (mPFC), inferior lateral prefrontal cortex (ilPFC), medial orbitofrontal cortex, lateral orbitofrontal cortex, frontal eye field (FEF), lateral premotor cortex (LPM), supplementary motor complex (SMC), and primary motor cortex (M1). The lateral and medial OFC were combined to create a single orbitofrontal cortex (OFC) ROI. Ranta ROIs were combined to permit examination of prefrontal cortex (PFC) ROIs (ACC, dlPFC, mPFC, ilPFC, and OFC), as done previously (Dirlikov et al., 2015). FreeSurfer parcellation quality was visually inspected for each subject.
Data Analysis
Data analysis was accomplished using SPSS Statistics Version 20 (IBM, Chicago). Analysis of GNG performance focused on error rates (proportion of omission errors for go stimuli and commission errors for no-go stimuli) and ex-Gaussian RT estimates, mu and sigma, measures of the speed and variability, respectively, in the normal part of the RT distribution, and tau, a measure of speed and variability of the exponential component of the RT distribution (Castellanos et al., 2006).1 Ex-Gaussian indicators were computed in Matlab version 7.1 (The Mathworks, Inc., Natick, MA) using the DISTRIB toolbox (Lacouture & Cousineau, 2008). Responses faster than 200ms were excluded from all RT analyses and we examined whether the fast go rate (i.e., proportion of go trials with RTs < 200 ms) differed across diagnostic groups and sex. Participants were excluded if the proportion of go trials with RTs < 200 ms exceeded .25 (n=2), if the omission error rate exceeded .50 (n=1), or if the ex-Gaussian fit index was poor (n=2) on either task (final n=202).
Repeated measures multivariate analysis of covariance (MANCOVA) was used to examine changes in performance during the standard and motivational GNG tasks. Specifically, a 2 (Diagnosis: ADHD vs. TD) × 2 (Sex: girls vs. boys) × 2 (Task: standard vs. motivational GNG) MANCOVA was employed with fast go rate, omission error rate, commission error rate, mu, sigma, and tau as the dependent variables and age and WISC-IV General Ability Index (GAI) as covariates. The GAI is a measure of general intellectual ability based on the verbal and perceptual reasoning abilities, while excluding performance on working memory and processing speed subtests. Among ADHD samples, GAI is thought to more accurately reflect intellectual ability without the contribution of cognitive deficits often associated with ADHD, such as working memory and processing speed (e.g., Jacobson et al., 2011). All analyses were also conducted without these covariates, given the problems with covarying variables that differ between groups (Miller & Chapman, 2001), and the primary findings remained the same. To reduce the number of comparisons, univariate ANCOVAs were only conducted following significant multivariate main or interaction effects (p<.05). For significant effects involving diagnosis, additional MANCOVAs were also conducted with only those participants with ADHD without a comorbid diagnosis of ODD to determine if diagnostic group differences remain. The results did not change with exclusion of ADHD participants with comorbid ODD; therefore models with the full sample of children with ADHD (including participants with comorbid ODD) are reported. For significant and trend-level findings, Cohen’s d is reported as a measure of effect size generally interpreted as d = 0.2, 0.5, and 0.8, indicating a small, medium, and large effect, respectively (Cohen, 1988).
For the subsample of participants with anatomical MRI (n=153), we examined whether diagnostic groups differed in PFC (ACC, dlPFC, mPFC, ilPFC, and OFC) surface area, as shown in our previous study reporting on ADHD-related sex differences in frontal lobe surface area (Dirlikov et al., 2015). In line with previous research, each participant’s frontal lobe ROI cortical measurements were normalized by multiplying the raw cortical metric by the ratio of their respective diagnostic group’s average total brain volume (TBV) and individual subject’s total brain volume (e.g. Subject ROI Surface Area*Mean ADHD TBV/Subject TBV) (Dirlikov et al., 2015; Kramer et al., 2007; Mahone et al., 2011; Ranta et al., 2009). Normalization was done to account for common findings of reduced cerebral/frontal gray matter volume in children with ADHD. More stringent methods of account for TBV (e.g., covarying for TBV) were not employed due to the expectation of subtle group differences.
Separate 2 (Diagnosis) × 2 (Sex) univariate ANCOVAs were run for total (i.e., sum of the respective ROIs) PFC surface area (SA) with age and GAI included as covariates. Next, we conducted exploratory analyses evaluating whether PFC SA was associated with GNG performance and change in performance across tasks using a planned hierarchical approach (e.g., Dirlikov et al., 2015). Change in performance across tasks was computed as ([Standard–Motivational]/Standard)×100, such that positive values indicate reductions in errors and response speed and variability during the motivational compared to the standard GNG. Larger values reflect greater percent change from performance during the standard GNG task. We were primarily interested in associations with change in commission error rate and tau, as we predicted improved response control as indicated by these two measures during the motivational compared to standard GNG task. However, given the lack of published findings on correlations among neuroanatomical measures and cognitive task performance (c.f., Mahone et al., 2011), we also examined correlations with each of the dependent variables separately for each GNG task. Since correlations with behavioral measures were conducted separately for each diagnostic group, unnormalized SA values were used.
