Abstract
Both type I and II interferons (IFNs) have been implicated in the pathogenesis of Sjogren's syndrome (SS). We aimed to explore the contribution of type I and II IFN signatures in the generation of distinct SS clinical phenotypes including lymphoma development. Peripheral blood (PB) from SS patients (n=31), SS patients complicated by lymphoma (n=13) and healthy controls (HC, n=30) were subjected to real-time PCR for 3 interferon inducible genes (IFIGs) preferentially induced by type I IFN, 2 IFIGs preferentially induced by IFNγ as well as for IFNα and IFNγ genes. The same analysis was performed in minor salivary gland tissues (MSG) derived from 31 SS patients, 10 SS-lymphoma patients and 17 sicca controls (SC). In PB and MSG tissues, overexpression of both type I and type II IFIGs was observed in SS patients versus HC and SC, respectively, with a predominance of type I IFN signature in PB and a type II IFN signature in MSG tissues. In SS-lymphoma MSG tissues, lower IFNα, but higher IFNγ and type II IFIG transcripts compared to both SS and SC were observed. In receiver operating characteristic curve analysis, IFNγ/IFNα mRNA ratio in MSG tissues showed the best discrimination for lymphoma development. Discrete expression patterns of type I and II IFN signatures might be related to distinct SS clinical phenotypes. Additionally, IFNγ/IFNα mRNA ratio in diagnostic salivary gland biopsies is proposed as a novel histopathological biomarker for the prediction of in situ lymphoma development in the setting of SS.
Keywords: Type I interferon, type II interferon, Sjogren's syndrome, lymphomagenesis, B cell activating factor
1. Introduction
Sjogren's syndrome (SS) or autoimmune epithelitis is a chronic autoimmune exocrinopathy [1] characterized by the highest risk for development of non-Hodgkin's lymphoma (NHL) among all autoimmune diseases [2]. Clinical, serological and histopathological features such as purpura, salivary gland enlargement, C4 hypocomplementemia, germinal center formation and the presence of interleukin-18 (IL-18) producing macrophages in minor salivary gland (MSG) tissues have been previously associated with lymphoma development in the setting of SS [3-5]. Additionally, mutations of the p53 gene, the presence of the t(14:18) translocations, genetic variants of the B cell activating factor (BAFF) and its receptor, as well as the tumor necrosis factor alpha-induced protein 3 (TNFAIP3) gene have been considered to confer increased risk for lymphoma development [6-10].
Growing evidence over the last decade points towards a significant role of the type I interferon (IFN) system in the pathogenesis of systemic and organ-specific disorders including SS [11-13]. Type I IFNs (IFN α/β) are proteins that normally provide protection from viral infections, through induction of hundreds of genes implicated in antiviral response, the so called “IFN signature” [14]. Microarray analysis of MSGs [15], peripheral mononuclear cells/whole blood [16] and peripheral blood (PB) CD14+ monocytes [13] revealed a prominent type I IFN signature in patients with SS, often in association with autoantibodies against Ro/SSA and La/SSB antigens [13]. Additionally, elevated plasma type I IFN activity has been also demonstrated in plasma from SS patients compared to healthy individuals[17]. Finally, plasmacytoid dendritic cells (pDCs) -the professional type I IFN producing cells- were found to be reduced in the periphery and preferentially recruited in MSGs derived from these patients [11, 18].
Though type I IFNs were proposed as predominant contributors in the pathogenesis of SS, recent data suggest a prevailing role of type II IFNs in disease pathogenesis [15, 19, 20]. Involvement of the type II IFN -IFNγ- in SS has been previously well demonstrated in both humans and animal models [11, 19, 21-24]. IFNγ is predominantly produced by T and NK cells -to a lesser extent by dendritic cells, macrophages and B cells [25]. Following ligation of the IFNγ receptor, induction of interferon II signature genes occurs, promoting antimicrobial protection (host defense), apoptosis, inflammation and tissue damage [26, 27].
Whether type I or II IFNs predominate in SS pathogenesis remains controversial. In a recent report by Hall et al, both type I and II IFNs seemed to contribute to distinct SS phenotypes [28]. Disease heterogeneity -which extends from mild to severe/life-threatening disease- [29] together with the significant overlap between the genes induced by types I and II IFNs [19] [28]or the type of sample (PB or tissue) implemented in different studies [11, 15, 16, 19, 30] might account for apparently discrepant findings between studies. In the current work, we aimed to clarify the role of type I and II IFNs in the generation of diverse clinical phenotypes of SS using both PB and MSG tissues from SS patients with well characterized clinical phenotypes.
2. Patients and Methods
2.1 Patients
2.1.1 Peripheral blood and salivary gland tissues
Whole blood samples (cohort 1) from 31 primary SS patients not complicated by lymphoma (SS-non lymphoma), 13 patients with primary SS complicated by lymphoma [SS-lymphoma, 12 out of 13 (92.3%) by mucosa associated lymphoid tissue (MALT) type and 1 (7.7%) by small lymphocytic lymphoma (SLL) type] and 30 healthy controls (HC) were collected into PAXgene Blood RNA tubes (PreAnalytiX GmbH, CH/BD Diagnostics) and stored according to manufacturer's instructions upon RNA extraction. MSG tissues (cohort 2) were also obtained from an independent cohort of 31 SS-non lymphoma, 10 SS-lymphoma (all of MALT type) and 17 patients presenting with sicca features without fulfilling classification criteria for SS (sicca controls, SC). Five patients were evaluated in both cohorts: 4 SS-non lymphoma and 1 SS-lymphoma. All SS patients -diagnosed according to previously published criteria [31]- were followed in the Rheumatology Department of General Hospital of Athens “G.Gennimatas” and the Department of Pathophysiology, University of Athens, both in Athens, Greece and they provided informed consent prior to their entry in the study.
Labial MSG biopsies were obtained with informed consent from patients and controls at the Department of Pathophysiology of the School of Medicine at the University of Athens, Athens, Greece, as a routine part of the diagnostic evaluation for SS. The biopsies were immediately frozen in -80°C. Focus score was determined for each MSG biopsy sample, as previously described [31]. 40 out of 41 SS patients and all SC were females between the ages of 16 and 76. The control group included individuals complaining of sicca symptoms, who did not fulfil the SS criteria (<1 foci/4 mm2). According to clinical and serological data the control group was further subdivided into autoimmune controls (AC) (n=12) or non-autoimmune controls (NAC) (n=5); autoimmune controls (AC) included 3 patients with SLE, 4 with Hashimoto thyroiditis, 3 with primary biliary cirrhosis (PBC), 1 with morphea and 1 with high titer of antinuclear antibodies. A commercial preparation of total RNA from normal salivary glands pooled from 24 male/female Caucasians (ages 15-60; cause of death: sudden death) was used as a source of healthy RNA (Clontech Laboratories, Inc).
2.1.2 Clinical, serological and histopathological characteristics of study participants
Clinical, serological and histopathological characteristics were recorded after thorough chart review. These included the presence of arthralgias, arthritis, subjective and objective measures of oral and ocular dryness, salivary gland enlargement, Raynaud's phenomenon, lung involvement (documented by pulmonary-function tests and X-ray and/or computed-tomography scans), interstitial nephritis or glomerulonephritis (documented by biopsy), liver involvement (documented by liver biopsy showing changes compatible with primary biliary cirrhosis or autoimmune cholangitis), including palpable purpura, vasculitis, peripheral neuropathy (verified by nerve-conduction studies), lymphoma development (histologically diagnosed), anti-Ro/SSA and/or anti-La/SSB autoantibodies, rheumatoid factor (RF), complement C3- and C4-levels, cryoglobulinemia, hypergammaglobulinemia (total gammaglobulins>2 g/L), leucopenia (white-blood-cell count<4000/mm3) and lymphopenia (number of lymphocytes<1000/mm3). C4 hypocomplementemia was defined as complement levels <20mg/dl and rheumatoid factor positivity as rheumatoid factor levels more than 20IU/ml. No evidence of monoclonality was detected in MSG SS-non lymphoma tissues included in the study.
2.2 Methods
2.2.1 RNA extraction
Our peripheral whole blood samples were extracted with PAXgene Blood RNA System (PreAnalytiX GmbH, BD/QIAGEN) which consolidates and integrates the key steps of whole blood collection, intracellular RNA profile stabilization and RNA purification. According to the manufacturer's instructions, 2.5ml of whole peripheral blood was collected in the PAXgene Blood RNA Tube and the RNA was stabilized and stored upon RNA extraction. Total RNA was extracted from the PAXgene RNA tubes with the PAXgene blood RNA and miRNA isolation kit (PreAnalytiX GmbH, CH/Qiagen, Germany), after elimination of red blood cells and collection of all types of white blood cells was performed.