First, partial correlations (controlling for age and GAI) were examined within diagnostic group among the primary dependent variables for each task (omission rate, commission rate, mu, sigma, and tau), as well as the percent change in commission error rate and tau and PFC surface area. Second, for significant findings only (p < .05), partial correlations were examined at the ROI level. Third, for significant findings, partial correlations were examined separately for each hemisphere at the ROI level. Finally, for significant findings at the lateralized ROI level, correlations were examined separately for girls and boys. Correction for multiple comparisons was not applied given the hierarchical, stepwise approach taken and the exploratory nature of these analyses.
Results
Sample Characteristics
Demographic information is provided in Table 1. The sample was drawn from largely middle class socioeconomic status and was 74% white, 14% African American, 10% biracial, and 2% Asian. The ADHD group tended to be younger (p = .083) and had lower FSIQ (p = .001), as is often seen in the childhood ADHD literature (Frazier, Demaree, & Youngstrom, 2004). GAI also differed between diagnostic groups (p = .021), but the difference was not as great as for FSIQ.
Within the ADHD group, girls and boys differed on parent ratings of ADHD inattentive symptoms standardized relative to same-sex children (CPRS DSM Inattention T-score: p < .001), with girls with ADHD showing greater levels of inattention than boys with ADHD. The comparison of boys with ADHD and TD boys indicated that boys with ADHD tended to be younger than TD boys (p = .086) and had lower FSIQ (p < .001) and GAI (p = .014). Girls with ADHD did not differ from TD girls in any of these demographic characteristics.
Demographic characteristics were also examined among the sample of 153 children included in the analyses with neuroanatomical data (76 ADHD, 29 girls; 77 TD, 29 girls; see Supplementary Table S1). The significant differences reported above were maintained in the MRI sample and there were no additional differences for each of the subgroup comparisons.
Go/No-Go Task Performance
A 2 (Diagnosis) × 2 (Sex) × 2 (Task) MANCOVA of GNG performance revealed multivariate effects of diagnosis, F(6, 191) = 4.5, p < .001, sex, F(6, 191) = 3.3, p = .004, and a Diagnosis × Sex × Task interaction, F(6, 191) = 2.6, p = .018. None of the remaining multivariate tests were significant: Diagnosis × Sex: F(6, 191) = 0.7, p = .624; task: F(6, 191) = 0.8, p = .591; Diagnosis × Task: F(6, 191) = 0.6, p = .716; Sex × Task: F(6, 191) = 0.9, p = .482. Examination of univariate tests indicated that there was a significant effect of diagnosis on omission rate, F(1, 196) = 6.5, p = .012, d = 0.36, and tau, F(1, 196) = 15.6, p <.001, d = 0.56, such that children with ADHD made more omission errors and had higher tau in general. There were also significant effects of sex on fast go rate, F(1, 196) = 10.5, p = .001, d = 0.48, commission rate, F(1, 196) = 9.6, p = .002, d = 0.46, mu, F(1, 196) = 11.3, p = .001, d = 0.50, and sigma, F(1, 196) = 4.3, p = .040, d = 0.31. Specifically, boys had a higher proportion of fast go responses and commission errors, and faster and less variable responses in the normal part of the RT distribution (mu and sigma) than did girls, regardless of diagnosis.
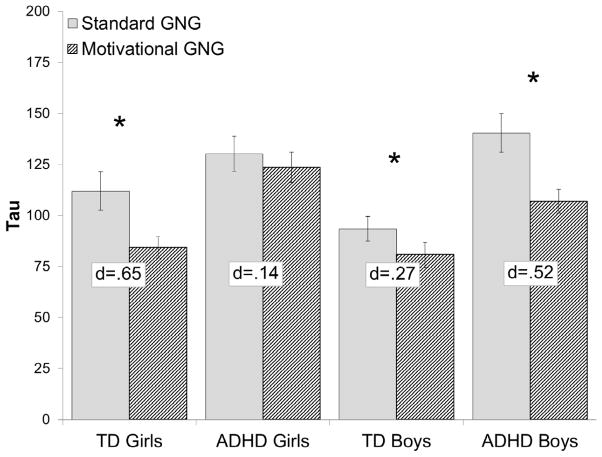
The Diagnosis × Sex × Task interaction was only significant for tau, F(1, 196) = 7.9, p = .005, d = 0.40, such that tau decreased during the motivational GNG compared to the standard GNG task for TD boys (p = .044, d = 0.27), TD girls (p = .001, d = 0.65), and ADHD boys (p < .001, d = 0.52), but not for girls with ADHD (p = .428, d = 0.14; Figure 2). Furthermore, girls with ADHD exhibited elevated tau compared to TD girls during the motivational GNG (p < .001, d = 1.06) but not during the standard GNG (p = .287, d = 0.34), whereas boys with ADHD exhibited higher tau compared to TD boys during both the standard GNG (p = .001, d = 0.74) and, to a lesser extent, during the motivational GNG (p = .021, d = 0.54).