Total RNA was isolated from MSG samples with Qiagen RNeasy mini kit (Qiagen, Chatsworth, CA) or Trizol Reagent (Ambion, Life Sciences, USA) according to the manufacturers' instructions. All samples were treated with DNAse I (Qiagen, Germany) prior to cDNA synthesis. The quantity and quality of RNA samples were spectrophotometrically tested (Biospec Nano, Japan).
2.2.2 cDNA synthesis
0.5μg of total RNA obtained from MSG samples and 0.25μg of total RNA from PAXgene samples were reverse-transcribed using the Superscript III reverse transcriptase system from Invitrogen (Carlsbad, CA). Oligo-dT primer (0.5μM) (Qiagen, Germany) was used to amplify mRNA and an RNAse inhibitor was included to prevent degradation (RNAseOUT, Invitrogen, USA). cDNA samples were diluted 1:10 and 1:5, respectively with nuclease free water (Qiagen, Germany) immediately after synthesis and stored at -20°C.
2.2.3 Quantitative Real-Time Polymerase Chain Reaction
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) was used to quantify specific cDNAs using the Bio-Rad IQ5 thermocycler and the IQ Bio-Rad SYBR Green Supermix (Bio-Rad, Hercules, CA). Specific primers for each gene were designed using the Beacon Designer software trying to amplify only cDNA (exon-intron spanning) and displayed in Table SI. Briefly, genes preferentially induced by type I IFNs according to recent data [13, 19] were selected and included the following: myxovirus (influenza virus) resistance 1 (MX-1), interferon-induced protein with tetratricopeptide repeats 1 (IFIT-1) and interferon- induced protein 44 (IFI44), as well as genes preferentially induced by type II INF [19, 32]: guanylate binding protein l(GBP-l) and chemokine (C-X-C motif) ligand 9 (CXCL9)/ Monokine induced by gamma interferon (MIG-1). Primers specific for IFNα, IFNγ, B cell acrivating factor (BAFF), TNF-related apoptosis-inducing ligand (TRAIL), tripartite motif containing 21 (TRIM21)/ribonucleoprotein Ro52 and tumor protein 53 (p53) were also designed. As an internal control and normalization gene we used the glyceraldehyde phosphate dehydrogenase (GAPDH). The reaction was carried out in a total volume of 25 μL per reaction. Reaction mixture included 2 μL of template cDNA, 0.4 μM of each primer, 12.5μl 2× IQ SYBR Green SuperMix (Bio-Rad), and sterile water. Amplification protocol started with 95°C for 4 min followed by 40 cycles at 95°C for 10 s and 60°C for 30 s and 72°C for 30 s. To assess product specificity, amplicons were checked by melting curve analysis. Melting curves were generated from 65°C to 95°C with increments of 0.5°C/cycle for 15s at each cycle and all inconsistent results were discarded. Then, the threshold values were recorded for each sample in the logarithmic portion of the amplification curve. All reactions were performed in duplicate. In addition, a reference sample was included in each PCR plate to ensure normalization across experiments. MSG healthy RNA pool was the reference sample for MSG tissues, whereas the RNA from peripheral blood of a healthy subject was the reference sample for peripheral blood samples. Each sample's threshold cycle values for the target and house-keeping genes were subtracted from the corresponding values of the reference sample. Finally, the target gene values were divided by the housekeeping gene values for each sample, and the result was the relative expression value for each unknown sample [33]. Type I and II IFN scores were calculated as previously described [33]. The mean and SD level of each IFN inducible gene in the HC group were used to standardize expression levels of each gene for each study subject. The standardized expression levels were subsequently summed for each patient to provide an IFN type I expression score as the sum of each study subject's relative expression for each of 3 genes preferentially induced by type I IFN and for each of 2 genes preferentially induced by IFN type II, respectively for the 3 distinct groups (SS-non lymphoma, SS-lymphoma, healthy/sicca controls). The cut-off for high type I IFN and II scores among patient peripheral samples was defined as the mean plus 3 SD of the corresponding IFN scores in HC (cut-off for high type I and II IFN scores: 8.07 and 4.60, respectively). An IFNγ/α ratio was also calculated based on the ratio of the IFNγ to IFNα mRNA expression at the level of MSG tissues.
2.2.4 Immunohistochemistry
Immunohistochemical detection of IFNα, IFNγ, BDCA-2 (a marker of plasmacytoid dendritic cells), CD20 (marker of B cells) and CD3 (marker of T cells) was performed by a standard immunoperoxidase technique using the Super Sensitive™ Polymer HRP IHC Detection System (BioGenex, USA) in 10 formalin-fixed paraffin-embedded MSG tissue sections (5 μm) derived from 5 SS-non lymphoma and 5 SS-lymphoma patients. Briefly, paraffin sections were rehydrated in successive baths of xylene, 100%, 96%, 80%, 70% ethanol, and water. The sections were washed with PBS (phosphate buffered saline) 3 times. Antigen retrieval was performed by microwaving for 10 minutes in 0.01 M Citrate buffer (pH 6.0). Incubation for 10 minutes at room temperature with Power block Universal Blocking Reagent (BioGenex, USA) and 10 minutes with 3% H2O2 (BioGenex, USA) were performed to block non-specific antibody binding and endogenous peroxidase activity, respectively. Incubation of serial sections with polyclonal rabbit anti-IFNα (dilution 1:250, sc-20106, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), polyclonal rabbit anti-IFNγ (dilution 1:250, sc-8308, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), polyclonal rabbit anti-BDCA-2 (dilution 1:1000, OSD00005W, Thermo Scientific, USA), monoclonal mouse anti-CD20 (dilution 1:2000, M075529, clone L26, DAKO, Denmark), polyclonal rabbit anti-CD3 (dilution 1:2000, A045229, DAKO, Denmark), and concentration-matched isotype control antibodies (PharMingen, San Diego, CA) was performed for 30 minutes at room temperature. Polymer–horseradish peroxidise (HRP) Reagent (BioGenex, USA) was applied for 30 minutes at room temperature and after a washing step the substrate diaminobenzidine (DAB) solution (BioGenex, USA) was performed for 5-10 minutes. Biopsy sections were counterstained with haematoxylin for 2 minutes (Mayers Haematoxylin solution, Sigma Aldrich Inc, USA), dehydrated in successive baths of water, 70%, 80%, 96%, 100% ethanol, and xylene and coverslip mounted with 2 drops of Aqueous Mounting Media (BioGenex, USA). Negative control staining was performed by replacing primary antibody with PBS. Positive immunoreactivity appears as brown color.
2.2.5 Determination of serum BAFF levels
B-cell activating factor (BAFF) serum protein levels were assessed by ELISA (Quantikine Human BAFF/BLyS, R&D Systems, Minneapolis, USA), according to manufacturer's instructions in 24 HC, 58 SS-non lymphoma and 22 SS-lymphoma patients.
2.2.6 Statistics
Statistical analysis was performed by SPSS v.21 package and Graph Pad PRISM. Two-group comparisons of continuous data were assessed using t-tests, or the Mann-Whitney test, when the data did not have a normal distribution. Correlation between gene expression data was determined using non-parametric Spearman's test. Receiver operating characteristic (ROC) curve and corresponding area under the curve (AUC) were calculated with SPSS v.21. Difference was considered statistically significant if p<0.05.
3. Results
3.1 Demographics, clinical, serological and histopathological characteristics of study participants
Table 1 displays the clinical, serological and histopathological characteristics of all study participants. We included PB samples from 31 primary SS-non lymphoma, 13 SS-lymphoma patients as well as 30 sex and age matched healthy controls (HC) and an independent cohort of MSG tissues from 31 SS-non lymphoma, 10 SS-lymphoma patients as well as 17 subjects with sicca complaints (sicca controls, SC) of similar sex and age distribution. Of note, 5 patients were common in both cohorts (4 SS-non lymphoma and 1 SS-lymphoma patient). In the PB cohort, SS-lymphoma patients had higher focus and Tarpley scores, as well as higher prevalence of Schirmer's test positivity, lymphopenia and rheumatoid factor positivity compared to the SS-non lymphoma group (p-values: 0.009, 0.0002, 0.03, 0.04 and 0.01, respectively). In the MSG cohort, SS-lymphoma patients displayed higher Tarpley scores and increased prevalence of salivary gland enlargement (SGE) (p-values: 0.002 and 0.03, respectively).