Figure 2.
Diagnosis × Sex × Task interaction for tau. Error bars represent standard error of the mean; *p<.05; d = Cohen’s d effect size estimate.
We also examined whether this pattern of findings was maintained when we limited the sample of boys with ADHD to be more comparable to the sample of girls with ADHD both in size and ADHD symptom severity (i.e., CPRS sex-normed T-scores for inattention and hyperactivity/impulsivity). In order to better match girls and boys with ADHD and standardized symptom severity, we excluded boys with ADHD with a CPRS T-score below 69 on the inattention scale and below 66 on the hyperactivity/impulsivity scale, resulting in a subsample of boys with ADHD (n = 29) that did not differ from the sample of girls with ADHD in either inattention symptom severity (p = .366) or hyperactivity/impulsivity symptom severity (p = .125). This subsample of boys with ADHD also did not differ from the full sample of girls with ADHD in other important demographic characteristics, such as age (p = .545), SES (p = .403), FSIQ (p = .166), or GAI (p = .838).
The Diagnosis × Sex × Task interaction remained significant for tau, F (1, 154) = 6.5, p = .012, such that tau decreased during the motivational GNG compared to the standard GNG task for TD boys (p = .055), TD girls (p = .001), and ADHD boys (p < .001), but not for girls with ADHD (p = .398). Furthermore, girls with ADHD exhibited elevated tau compared to TD girls during the motivational GNG (p < .001) but not during the standard GNG (p = .259), whereas boys with ADHD exhibited higher tau compared to TD boys during both the standard (p = .003) and motivational (p = .035) GNG tasks.
Prefrontal Cortex Surface Area
A 2 (Diagnosis) × 2 (Sex) ANCOVA for PFC SA revealed a significant effect of diagnosis, F(1, 147) = 5.5, p = .021, d = 0.38, such that total PFC SA was reduced in the ADHD group. In contrast to our previous findings in a larger sample (n=226; Dirlikov et al., 2015), there were no significant sex differences, sex: F(1, 147) = 0.01, p = .938, and Diagnosis × Sex: F(1, 147) = 0.60, p = .439. A detailed examination of the effects of diagnosis and sex for the specific PFC ROIs is beyond the scope of this paper, although this information is presented and discussed in great detail in Dirlikov et al. (2015). Follow-up analyses examining diagnosis and sex effects for SA of the ROIs differentially associated with GNG task performance among ADHD and TD groups are presented below.
Neuroanatomical Correlates of GNG Performance
Examination of partial correlations (controlling for age and GAI) among GNG task performance and total PFC SA within diagnostic group revealed lack of significant correlations in the TD group (n = 77; all rs < .14 and ps > .23). Among children with ADHD (n = 76), a greater change in tau across tasks was associated with greater PFC SA (r = .251, p = .031). None of the remaining correlations approached significance in the ADHD group (rs < .23, ps > .05).
To further explore the significant correlation between change in tau across tasks and PFC SA in the ADHD group, we examined whether this relationship was associated with a specific PFC ROI (ACC, dlPFC, mPFC, ilPFC, and OFC). Among children with ADHD, change in tau across tasks was associated with mPFC (r = .252, p = .031) and OFC (r = .249, p = .032) SA (see Figure 3). Next, these associations were examined separately for left and right mPFC and OFC among the ADHD group indicating that the association at the broader ROI level was driven by right mPFC (r = .328, p = .004) and right OFC (r = .274, p = .018) and were not significant for left mPFC (r = .106, p = .368) and left OFC (r = .177, p = .132). Finally, we examined whether the association among right mPFC and right OFC SA and change in tau across tasks was significant within sex in the ADHD group only. We found that right OFC SA was significantly associated with change in tau among boys with ADHD (n = 47; r = .336, p = .024), but not among girls with ADHD (n = 29; r = .048, p = .813). In addition, right mPFC SA was not significantly associated with change in tau in either boys (r = .266, p = .077) or girls (r = .306, p = .121) with ADHD.
Figure 3.
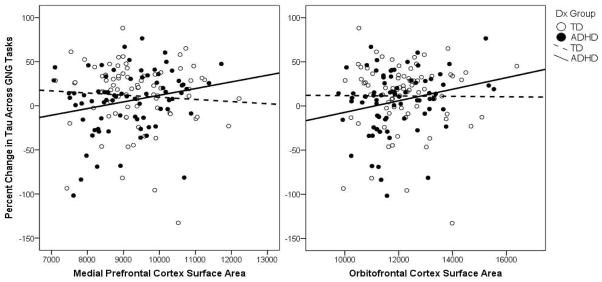
Associations among change in tau across GNG tasks and medial prefrontal cortex and orbitofrontal cortex surface area for the TD and ADHD groups.