Table 1.
Demographics, clinicopathological and serological parameters of study participants.
| Peripheral Blood (PB) | Minor salivary gland (MSG) tissues | |||||||
|---|---|---|---|---|---|---|---|---|
| Disease parameters | Healthy Controls (n=30) |
SS-non lymphoma (n=31) |
SS- lymphoma (n=13) |
p- value1 |
Sicca Controls (n=17) |
SS-non lymphoma (n=31) |
SS- lymphoma (n=10) |
p- value2 |
| Gender/ Female, n (%) | 26/30 (86.7) | 27/31 (87.1) | 12/13 (92.3) | ns | 17/17 (100) | 30/31 (97.8) | 10/10 (100) | ns |
| Mean age (years) (±SD) | 51.9±14. 5 | 54.0±17.2 | 55.8 ±15.1 | ns | 48.0±12.1 | 53.2±13.6 | 53.2±15.7 | ns |
| Disease duration (years) (mean±SD) | na | 7.7±5.4 | 6.3±4.9 | ns | 9.9±1.2 | 8.1±5.9 | 11.9±7.6 | |
| Dry mouth, n (%) | na | 26/31 (83.9) | 13/13 (100) | ns | 15/17 (88.2) | 27/29 (93.1) | 10/10 (100) | |
| Dry eyes, n (%) | na | 27/31 (87.1) | 12/13 (92.3) | ns | 4/17 (23.5) | 26/29 (89.7) | 7/9 (77.8) | ns |
| Shirmer's test positive, n (%) | na | 20/29 (69.0) | 12/12 (100) | 0.03 | na | 22/27 (81.5) | 9/9 (100) | ns |
| Rose Bengal test positive, n (%) | na | 6/18 (33.3) | 6/12 (50.0) | ns | na | 14/23 (60.9) | 6/9 (66.7) | ns |
| Focus score (mean±SD) | na | 1.8±2.5 | 4.4±2.5 | 0.13±0.03 | 2.0±1.1 | 3.4±2.8 | ||
| Tarpley score (mean±SD) | na | 1.2±1.0 | 2.7±0.9 | na | 2.4±1.1 | 3.8±0.4 | ||
| Salivary gland enlargement, n (%) | na | 7/30 (23.3) | 7/13 (53.8) | 0.08 | na | 11/31 (35.5) | 8/10 (80.0) | 0.03 |
| Arthralgia, n (%) | na | 16/31 (51.6) | 7/13 (53.8) | ns | na | 18/29 (68.1) | 3/9 (33.3) | ns |
| Raynaud's, n (%) | na | 9/31 (29.0) | 6/13 (46.2) | ns | na | 7/30 (23.3) | 3/10 (30.0) | ns |
| Palpable purpura, n (%) | na | 7/31 (22.6) | 7/13 (53.8) | 0.07 | na | 5/30 (16.7) | 4/10 (40.0) | ns |
| Lymphadenopathy, n (%) | na | 9/30 (30.0) | 3/13 (23.1) | ns | na | 1/25 (4.0) | 3/10 (30.0) | 0.06 |
| Lung brochocentric disease, n (%) | na | 7/31 (22.6) | 2/13 (15.4) | ns | na | 1/30 (3.3) | 0/10 (0.0) | ns |
| Leucopenia, n (%) | na | 5/31 (16.1) | 2/13 (15.4) | ns | na | 6/31 (19.4) | 3/10 (30.0) | ns |
| Lymphopenia, n (%) | na | 3/31 (9.7) | 5/13 (38.5) | 0.04 | na | 6/31 (19.4) | 2/9 (22.2) | ns |
| Anti-Ro/SSA, n (%) | na | 25/31 (80.6) | 12/13 (92.3) | ns | na | 21/29 (72.4) | 9/10 (90.0) | ns |
| Anti-La/SSB, n (%) | na | 15/28 (53.6) | 8/13 (61.5) | ns | na | 15/29 (51.7) | 6/10 (60.0) | ns |
| Low complement C4, n (%) | na | 18/31 (58.1) | 9/13 (69.2) | ns | na | 14/30 (46.7) | 6/10 (60.0) | ns |
| Hypergammaglobulinemia, n (%) | na | 20/29 (69.0) | 8/13 (61.5) | ns | na | 15/28 (53.6) | 7/10 (70.0) | ns |
| Positive Rheumatoid Factor, n (%) | na | 13/26 (50.0) | 12/13 (92.3) | 0.01 | na | 19/31 (61.3) | 9/10 (90.0) | ns |
| Erythrocyte sedimentation rate (mean±SD) | na | 37.1±28.8 | 45.1±24.6 | ns | na | 42.1±28.4 | 41.7±24.8 | ns |
| Hydroxychloroquine use, n (%) | na | 5/31 (16.1) | 2/13 (15.4) | ns | na | 3/31 (9.7) | 1/10 (10) | ns |
p-value: SS-non lymphoma vs SS-lymphoma patients in PB,
p-value: SS-non lymphoma vs SS-lymphoma patients in MSG tissues, na: not applicable, ns: not significance, n: number
The prevalence of hydroxychloroquine use in the peripheral blood cohort was 16.1% and 15.4% in SS-non lymphoma and SS lymphoma group respectively. The corresponding figures for the MSG cohort were 9.7% and 10% (Table 1).
3.2 Relative mRNA expression of IFN type I and II genes in PB samples from SS patients and healthy controls
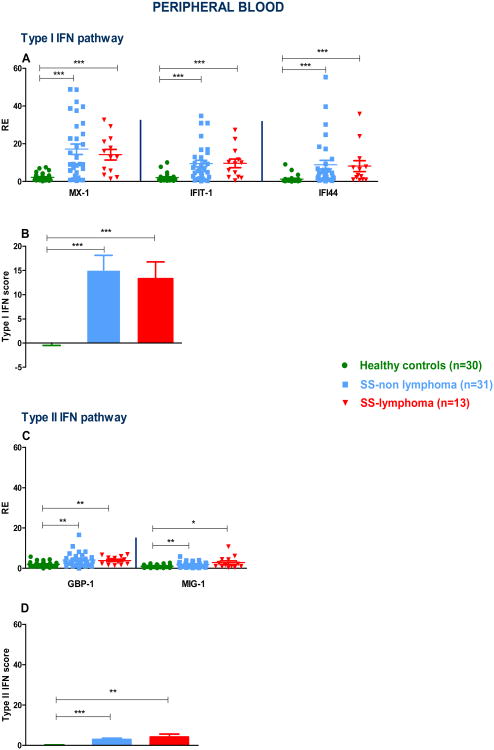
In order to explore the contribution of type I IFN in the generation of distinct clinical SS phenotypes, we measured the relative expression of genes preferentially induced by type I IFN such as MX-1, IFIT-1 and IFI44 in the PB samples from 31 primary SS-non lymphoma, 13 SS- lymphoma patients as well as 30 HC. As shown in Fig. 1A, transcript levels of those genes were significantly higher in all SS patients compared to HC, with no significant differences between the SS subgroups. In Fig. 1B, type I IFN score was found to be significantly higher in both SS subgroups complicated or not by lymphoma compared to HC (mean±SD: 2.3×10-7±2.7 versus 15.1±16.7 and 13.3±12.5, p-values: <0.0001 for both comparisons). The preferentially induced by IFNγ gene transcripts guanylate binding protein 1 (GBP-1) and chemokine (C-X-C motif) ligand 9 (CXCL9)/ Monokine induced by gamma interferon (MIG-1) were also increased in both SS subgroups compared to the HC group (Fig. 1C). Accordingly, the type II IFN score was found to be significantly increased in both SS subgroups complicated or not by lymphoma compared to HC (mean±SD: 2.9±3.6 vs 4.1±5.4 vs 5.6×10-7±1.5, p-values: 0.002 and 0.0004, respectively). No significant differences between SS subgroups were noted (Fig. 1D). When SS patients were stratified based on the presence of high or low type I and II IFN scores (see methods for definitions), 56.8% of SS patients had a high type I IFN score in accord with previous observations [13, 34] and 31.8% displayed high type II IFN scores. Interestingly, one fourth of patients showed both high type I and II IFN scores, 31.8% showed only high type I IFN score and 6.8% only high type II IFN score. Only one of the healthy individuals exhibited high type I IFN score (3.3%) and none type II IFN score (data not shown). Taken together, our data indicate that while both classes of IFN inducible genes (IFIGs) were upregulated in PB from SS individuals compared to HC, type I preferentially predominates over type II IFN signature.
Fig. 1. Type I and II interferon inducible genes (IFIGs) as well as type I and II interferon (IFN) scores in peripheral blood (PB) of Sjogren's syndrome (SS) patients and healthy controls (HC).
A. Normalized relative mRNA expression of preferentially type I IFIGs in PB samples derived from 30 HC, 31 SS-non lymphoma and 13 SS-lymphoma patients. Transcript levels of the MX-1, IFIT-1 and IFI44 genes were lower in HC compared to both SS non-lymphoma (p-value: <0.0001 for all comparisons) and SS-lymphoma groups (p-values: <0.0001, 0.0004 and 0.0002, respectively). No statistically significant differences were detected between the SS subgroups. B. PB type I IFN score was found to be significantly higher in both SS subgroups compared to HC (p-values: <0.0001 for both comparisons), with no significant differences between SS subgroups. C. Normalized relative mRNA expression of the preferentially type II IFIGs GBP-1 and MIG-1 in PB from 30 HC, 31 SS-non lymphoma and 13 SS-lymphoma patients. Transcript levels of the GBP-1 and MIG-1 genes were lower in HC compared to both SS non-lymphoma (p-values: 0.001 and 0.008, respectively) and SS-lymphoma groups (p-values: 0.003 and 0.02, respectively), with no detectable differences between SS subgroups. D. PB type II IFN score was found to be significantly increased in both SS subgroups complicated or not by lymphoma compared to HC, p-values: 0.004 and 0.0005, respectively. (*p<0.05, ** p<0.01, *** p<0.001). RE: Relative expression.
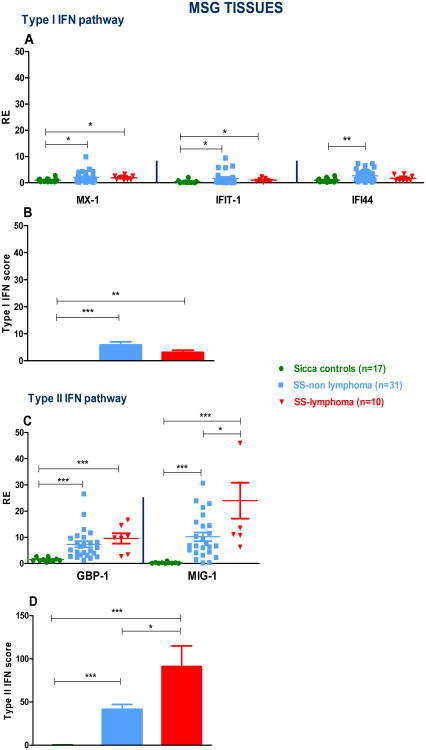
3.3 Relative mRNA expression of IFN type I and II gene signatures in MSG tissues
To investigate the role of type I and II IFN pathways at the level of tissue injury, we next measured mRNA expression of genes preferentially induced by either type I or type II IFNs in MSG biopsies derived from 31 SS-non lymphoma and 10 SS-lymphoma patients as well as 17 SC. In Fig.2A, transcripts preferentially induced by type I IFN were higher in SS patients compared to SC, while no significant differences were observed between SS subtypes. As shown in Fig. 2B, type I IFN score was significantly increased in both SS subgroups complicated or not by lymphoma compared to SC (mean±SD: 3.1±2.6 vs 5.8±6.6 vs 3.5×10-3±1.8, p-values: 0.002 and 0.0002, respectively). mRNA expression of the preferentially type II IFIGs were higher in both SS subgroups compared to SC. Of note, MIG-1 transcript levels were higher in SS-lymphoma compared to the SS-non lymphoma group (p-value:<0.05). Type II IFN score significantly differed between SC and SS subgroups as well as between SS subgroups (mean±SD: SC: 1.0×10-5±1.3, versus SS-non lymphoma: 41.5±28.2 vs SS-lymphoma: 91.2±63.5, p-values: 0.0001, <0.0001 and 0.04, respectively, Fig. 2D). Thus, an opposite pattern was observed in MSG tissues of SS patients compared to PB, with type II IFN predominating over type I IFN signature.
Fig. 2. Type I and II IFN inducible genes (IFIGs) as well as type I and II interferon (IFN) scores in minor salivary glands (MSG) from Sjogren's patients (SS) and sicca controls (SC).
A. Normalized relative mRNA expression of the preferentially type I IFIGs MX-1, IFIT-1 and IFI44 in MSG tissues from 17 SC, 31 SS-non lymphoma and 10 SS-lymphoma patients. Transcript levels of the MX-1, IFIT-1 and IFI44 genes were lower in SC compared to both SS non-lymphoma (p-value: 0.02, 0.02 and 0.005, respectively) and SS-lymphoma groups (p- values: 0.01, 0.04 and 0.07, respectively), while no statistically significant differences were observed between SS subgroups. B. Type I IFN score was significantly increased in both SS subgroups complicated or not by lymphoma compared to SC (p-values: 0.004 and 0.0003, respectively), with no significantly detectable differences between SS subgroups. C. Normalized relative mRNA expression of the preferentially type II IFIGs GBP-1 and MIG-1 in MSGs from 17 SC, 31 SS-non lymphoma and 10 SS-lymphoma patients. Transcript levels of the GBP-1 and MIG-1 genes were significantly decreased in SC vs. both SS non-lymphoma (p-values: <0.0001 for both genes) and SS-lymphoma (p-values: 0.0002 and 0.0001, respectively); of note, significantly increased MIG-1 transcript levels were observed in SS-lymphoma compared to SS-non lymphoma group (p-value: <0.05). D. Type II IFN score was found to be significantly increased in both SS subgroups complicated or not by lymphoma compared to SC (p-values: 0.0001 and <0.0001, respectively), as well as in SS-lymphoma patients compared to the SS-non lymphoma group, p-value: 0.045. (*p<0.05, **p<0.01, ***p<0.001). RE: Relative expression.
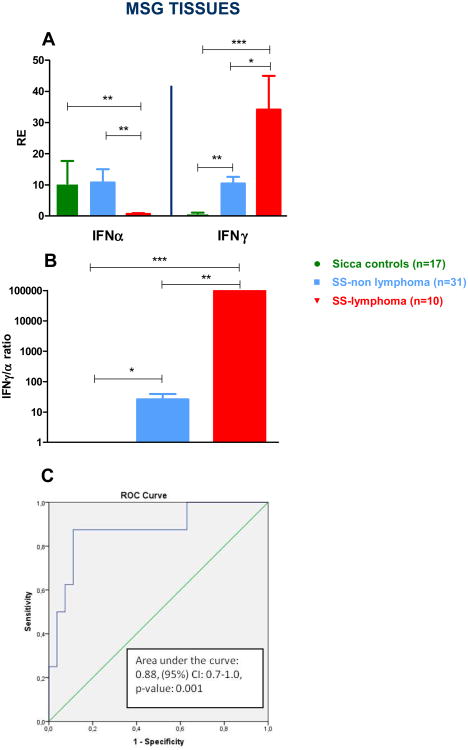
3.4 IFNγ/IFNα mRNA ratio in MSG tissues
We next sought to investigate the potential role of IFNα and IFNγ transcript levels in MSG tissues as biomarkers of malignant transformation in patients with SS. As depicted in Fig. 3A, IFNα mRNA expression was significantly reduced in SS-lymphoma cases compared to both SS-non lymphoma and SC groups (mean±SD: 0.6±0.9 versus 10.8±23.5 and 10.5±29.7, p-values: 0.003 and 0.005 respectively). On the other hand, IFNγ transcript levels were significantly increased in both SS subgroups compared to SC (mean±SD: SS-lymphoma group: 34.2±30.6 versus SS-non lymphoma: 10.5±11.0 versus SC: 0.9±0.6, p-values: <0.0001 and 0.007 respectively), as well as in the SS-lymphoma compared to the SS-non lymphoma group (p-value: 0.02). Following this observation, IFNγ/α ratio was calculated and found to be significantly higher in MSG tissues derived from SS patients complicated by lymphoma compared to both SS-non lymphoma patients and SC (mean±SD: 4.5×107±1.3×108 vs 26.3±66.9 vs 1.0±1.3, p-values: 0.002 and 0.0003, respectively). Additionally, SS-non lymphoma patients exhibited significantly higher mRNA IFNγ/α ratio compared to SC (p: 0.03). Receiver-operating characteristic analysis (ROC) showed that the IFNγ/α ratio in MSG tissues has the potential to distinguish between SS-lymphoma and SS-non lymphoma patients (area under the curve (AUC) = 0.88, p-value: 0.001, 95% CI: 0.72-1.00) (Fig. 3C). For cut-off values > 22.3 the IFNγ/α ratio displays specificity of 87.5% and sensitivity of 88.9%. Collectively, these results imply a putative role of the IFNγ/α ratio in MSG tissues as a biomarker for identifying SS patients complicated by lymphoproliferative disease at the time of performance of a diagnostic salivary gland biopsy. When ROC curves were constructed to evaluate the distinguishing ability of SS-high risk/lymphoma patients versus SS low-risk[3], the corresponding AUC value was 0.80, p-value: 0.002, 95% CI: 0.66-0.95 (data not shown).