Follow-up analyses were conducted examining whether there were diagnosis or sex differences in SA for the right mPFC and right OFC given their specific association with change in tau across GNG tasks among children with ADHD. As for the PFC analysis reported above, a 2 (Diagnosis) × 2 (Sex) ANCOVA was employed to test for SA differences for the right mPFC and right OFC. For the right mPFC, there were no significant effects of diagnosis, F(1, 147) = 1.7, p = .190, sex, F(1, 147) = 0.06, p = .800, or their interaction, Diagnosis × Sex: F(1, 147) = 1.4, p = .237. Examination of unprotected univariate tests suggested that although right mPFC SA did not significantly differ among girls with ADHD compared to TD girls, F(1, 147) = 2.6, p = .110, effect size estimates suggest a medium effect (d = 0.46), whereas there was no evidence of differences in right mPFC SA among boys with ADHD compared to TD boys, F(1, 147) = .01, p = .907, d = 0.02. In contrast, for the right OFC, there was a strong effect of diagnosis, F(1, 147) = 9.1, p = .003, d = 0.43, such that right OFC SA was reduced in the ADHD group relative to the TD group. There was no effect of sex, F(1, 147) = 0.7, p = .397, and no Diagnosis × Sex interaction F(1, 147) = 0.3, p = .558. Examination of unprotected univariate tests suggested that right OFC SA was significantly reduced among boys with ADHD compared to TD boys, F(1, 147) = 8.3, p = .004, d = 0.44. Although right OFC SA among girls with ADHD did not significantly differ from TD girls, F(1, 147) = 2.5, p = .116, effect size estimates suggest a medium effect (d = 0.41) comparable to the difference among boys. Given the much smaller sample of girls than boys, examination of effect sizes provides important information. Effect size estimates indicate that reduced right mPFC and right OFC SA observed in girls with ADHD compared to TD girls would have likely approached traditional levels of significance if the sample of girls was as large as the sample of boys. In contrast, reduced SA was only evident in the right OFC, but not the right mPFC, among boys with ADHD compared to TD boys.
Discussion
Contingency management involving reinforcement and punishment is an evidence-based treatment for ADHD (Evans et al., 2013; Fabiano et al., 2009; Pelham & Fabiano, 2008). In addition, children with ADHD demonstrate a variety of cognitive deficits including difficulties with inhibitory control, working memory, and attention regulation, thought to contribute to greater ISV, compared to TD children (Coghill, Seth, & Matthews, 2013; Kofler et al., 2013; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Etiologic models of ADHD increasingly emphasize atypical motivation and response to reinforcement as contributing to the behavioral symptoms of ADHD (see review by Luman et al., 2010). It follows that contingency management may improve the behavioral symptoms of ADHD by improving the cognitive deficits implicated in this disorder, although the mechanism by which motivational factors improve cognitive deficits in children with ADHD remains unclear. The current study evaluated the impact of motivational contingencies (i.e., immediate feedback paired with monetary gain and loss) on response control during a GNG task as indicated by error rates and ex-Gaussian measures of response speed (mu) and variability in the normal (sigma) and exponential (tau) portion of the RT distribution in girls and boys with and without ADHD. Associations with PFC surface area were also examined using a functionally defined atlas.
Our findings suggest that boys with ADHD demonstrated similar improvements in ISV as TD boys and girls when motivational contingencies were offered for performance relative to a standard GNG task without feedback or contingencies, whereas girls with ADHD did not show reduced ISV with motivational contingencies. Furthermore, PFC surface area was reduced in the ADHD group and, within the ADHD group, greater PFC surface area was associated with a greater reduction in tau during the motivational GNG compared to the standard GNG. This association was particularly strong with the right mPFC and, among boys with ADHD only, the right OFC, a region in which surface area was reduced among children with ADHD compared to TD children. Finally, examination of effect sizes suggests that girls with ADHD displayed reduced right mPFC and right OFC SA compared to TD girls (medium effect) whereas boys with ADHD compared to TD boys only displayed reduced SA for the right OFC, but not the right mPFC.
The finding that motivational contingencies reduced ISV for most participants is consistent with neurocognitive models suggesting the interaction of neural systems involved in cognition and motivation (Haber & Knutson, 2010; Sallet et al., 2011). Interestingly, ISV was not differentially improved in children with ADHD and diagnostic group differences remained when motivational contingencies were in place. Thus, there was no evidence of a differential impact of reinforcement on cognitive task performance in children with ADHD as postulated in some reinforcement dysfunction models and as has been previously shown (e.g., Luman et al., 2005; Rosch et al., in press; Rosch & Hawk, 2013; Strand et al., 2012), although this is not consistently reported. In addition, none of the other performance measures (i.e., error rates, mu, and sigma) were improved during the motivational GNG task. This pattern of findings may be specific to the contingency structure applied in this study, which reinforced going quickly and punished failed inhibition. Perhaps children were more sensitive to reinforcement than to punishment and therefore prioritized responding efficiently to go stimuli, thereby reducing tau, over inhibiting to no-go stimuli, which would have reduced commission errors. It will be important for future research to vary the contingency structure in order to determine whether altering the contingencies produces similar results and whether directly reinforcing response variability produces a greater reduction in tau among children with ADHD.
Furthermore, this is the first study, to our knowledge, that has demonstrated sex differences in the effects of motivational contingencies, consisting of immediate feedback paired with monetary gain and loss, on cognitive task performance. Previous studies reporting a significant reduction in ISV with contingencies included primarily boys with ADHD (at least 90% male) (Andreou et al., 2007; Douglas & Parry, 1983; Uebel et al., 2010), whereas studies that did not consistently find an effect of contingencies on ISV included more girls (72–75% male) (Epstein et al., 2011; Scheres et al., 2001; Shanahan et al., 2008). Our data suggest that including a larger percentage of girls with ADHD may weaken the effect of motivational contingencies on performance. Furthermore, only one of these studies examined whether boys and girls with ADHD differed in their response to reinforcement (Shanahan et al., 2008), and they did not find evidence of a Diagnosis×Sex interaction. Our ADHD sample was deliberately oversampled for girls providing greater power to test for sex differences in children with ADHD than in previous studies, and this interaction emerged, although it was specific to ISV as measured by tau. Replication of these findings in studies with larger samples of girls and on different cognitive tasks will be important to determine whether these findings are specific to the current sample and task.
One possible interpretation for the observed sex differences is that motivational factors contribute to greater ISV in boys with ADHD more so than in girls with ADHD. Motivational models of ADHD emphasize associations among reinforcement sensitivity and hyperactive/impulsive symptoms of ADHD (Sagvolden, Johansen, Aase, & Russell, 2005), which tend to be greatest in boys (Rucklidge, 2008b). However, boys and girls with ADHD in the current sample showed equivalent levels of hyperactive, impulsive, and inattentive symptoms, although they did differ in sex-normed T-scores of ADHD symptoms (see Table 1), suggesting that a differential response to reinforcement in boys and girls with ADHD is not simply a function of symptom presentation. Supplementary analyses indicated that this pattern of findings was maintained when we limited the sample of boys with ADHD to be more comparable to the sample of girls with ADHD both in size and ADHD sex-normed symptom severity (i.e., CPRS sex-normed T-scores for inattention and hyperactivity/impulsivity). Thus, these ADHD-related sex differences do not appear to be a function of different symptom profiles among girls and boys with ADHD. These findings have implications for effective implementation of behavioral treatments, which center around contingency management, such that girls with ADHD may require an alternative contingency structure than boys with ADHD.
It may also be that motivational contingencies did not impact ISV among girls with ADHD because response control was not impaired in girls with ADHD during the standard GNG task, thereby reducing the potential for motivational contingencies to have an effect. However, motivational contingencies improved ISV among TD children despite strong baseline performance. Findings from a recent study (Seymour et al., 2015) suggest that impaired response control among girls with ADHD is only present during a task with greater executive function demands, during which working memory was necessary to guide response control, but not during a response control task with minimal cognitive demands. In contrast, boys with ADHD demonstrated elevated ISV during tasks with minimal and greater cognitive demands, as shown in a previous study (Seymour et al., 2015) and on both the standard and motivational GNG compared to TD boys in the current study. This divergent pattern of findings regarding impaired performance during the standard GNG and change in performance with motivational contingencies might suggest different underlying causes of ISV in boys and girls with ADHD. Specifically, it may be that greater basic motor impairments in boys with ADHD contribute to poorer performance on response control tasks, regardless of cognitive demand, whereas motor dysfunction is less common in girls with ADHD such that impaired response control is only evident during tasks with greater executive function demands. This interpretation is consistent with recent neuroanatomical findings of reduced cortical surface area in premotor regions in boys, but not girls, with ADHD, and more widespread surface area reductions in prefrontal regions in girls compared to boys with ADHD (Dirlikov et al., 2015). Furthermore, it may be that girls with ADHD may show improved performance with motivational contingencies during response control tasks with greater cognitive demands. Consideration of the current findings within the context of the broader empirical and theoretical literature suggests greater involvement of ‘cool’ rather than ‘hot’ EF in the pathophysiology of ADHD among girls. However, it may also be that reward and punishment contingencies are only effective for girls with ADHD during tasks and activities that require greater cognitive demand.
It is also important to consider whether other differences between the tasks influenced performance as hypothesized by the cognitive-energetic model of ADHD (Sergeant, 2000, 2005), which suggests that not only the motivational contingencies affect performance, but also the event rate and relative engagingness of the task. The motivational GNG was likely perceived by participants as more engaging than the standard GNG because of the presentation of feedback stimuli in between go/no-go stimulus presentations, which also altered the event rate (i.e., interstimulus interval). Although participants actually had a longer time to respond during the motivational GNG task, variability in their RTs may have been reduced due to the faster event rate as a result of presentation of feedback stimuli in between go/no-go stimuli. Thus, it may be that the performance of girls with ADHD was less influenced by motivational contingencies, or how engaging the task was, or the faster event rate in comparison to TD boys and girls and boys with ADHD, who all showed reduced ISV during the motivational GNG. One challenge of testing the cognitive-energetic model is that a task that includes motivational contingencies is likely to be more engaging, making it difficult to separate the effects of task engagement more broadly and direct effects of reinforcement or punishment on cognition. The current paradigm is not able to disentangle these possible effects, but this is an important question for future research.