Fig. 3. IFNγ and IFNα mRNA expression in MSG tissues.
A. Normalized relative mRNA expression of IFNα and IFNγ genes in MSG tissues from 17 SC, 31 SS-non lymphoma and 10 SS-lymphoma patients. IFNα mRNA expression was significantly reduced in SS-lymphoma cases compared to both SS-non lymphoma and SC groups (p-values: 0.003 and 0.005, respectively). On the other hand, IFNγ transcript levels were significantly increased in both SS subgroups complicated or not by lymphoma compared to SC (p-values: <0.0001 and 0.007 respectively), as well as in the SS-lymphoma compared to the SS-non lymphoma group (p-value: 0.02). B. A significantly higher IFNγ/α ratio normalized relative mRNA expression was observed in MSG tissues derived from SS patients complicated by lymphoma compared to both SS-non lymphoma patients and SC (p-values: 0.004 and 0.0007, respectively). C. Area under the receiver operating characteristic (AUC-ROC) curves for gene transcripts in SS patients at the level of MSGs tissues. AUC-ROC curves for the ratio of the IFNγ/IFNα mRNA expression discriminates the SS lymphoma group (n=10) from SS-non lymphoma patients (n=31), (AUC=0.88, 95%CI: 0.7-1.0, p-value: 0.001). * p<0.05, ** p<0.01, *** p<0.001, RE: Relative expression.
Calculation of the IFN-II/I ratio in peripheral blood cohort was also performed. No statistically significant difference between SS non lymphoma and SS lymphoma patients was detected (Fig. S1).
3.5 IFNα and IFNγ protein expression and IFNα and IFNγ producing cells in MSG tissues from SS patients complicated or non by lymphoma
IFNα expressing cells -most of which were also stained positive for BDCA-2 (a specific marker for PDCs)- were detected by immunohistochemistry in SS MSG tissues from patients uncomplicated by lymphoma (Fig.4A, B). These findings imply pDCs as a major source of IFNa in SS affected tissues. On the other hand, SS-lymphoma patients showed strong cytoplasmic IFNγ expression in both T and B cells and a weaker signal in salivary ductal cells (Fig. 4C-E).
Fig. 4. Immunohistochemical detection of IFNα, BDCA-2, IFNγ, CD3 and CD20 protein in MSG tissues from an SS patient without lymphoma (A, B) and an SS patient complicated by lymphoma (C-E).
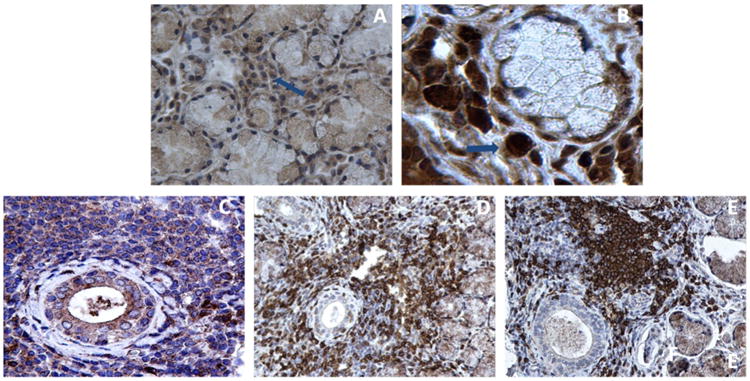
A) IFN-α stained positive cells in a non lymphoma SS patient (representative cell indicated by arrow, ×400 magnification). B) Positive BDCA-2 (a specific marker for PDCs) stained cells in the same patient (representative cell indicated by arrow, ×630 magnification). C) Positive IFN-γ staining within lymphocytic infiltrates and salivary gland epithelia (×400 magnification) in representative tissue from an SS patient complicated by lymphoma. D) CD3+ T-lymphocytes and E) CD20+ B-cells in the same patient (×400 magnification).
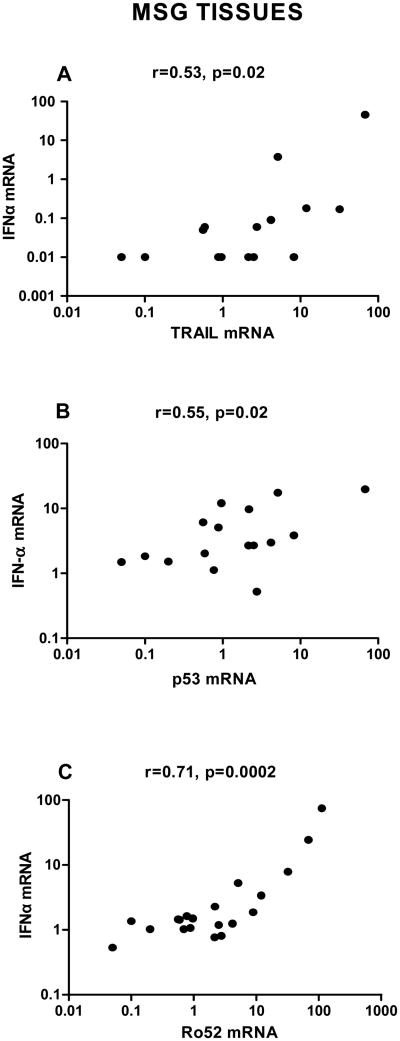
3.6 Correlation of IFNα mRNA expression with apoptotic gene transcripts at the level of MSG tissues
In order to explore potential underlying mechanisms through which reduced IFNα transcript levels in MSG tissues derived from SS patients complicated by lymphoma could predispose to malignant transformation in the setting of SS, we further measured transcript levels of previously reported IFNα related apoptotic molecules including TRAIL [35, 36]; p53, an intrinsic apoptotic inducer and gatekeeper of cancer development; and Ro52/TRIM21 -an IFNα induced, SS related, E3 ubiquitin ligase with a prominent regulatory role in inflammation as well as with anti-proliferative and pro-apoptotic properties [37, 38]. A strong positive correlation of IFNα mRNA levels with TRAIL, p53 and Ro52/TRIM21 mRNA levels (r=0.53/p:0.02, r=0.55/p:0.02 and r=0.71/p:0.0002, respectively) was detected, indicating that low IFNα levels might be related to lower expression of apoptotic/antitumor genes (Fig. 5A-C).
Fig. 5. Association of IFNα mRNA transcripts with apoptotic gene mRNA expression at the level of MSG tissues.
Relative expression of IFNα as well as TRAIL, p53 and Ro52 (TRIM21) was measured by real-time PCR in MSG tissues obtained from SS patients. Associations between IFNα mRNA and the apoptotic genes were tested using Spearman's non-parametric correlation test. IFNα transcript levels were positively correlated with the mRNA transcripts of the pro-apoptotic genes TRAIL and p53 (r=0.53, p-value: 0.02 and r=0.55, p-value: 0.002, respectively, panels A and B), as well as the mRNA transcripts of the Ro52 (TRIM21) (r=0.71/p=0.0002, panel C) at the level of MSG tissues. RE: Relative expression.
3.7 Association of type I and II IFN scores in PB and MSG tissues with B cell activating factor levels and disease characteristics
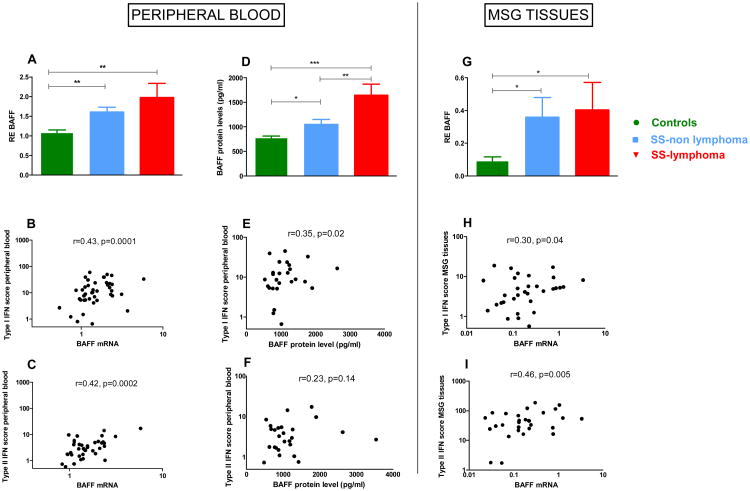
Given that B cell activating factor (BAFF) has been previously shown to be a critical cytokine for SS development with dual regulation by both type I and type II IFNs [13, 39-41], we next evaluated both BAFF mRNA expression in whole PB, MSG tissues as well as serum protein levels in our study participants. Both type I and II IFN scores were significantly associated with PB and MSG BAFF transcript levels (Fig. 6 panels B-C and H-I), while weaker associations were depicted between peripheral type I IFN score and serum BAFF levels (Fig. 6E).
Fig.6. Association of type I and II IFN scores with B cell activating Factor (BAFF) mRNA expression and serum protein levels.
A. Normalized relative mRNA expression of the BAFF gene at the level of peripheral blood samples from 30 HC, 31 SS-non lymphoma and 13 SS-lymphoma patients. BAFF transcript levels were significantly increased in both SS subgroups complicated or non by lymphoma compared to HC (mean±SD: 1.97±1.32 vs 1.61±0.70 vs1.09±0.36, p-values: 0.005 and 0.002, respectively), with no significantly detectable difference between SS subgroups B. Peripheral blood BAFF mRNA transcripts were positively correlated with type I IFN score in SS patients (r=0.43, p=0.0001). C.. Peripheral blood BAFF mRNA transcripts were also positively correlated with type II IFN score in SS patients (r=0.44, p=0.0002). D. BAFF protein levels in serum samples from 24 HC, 62 SS-non lymphoma and 23 SS-lymphoma patients. SS-lymphoma patients had higher BAFF serum levels compared to both SS-non lymphoma and HC (mean ±SD: 1648.00±1178.00 pg/ml versus 1052.00±793.20 pg/ml versus 781.5±147.5 pg/ml, p-values: 0.002 and <0.0001, respectively), while SS-non lymphoma patients showed also increased serum BAFF levels compared to HC (p=0.03). E-F. A relatively weak correlation was observed between BAFF serum protein levels with peripheral blood type I IFN score (r=0.30, p-value=0.04), while type II IFN score was not correlated with BAFF serum levels in SS patients. G. BAFF normalized relative mRNA expression in MSG tissues from 17 SC, 31 SS-non lymphoma and 10 SS-lymphoma patients. BAFF mRNA levels were found to be increased in both SS subgroups complicated or not by lymphoma compared to SC (p-value: 0.02, for both comparisons). H-I. BAFF mRNA transcripts in MSG tissues were found to positively correlate with both type I and II IFN MSG tissue scores (r=0.3/ p-value =0.04 and r=0.46/ p-value=0.005, respectively). *p<0.05, **p<0.01, ***p<0.001, RE: Relative expression
In order to identify potential associations of type I and II IFN signatures with disease characteristics, all SS patients were stratified according to the presence or absence of common SS related clinical and serological features. SS patients with salivary gland enlargement (SGE), positive anti-Ro/SSA titers and lymphopenia exhibited increased peripheral type I IFN scores, while patients with hypergammaglobulinemia had both higher type I and II IFN scores (Fig. S2 panels A-D). In MSG tissues, both type I IFN score and IFNα transcripts were higher in patients with arthralgias (Fig. S3A and Fig. S4A), while IFNγ mRNA levels were increased in patients with Raynaud's phenomenon, purpura and low C4 levels (Fig. S4 panels B, D). No other significant associations were detected.
4. Discussion
The aim of the current study was to clarify the contributory role of both type I and II IFN signatures in the generation of SS clinical phenotypes, including lymphoma development. The first observation was the predominance of the type I IFN signature in PB of SS patients in contrast to type II IFN signature in SS MSG biopsies. Second, the concomitant presence of low IFNα and high IFNγ mRNA in MSG tissues is strongly associated with lymphomagenesis in the setting of SS. In this context, IFNγ/α mRNA ratio in MSG diagnostic biopsies could serve as a putative novel tissue biomarker in the diagnosis of salivary extranodal SS-related NHL lymphoma.
The type I IFN signature has been proposed over the last decade as a central contributor in many autoimmune diseases including lupus, dermatomyositis, autoimmune thyroiditis (ATD) and SS [14, 42-44]. In accord with previous reports, we also confirm the presence of a prominent type I IFN signature in PB in more than half of the SS population in association with lymphopenia and markers of B-cell overeactivity, such as anti-Ro/SSA antibodies and hypergammaglobulinemia [13]. The latter can be attributed to type I IFN- induced BAFF overproduction, as suggested by the association detected between peripheral type I IFN activity or signature and BAFF at both mRNA[17] [13] and -to a lesser extent- protein level. The latter association has not been previously detected [13].
At the MSG tissue level, IFNα transcript levels per se were remarkably reduced in patients complicated by NHL lymphoma compared to both uncomplicated SS patients and SC. On the other hand, type I IFIGs were found to be upregulated in all SS patients studied, as previously suggested in MSG tissue microarray studies, though to a much lesser extent compared to the periphery [11, 15]. This apparent discrepancy might be related to the increased number of infiltrating cells seen in salivary gland tissues derived from both SS patient groups compared to SC. Given the previously reported antitumor activities of IFNα -besides its major antiviral role-we next aimed to identify potential operating mechanisms contributing to SS-related lymphomagenesis. IFNα has been widely used as an antineoplastic agent [45], mainly through pro-apoptotic effects on several tumor cells, including myeloma, glioma and melanoma cells [46], and potentiation of natural killer (NK) cell cytotoxicity through induction of the Fas/FasL pathway [47]. The tumor suppressor gene p53 [36], the extrinsic apoptotic molecule TRAIL [35] and the Ro52 autoantigen –a major SS autoantigen known to negatively regulate the anti-apoptotic protein Bcl-2- [38] have been previously shown to be induced by IFNα, possibly accounting for its antiproliferative properties. In line with these observations, IFNα transcript levels at the level of SS MSG tissues in our study were strongly correlated with mRNA expression of pro-apoptotic molecules TRAIL, p53 and Ro52, indicating suppressed IFNα as an important contributor to the survival of malignant B-cell populations in the setting of SS.
When we compared IFNα mRNA expression between the non-lymphoma SS group and SC, no significant difference was observed; this was attributed to the inclusion among the SC of lupus and ATD patients, who demonstrated heightened IFNα transcript levels in their MSG tissues in the absence of lymphocytic infiltrates (data not shown). Recruitment of PDCs -the main IFNα producers- to MSG tissues of autoimmune patients with sicca symptomatology remains a possibility which needs to be further explored.
When we sought to identify potential associations between type I IFN score as well as IFNα mRNA expression in MSG tissues with SS-related clinical and serological features, patients with arthralgias were found to exhibit both significantly higher IFNα transcript levels and type I IFN score, implying a potential contributory role of this cytokine in the generation of musculoskeletal manifestations. In support of this observation, administration of hydroxychloroquine -an inhibitor of IFNα production by PDCs through alterations of endodomal function- is beneficial in the management of musculoskeletal complaints in the setting of both lupus and SS [48] [49].
While microarray gene expression data from PB of SS patients revealed a prominent type I IFN signature [13, 16], MSG tissue-derived data revealed the presence of both type I and II IFN signatures with a predilection towards IFNγ-related genes [11, 19, 30]. In the present report, we have also demonstrated the predominance of a type I IFN signature in the periphery together with a much less prominent upregulation of type II IFIGs compared to healthy individuals (approximately 2-fold, which might be undetectable in microarray gene expression studies). This peripheral type II IFN signature was also found to be associated with the presence of hypergammaglobulinemia and BAFF mRNA levels, in accord with the dual regulation of BAFF by both types of IFNs [17, 40, 41].