We also evaluated the neuroanatomical correlates of changes in cognitive task performance with motivational contingencies. We found that PFC SA was reduced in children with ADHD, particularly in the right OFC, consistent with prior research (Dirlikov et al., 2015; Shaw et al., 2012). Although girls with and without ADHD did not significantly differ in either right mPFC or right OFC SA, this appears to be an issue of power as examination of effect size estimates indicated a medium effect (d ~ 0.40) for reduced SA in both regions among girls with ADHD compared to TD girls. In addition, within the ADHD group, greater PFC SA was associated with a greater reduction in ISV during the task with motivational contingencies after adjusting for baseline performance among children with ADHD. This association was particularly strong with the right OFC among boys with ADHD, who also showed the greatest improvement in ISV with motivational contingencies, and with the right mPFC among the overall group of children with ADHD. These results are consistent with multiple pathway models of ADHD implicating brain regions involved in both ‘cool’ and ‘hot’ EF (Castellanos et al., 2006; Haber & Knutson, 2010). They also suggest that the OFC, in control of voluntary, goal-directed behavior (Tremblay & Schultz, 2000), may be particularly important for improved attention regulation among children with ADHD. Furthermore, the broader anatomical differences involving both the mPFC and OFC among girls with ADHD may underpin their lack of response to motivational contingencies.
It is important to consider these findings within a developmental context because children in this age range are undergoing significant synaptic folding to create efficient neural networks. Research has shown that the mean age by which 50% of the of the cortical vertices in the right PFC attained peak surface area for individuals with ADHD was 14.6 years whereas it was 12.7 years for the TD group (Shaw et al., 2012). Thus, given the age range for our sample of 8–12 years, reduced PFC surface area in the ADHD group might suggest delayed cortical maturation. Furthermore, it may be that children with ADHD that have undergone greater maturation of the PFC, particularly in the OFC and mPFC, were most capable of exerting effortful control to regulate attention in a motivational context. The finding that the association with OFC was particularly strong among boys with ADHD, who also showed improved ISV with motivational contingencies at the group level, suggests that those boys with ADHD with a more mature right OFC showed the greatest response to motivational contingencies. In addition, maturation of the mPFC may be associated with improved cognitive performance under conditions of motivational contingencies among children with ADHD, regardless of sex.
This study provides novel information regarding sex differences in the impact of motivational contingencies on ISV in children with ADHD and association with prefrontal anatomy, although the limitations of this study are worth noting. Regarding the motivational GNG task, the combination of feedback and monetary gain/loss does not permit isolation of the separate effects of feedback compared to reward and punishment contingencies. Based on the current pattern of findings, we would predict that a feedback only condition would not improve performance among girls with ADHD, but it is less clear whether the performance of boys with ADHD would improve with feedback only. In addition, the motivational GNG task was always performed after the standard GNG task, which may produce practice or order effects. This study is also limited in its focus on a single cognitive task, particularly given the heterogeneity of cognitive deficits implicated in ADHD (Willcutt et al., 2005). Similarly, the focus of the neuroanatomical correlates was limited to specific ROIs in the prefrontal cortex without consideration of subcortical regions involved in reward and motivational processes, such as the ventral striatum. Finally, this study did not include dimensional measures of temperament or personality that are associated with behavioral responses to reward and punishment, such as sensitivity to punishment and reward (Luman, van Meel, Oosterlaan, & Geurts, 2012), which may be associated with the extent to which individuals with ADHD show improvements in task performance when motivational contingencies are introduced (e.g., Fosco, Hawk, Rosch, & Bubnik, 2015).
Further research on ADHD-related sex differences in cognitive functioning and response to motivational contingencies is necessary to understand whether these findings are specific to ISV or apply to other cognitive deficits implicated in this disorder. Similarly, examination of other brain regions, particularly those regions primarily implicated in reward processing (e.g., the ventral striatum) and motor control should be considered in future research. It may also be beneficial to use functional MRI to examine whether PFC or ventral striatum activation predicts improved performance on cognitive tasks in the context of motivational contingencies. These findings support etiological models of ADHD postulating an interaction between cognition and motivation and draw attention to consideration of sex differences in children with ADHD. There are also implications of these findings for effective implementation of behavioral treatments, which center around contingency management, such that girls with ADHD may require an alternative contingency structure than boys with ADHD. Further research is required to determine whether these sex differences in response to contingencies are also present in behavioral treatments.
Supplementary Material
Table S1. Demographic characteristics for TD and ADHD girls and boys in the sample with anatomical MRI data.
Highlights.
Motivational contingencies improved response control in boys, not girls, with ADHD.
Prefrontal cortex (PFC) surface area (SA) was reduced in children with ADHD.
PFC SA was associated with improvement in response variability with reward in ADHD.
Different factors may influence response control in boys and girls with ADHD.