On the other hand and in accord with previously published data, upregulation of both type II IFIGs and IFNγ per se was detected in SS MSG tissues compared to those derived from SC, with a clear predominance compared to type I IFN signature. Moreover, both type I IFN score and IFNγ transcript levels were remarkably increased in MSG tissues derived from SS-lymphoma patients compared to both SS-non lymphoma and SC, implying a direct role of IFNγ in SS-related lymphomagenesis. These findings are in line with recently reported observations by Hall et al, according to which type II IFN signatures have been found to be associated with higher focus scores [28], recently suggested as marker for lymphoma development[50]. Accordingly, IFNγ transcript levels were significantly increased in patients sharing previously described adverse predictors for lymphoma development such as purpura and low C4 levels [3] as well as with Raynaud's phenomenon, previously associated with increased number of IFN-γ-secreting peripheral mononuclear cells [51].
T-helper 1 cytokines, including IFNγ, have been previously associated with more severe forms of SS, characterized by high grade infiltration of lymphocytes, macrophages and dendritic cells [4, 52] as well as with gastric NHL lymphomas arisen in a background of Helicobacter pylori-induced chronic inflammation [53, 54]. NK cells -another potential source of IFNγ at the level of SS MSG tissue- have been recently shown to correlate with heavier lymphocytic infiltration in the inflamed salivary gland SS tissue [55], while B-cell derived IFNγ has been shown to promote gastric lymphoid follicle formation in the setting of Helicobacter suis-induced gastric lymphoma [56]. Our immunohistochemical data support B and T cells as important contributors to the IFNγ related signature locally, at the level of salivary gland tissues.
However, our findings should be interpreted in the context of potential limitations. First, the relatively small number of SS patients in our PB and MSG tissues cohorts. Second, the use of independent PB and MSG cohorts, with only 5 shared SS patients. Finally, the sicca control group in our MSG cohort included patients with other than SS underlying autoimmune disease, due to the difficulty of obtaining MSG tissues from healthy volunteers. The need for independent validation of our findings through multicentric efforts is well recognized, though SS complicated by lymphoma is rather rare in the general population. On the other hand, the complete and careful clinical, serological, histopatholological characteristics of our study participants and the similar age and sex distribution across groups are of highly importance.
An interesting observation in our study was the unique pattern of increasing IFNγ and decreasing IFNα with disease severity, with the SS-lymphoma group showing the highest IFNγ expression (as well as type II IFN signature) and the lowest IFNα expression (as well as type I signature, though non significant). As a result, the ratio of the mRNA expression of IFNγ/IFNα at the level of diagnostic salivary gland biopsy could serve as a putative histological biomarker for differentiating benign from malignant SS lesions in clinical practice. Although the underlying mechanisms leading to lymphomagenesis remain unclear, we postulate that the reduced levels of the antiproliferative IFNα in a context of IFNγ-related chronic inflammation could be a major driver of malignant transformation in the setting of SS. On the other hand, it has been been suggested that activation of NFkB signaling pathways -recently shown to be activated in SS- [10], leads to decreased IFNα levels through proteasomal degradation of Interleukin-1 receptor-associated kinase 1 (IRAK1) impairing phosphorylation of the interferon regulatory factor 7 (IRF7), an essential transcription factor for IFNα production [57].
5. Conclusions
Taken together, in the current report, we attempted to clarify the contribution of both type I and II IFN signatures in generation of distinct clinical phenotypes of SS, including lymphoma development. Additionally, we propose IFNγ/α ratio as a potential novel -easy to use biomarker- which allows the distinction between SS benign histopathological lesions from in situ lymphoma development.
Supplementary Material
Caption for Suppl. Fig.1 (S1): No statistically significant difference in IFN II/IFN I score between SS non lymphoma and SS lymphoma patients was detected.
Caption for Suppl. Fig.2 (S2): Peripheral blood type I and II Interferon (IFN) scores according to the presence (+) or absence (-) of SS-related clinical and serological parameters. A-C. SS patients with salivary gland enlargement (SGE) showed significantly increased type I IFN score (mean±SD: 23.4±18.7 versus 10.1±12.0, p-value: 0.02), as well as anti-Ro/SSA positive patients (mean±SD: 16.9±16.4 versus 5.0±7.2.2, p-value <0.05), patients with elevated γ globulins (mean±SD: 16.9±14.2 versus 8.4±13.8, p-value: 0.04) and patients with lymphopenia (mean±SD: 25.6±17.1 versus 12.1±14.2, p-value: 0.04). B-D. Significantly higher type II IFN score was found only among patients with elevated γ globulins (mean±SD: 4.0±4.3 versus 1.7±4.3, p-value: 0.04). * p<0.05, RE: Relative expression.
Caption for Suppl. Fig.3 (S3): Minor Salivary Gland (MSG) tissue type I and II IFN scores according to the presence (+) or absence (-) of SS-related clinical and serological parameters. A-C. Higher type I IFN score was observed in patients with arthralgias (mean±SD: 6.4±5.3 versus 1.7±2.8, p-value: 0.03). B-D. Higher type II IFN score was only found in patients with dry eye symptoms (mean±SD: 66.8±42.6 versus 30.2±15.8, p-value: 0.03). * p<0.05, RE: Relative expression.
Caption for Suppl. Fig.4 (S4): IFNα and IFNγ mRNA expression in MSG tissues according to the presence (+) or absence (-) of SS-related clinical and serological parameters. A-C. Higher IFNα transcript levels were found in patients with arthralgias (mean±SD: 3.8±8.2 versus 0.3± 0.6, p-value: 0.003). B-D. Significantly increased IFNγ transcript levels were detected in patients with Raynaud's phenomenon (mean±SD: 40.4±27.4 versus 11.5±11.0, p-value <0.05), purpura (mean±SD: 30.9±22.9 versus 14.3±20.4, p-value <0.05) and low C4 levels (mean±SD: 22.8±19.4 versus 13.9±25.5, p-value: 0.04). * p<0.05, ** p<0.01, RE: Relative expression.
Supplementary Table 1: Specific genes and primers sequence for the gene expression analysis.
Highlights.
Type I IFN signature predominates in the peripheral blood of primary SS patients.
Type II IFN signature prevails in minor salivary gland tissues of primary SS patients.
IFNγ/α mRNA ratio in MSG biopsies can serve as a biomarker of prediction of in situ SS-related lymphoma.
Type I and II IFN signatures were related to distinct SS clinical/serological phenotypes.
Acknowledgments
We would like to thank Prof. HM Moutsopoulos for providing minor salivary gland tissues from SS patients and for his invaluable comments and suggestions.
Funding: This study was supported by a Stavros Niarchos Fellowship grant through the Arthritis Foundation, New York Chapter and an Hellenic Rheumatology Association grant to CPM; a Stavros Niarchos Foundation Research Grant to Department of Physiology, University of Athens; and a NIH R01AI059893, a novel research grant from the Lupus Research Institute, a Target Identification in Lupus grant from the Alliance for Lupus Research, and the Mary Kirkland Center for Lupus Research to MKC.