Acknowledgments
This work was supported by NIH/NINDS: RO1 MH078160; RO1 MH085328, K23 MH101322, and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award program UL1 TR 000424-06, and was carried out in part at the F.M. Kirby Center for Functional Brain Imaging, using resources provided under P41 EB015909.
Footnotes
Traditional RT measures including the mean and standard deviation of reaction time (MRT, SDRT) were also examined. MRT was not reported because it is greatly impacted by outlying RT values and highly correlated with SDRT. The findings for SDRT were very similar to the findings for tau, although they were slightly weaker. To reduce the number of measures, we do not report MRT and SDRT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, Kuntsi J. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37(12):1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Batty Martin J, Liddle Elizabeth B, Pitiot Alain, Toro Roberto, Groom Madeleine J, Scerif Gaia, Hollis Chris. Cortical Gray Matter in Attention-Deficit/Hyperactivity Disorder: A Structural Magnetic Resonance Imaging Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):229–238. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bubnik MG, Hawk LW, Pelham WE, Waxmonsky JG, Rosch KS. Reinforcement Enhances Vigilance Among Children With ADHD: Comparisons to Typically Developing Children and to the Effects of Methylphenidate. Journal of Abnormal Child Psychology. 2015;43(1):149–161. doi: 10.1007/s10802-014-9891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychological Medicine. 2013:1–13. doi: 10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71(19):1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales- Revised. Toronto: Multi-Health Systems, Inc; 2002. [Google Scholar]
- Conners CK. Conners 3. North Tonawanda, NY: Multi-Health Systems, Inc; 2008. [Google Scholar]
- Dirlikov B, Rosch KS, Crocetti D, Denckla MB, Mahone EM, Mostofsky SH. Distinct Frontal Lobe Morphology in Girls and Boys with ADHD. Neuroimage: Clinical. 2015;7:222–229. doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas VI. Cognitive control processes in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. New York: Kluwer Academic/Plenum Publishing; 1999. pp. 105–138. [Google Scholar]
- Douglas VI, Parry PA. Effects of reward on delayed reaction time task performance of hyperactive children. Journal of Abnormal Child Psychology. 1983;11(2):313–326. doi: 10.1007/BF00912094. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV. New York, NY: Guilford Press; 1998. [Google Scholar]
- Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, Altaye M. Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology. 2011;36(5):1060–1072. doi: 10.1038/npp.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SW, Owens JS, Bunford N. Evidence-Based Psychosocial Treatments for Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Journal of Clinical Child and Adolescent Psychology. 2013 doi: 10.1080/15374416.2013.850700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Jr, Coles EK, Gnagy EM, Chronis-Tuscano A, O’Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev. 2009;29(2):129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fosco WD, Hawk LW, Jr, Rosch KS, Bubnik MG. Evaluating cognitive and motivational accounts of greater reinforcement effects among children with attention-deficit/hyperactivity disorder. Behav Brain Funct. 2015;11(1):20. doi: 10.1186/s12993-015-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36(8):1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Getahun D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, Rhoads GG. Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA Pediatr. 2013;167(3):282–288. doi: 10.1001/2013.jamapediatrics.401. [DOI] [PubMed] [Google Scholar]
- Gopin CB, Berwid O, Marks DJ, Mlodnicka A, Halperin JM. ADHD Preschoolers with and without ODD: do they act differently depending on degree of task engagement/reward? Journal of Attention Disorders. 2013;17(7):608–619. doi: 10.1177/1087054711432140. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. An Integrative Theory of Anterior Cingulate Cortex Function: Option Selection in Hierarchical Reinforcement Learning. In: Mars RB, Sallet J, Rushworth MFS, Yeung N, editors. Neural Basis of Motivational and Cognitive Control. Cambridge, Massachusetts: The MIT Press; 2011. pp. 333–349. [Google Scholar]
- Jacobson LA, Ryan M, Martin RB, Ewen J, Mostofsky SH, Denckla MB, Mahone EM. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychol. 2011;17(3):209–224. doi: 10.1080/09297049.2010.532204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT. Annual research review: Reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J Child Psychol Psychiatry. 2014;55(6):685–710. doi: 10.1111/jcpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Scheres A, Sonuga-Barke EJ, Castellanos FX. Functional neuroimaging of reward and motivational pathways in ADHD. In: Fitzgerald M, Bellgrove M, Gill M, editors. The Handbook of Attention Deficit Hyperactivity Disorder. John Wiley & Sons Ltd; 2007. pp. 209–235. [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clinical Psychology Review. 2013;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Injury. 2000;14(10):859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Quitania L, Dean D, Neuhaus J, Rosen HJ, Halabi C, Miller BL. Magnetic resonance imaging correlates of set shifting. J Int Neuropsychol Soc. 2007;13(3):386–392. doi: 10.1017/S1355617707070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology. 2008;4(1):35–45. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104(2):167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lin HY, Gau SS, Huang-Gu SL, Shang CY, Wu YH, Tseng WY. Neural substrates of behavioral variability in attention deficit hyperactivity disorder: based on ex-Gaussian reaction time distribution and diffusion spectrum imaging tractography. Psychological Medicine. 2014;44:1751–1764. doi: 10.1017/S0033291713001955. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neuroscience and Biobehavioral Reviews. 2010;34(5):744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Luman M, van Meel CS, Oosterlaan J, Geurts HM. Reward and punishment sensitivity in children with ADHD: validating the Sensitivity to Punishment and Sensitivity to Reward Questionnaire for children (SPSRQ-C) J Abnorm Child Psychol. 2012;40(1):145–157. doi: 10.1007/s10802-011-9547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Li SC, Backman L. Neural underpinnings of within-person variability in cognitive functioning. Psychology and Aging. 2009;24(4):792–808. doi: 10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Ranta ME, Crocetti D, O’Brien J, Kaufmann WE, Denckla MB, Mostofsky SH. Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2011;17(6):1047–1057. doi: 10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JA, Kerns KA, Mateer CA. The effect of reinforcement variables on inhibition in children with ADHD. Neuropsychology, Development, and Cognition: Section C, Child Neuropsychology. 2005;11(3):295–302. doi: 10.1080/092970490911270. [DOI] [PubMed] [Google Scholar]
- Miller Gregory A, Chapman Jean P. Misunderstanding Analysis of Covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2008;37(1):184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Ranta ME, Chen M, Crocetti D, Prince JL, Subramaniam K, Fischl B, Mostofsky SH. Automated MRI parcellation of the frontal lobe. Hum Brain Mapp. 2014;35(5):2009–2026. doi: 10.1002/hbm.22309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta ME, Crocetti D, Clauss JA, Kraut MA, Mostofsky SH, Kaufmann WE. Manual MRI parcellation of the frontal lobe. Psychiatry Res. 2009;172(2):147–154. doi: 10.1016/j.pscychresns.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- Rosch KS, Fosco WD, Pelham WE, Jr, Waxmonsky JG, Bubnik MG, Hawk LW., Jr Reinforcement and stimulant medication ameliorate deficient response inhibition in children with Attention-Deficit/Hyperactivity Disorder. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-015-0031-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch KS, Hawk LW., Jr The effects of performance-based rewards on neurophysiological correlates of stimulus, error, and feedback processing in children with ADHD. Psychophysiology. 2013;50(11):1157–1173. doi: 10.1111/psyp.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge JJ. Gender differences in ADHD: implications for psychosocial treatments. Expert Rev Neurother. 2008a;8(4):643–655. doi: 10.1586/14737175.8.4.643. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Gender differences in ADHD: implications for psychosocial treatments. Expert Review of Neurotherapeutics. 2008b;8(4):643–655. doi: 10.1586/14737175.8.4.643. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33(2):357–373. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419–368. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Quilodran R, Procyk E, Petrides M, Rushworth MF. Neuroanatomical Basis of Motivational and Cognitive Control: A Focus on the Medial and Lateral Prefrontal Cortex. In: Mars RB, Sallet J, Rushworth MF, Yeung N, editors. Neural Basis of Motivational and Cognitive Control. Cambridge, Massachusetts: The MIT Press; 2011. pp. 5–20. [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response inhibition in children with DSM-IV subtypes of AD/HD and related disruptive disorders: the role of reward. Child Neuropsychology. 2001;7(3):172–189. doi: 10.1076/chin.7.3.172.8746. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57(11):1248–1255. doi: 10.1016/j.bps.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioral Reviews. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Seymour KE, Mostofsky SH, Rosch KS. Cognitive Load Differentially Impacts Response Control in Girls and Boys with ADHD. Journal of Abnormal Child Psychology. 2015 doi: 10.1007/s10802-015-9976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Willcutt EW. Do motivational incentives reduce the inhibition deficit in ADHD? Dev Neuropsychol. 2008;33(2):137–159. doi: 10.1080/87565640701884238. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(3):191–197. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Spencer SV, Waschbusch DA. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36(6):903–913. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels Rosch K, Dirlikov B, Mostofsky SH. Increased intrasubject variability in boys with ADHD across tests of motor and cognitive control. J Abnorm Child Psychol. 2013;41(3):485–495. doi: 10.1007/s10802-012-9690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Stevens J, Quittner AL, Zuckerman JB, Moore S. Behavioral inhibition, self-regulation of motivation, and working memory in children with attention deficit hyperactivity disorder. Developmental Neuropsychology. 2002;21(2):117–139. doi: 10.1207/S15326942DN2102_1. [DOI] [PubMed] [Google Scholar]
- Strand MT, Hawk LW, Jr, Bubnik M, Shiels K, Pelham WE, Jr, Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. J Abnorm Child Psychol. 2012;40(7):1193–1207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(10):1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. Journal of Neurophysiology. 2000;83(4):1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, Banaschewski T. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51(2):210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Individual Achievement Test - Second Edition (WIAT-II) San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. J Clin Exp Neuropsychol. 2007;29(4):345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic characteristics for TD and ADHD girls and boys in the sample with anatomical MRI data.