Footnotes
Competing interests: MKC has served as a consultant for Glaxo Smith-Kline, MedImmune, and Roche-Genentech.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mavragani CP, Moutsopoulos HM. Sjogren's syndrome. Annual review of pathology. 2014;9:273–85. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 2.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Archives of internal medicine. 2005;165:2337–44. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 3.Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren's syndrome. Arthritis Rheum. 2002;46:741–7. doi: 10.1002/art.10221. [DOI] [PubMed] [Google Scholar]
- 4.Manoussakis MN, Boiu S, Korkolopoulou P, Kapsogeorgou EK, Kavantzas N, Ziakas P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjogren's syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–88. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- 5.Theander E, Vasaitis L, Baecklund E, Nordmark G, Warfvinge G, Liedholm R, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjogren's syndrome. Annals of the rheumatic diseases. 2011;70:1363–8. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapinos NI, Polihronis M, Moutsopoulos HM. Lymphoma development in Sjogren's syndrome: novel p53 mutations. Arthritis Rheum. 1999;42:1466–72. doi: 10.1002/1529-0131(199907)42:7<1466::AID-ANR21>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Pisa EK, Pisa P, Kang HI, Fox RI. High frequency of t(14;18) translocation in salivary gland lymphomas from Sjogren's syndrome patients. J Exp Med. 1991;174:1245–50. doi: 10.1084/jem.174.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nezos A, Papageorgiou A, Fragoulis G, Ioakeimidis D, Koutsilieris M, Tzioufas AG, et al. B-cell activating factor genetic variants in lymphomagenesis associated with primary Sjogren's syndrome. J Autoimmun. 2014;51:89–98. doi: 10.1016/j.jaut.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Nocturne G, Boudaoud S, Miceli-Richard C, Viengchareun S, Lazure T, Nititham J, et al. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren's syndrome. Blood. 2013;122:4068–76. doi: 10.1182/blood-2013-05-503383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papageorgiou A, Mavragani C, Nezos A, Zintzaras E, Quartuccio L, De Vita S, et al. A B-cell activating factor receptor (BAFF-R) His159Tyr mutation in Sjögren's Syndrome related lymphoproliferation. Arthritis Rheum. 2015 doi: 10.1002/art.39231. in press. [DOI] [PubMed] [Google Scholar]
- 11.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2770–5. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vakaloglou KM, Mavragani CP. Activation of the type I interferon pathway in primary Sjogren's syndrome: an update. Current opinion in rheumatology. 2011;23:459–64. doi: 10.1097/BOR.0b013e328349fd30. [DOI] [PubMed] [Google Scholar]
- 13.Brkic Z, Maria NI, van Helden-Meeuwsen CG, van de Merwe JP, van Daele PL, Dalm VA, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren's syndrome and association with disease activity and BAFF gene expression. Annals of the rheumatic diseases. 2013;72:728–35. doi: 10.1136/annrheumdis-2012-201381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavragani CP, Crow MK. Activation of the type I interferon pathway in primary Sjogren's syndrome. Journal of autoimmunity. 2010;35:225–31. doi: 10.1016/j.jaut.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534–44. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 16.Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, et al. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes Immun. 2009;10:285–96. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren's syndrome treated with etanercept. Arthritis Rheum. 2007;56:3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildenberg ME, van Helden-Meeuwsen CG, van de Merwe JP, Drexhage HA, Versnel MA. Systemic increase in type I interferon activity in Sjogren's syndrome: a putative role for plasmacytoid dendritic cells. European journal of immunology. 2008;38:2024–33. doi: 10.1002/eji.200738008. [DOI] [PubMed] [Google Scholar]
- 19.Hall JC, Casciola-Rosen L, Berger AE, Kapsogeorgou EK, Cheadle C, Tzioufas AG, et al. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci U S A. 2012;109:17609–14. doi: 10.1073/pnas.1209724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertzog P, Forster S, Samarajiwa S. Systems biology of interferon responses. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2011;31:5–11. doi: 10.1089/jir.2010.0126. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren's syndrome. Arthritis Rheum. 2002;46:2730–41. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 22.Ronnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Current opinion in rheumatology. 2013;25:248–53. doi: 10.1097/BOR.0b013e32835c7e32. [DOI] [PubMed] [Google Scholar]
- 23.Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U, et al. A dual role for interferon-gamma in the pathogenesis of Sjogren's syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60:552–65. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen CQ, Peck AB. The Interferon-Signature of Sjogren's Syndrome: How Unique Biomarkers Can Identify Underlying Inflammatory and Immunopathological Mechanisms of Specific Diseases. Front Immunol. 2013;4:142. doi: 10.3389/fimmu.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-gamma and systemic autoimmunity. Discovery medicine. 2013;16:123–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine & growth factor reviews. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–50. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall JC, Baer AN, Shah AA, Criswell LA, Shiboski CH, Rosen A, et al. Arthritis & rheumatology. Hoboken, NJ: 2015. Molecular subsetting of interferon pathways in Sjogren's syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavragani CP, Moutsopoulos HM. Sjogren syndrome. Cmaj. 2014 doi: 10.1503/cmaj.122037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakamatsu E, Nakamura Y, Matsumoto I, Goto D, Ito S, Tsutsumi A, et al. DNA microarray analysis of labial salivary glands of patients with Sjogren's syndrome. Annals of the rheumatic diseases. 2007;66:844–5. doi: 10.1136/ard.2006.063370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Annals of the rheumatic diseases. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–16. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 33.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–67. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 34.Maria NI, Brkic Z, Waris M, van Helden-Meeuwsen CG, Heezen K, van de Merwe JP, et al. MxA as a clinically applicable biomarker for identifying systemic interferon type I in primary Sjogren's syndrome. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar-Sinha C, Varambally S, Sreekumar A, Chinnaiyan AM. Molecular cross-talk between the TRAIL and interferon signaling pathways. J Biol Chem. 2002;277:575–85. doi: 10.1074/jbc.M107795200. [DOI] [PubMed] [Google Scholar]
- 36.Porta C, Hadj-Slimane R, Nejmeddine M, Pampin M, Tovey MG, Espert L, et al. Interferons alpha and gamma induce p53-dependent and p53-independent apoptosis, respectively. Oncogene. 2005;24:605–15. doi: 10.1038/sj.onc.1208204. [DOI] [PubMed] [Google Scholar]
- 37.Strandberg L, Ambrosi A, Espinosa A, Ottosson L, Eloranta ML, Zhou W, et al. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies. Journal of clinical immunology. 2008;28:220–31. doi: 10.1007/s10875-007-9157-0. [DOI] [PubMed] [Google Scholar]
- 38.Jauharoh SN, Saegusa J, Sugimoto T, Ardianto B, Kasagi S, Sugiyama D, et al. SS-A/Ro52 promotes apoptosis by regulating Bcl-2 production. Biochemical and biophysical research communications. 2012;417:582–7. doi: 10.1016/j.bbrc.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Mariette X, Roux S, Zhang J, Bengoufa D, Lavie F, Zhou T, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren's syndrome. Ann Rheum Dis. 2003;62:168–71. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavie F, Miceli-Richard C, Ittah M, Sellam J, Gottenberg JE, Mariette X. B-cell activating factor of the tumour necrosis factor family expression in blood monocytes and T cells from patients with primary Sjogren's syndrome. Scandinavian journal of immunology. 2008;67:185–92. doi: 10.1111/j.1365-3083.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 42.Kirou KA, Mavragani CP, Crow MK. Activation of type I interferon in systemic lupus erythematosus. Expert Rev Clin Immunol. 2007;3:579–88. doi: 10.1586/1744666X.3.4.579. [DOI] [PubMed] [Google Scholar]
- 43.Mavragani CP, Niewold TB, Chatzigeorgiou A, Danielides S, Thomas D, Kirou KA, et al. Increased serum type I interferon activity in organ-specific autoimmune disorders: clinical, imaging, and serological associations. Front Immunol. 2013;4:238. doi: 10.3389/fimmu.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dastmalchi M, Grundtman C, Alexanderson H, Mavragani CP, Einarsdottir H, Helmers SB, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Annals of the rheumatic diseases. 2008;67:1670–7. doi: 10.1136/ard.2007.077974. [DOI] [PubMed] [Google Scholar]
- 45.Markowitz J, Luedke EA, Grignol VP, Hade EM, Paul BK, Mundy-Bosse BL, et al. A phase I trial of bortezomib and interferon-alpha-2b in metastatic melanoma. Journal of immunotherapy. 2014;37:55–62. doi: 10.1097/CJI.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thyrell L, Erickson S, Zhivotovsky B, Pokrovskaja K, Sangfelt O, Castro J, et al. Mechanisms of Interferon-alpha induced apoptosis in malignant cells. Oncogene. 2002;21:1251–62. doi: 10.1038/sj.onc.1205179. [DOI] [PubMed] [Google Scholar]
- 47.Kirou KA, Vakkalanka RK, Butler MJ, Crow MK. Induction of Fas ligand-mediated apoptosis by interferon-alpha. Clin Immunol. 2000;95:218–26. doi: 10.1006/clim.2000.4866. [DOI] [PubMed] [Google Scholar]
- 48.Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R155. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manoussakis MN, Moutsopoulos HM. Antimalarials in Sjogren's syndrome--the Greek experience. Lupus. 1996;5(Suppl 1):S28–30. [PubMed] [Google Scholar]
- 50.Carubbi F, Alunno A, Cipriani P, Bartoloni E, Baldini C, Quartuccio L, et al. A retrospective, multicenter study evaluating the prognostic value of minor salivary gland histology in a large cohort of patients with primary Sjogren's syndrome. Lupus. 2015;24:315–20. doi: 10.1177/0961203314554251. [DOI] [PubMed] [Google Scholar]
- 51.Willeke P, Schluter B, Schotte H, Domschke W, Gaubitz M, Becker H. Interferon-gamma is increased in patients with primary Sjogren's syndrome and Raynaud's phenomenon. Semin Arthritis Rheum. 2009;39:197–202. doi: 10.1016/j.semarthrit.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, et al. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–8. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riedel S, Kraft M, Kucharzik T, Pauels HG, Tiemann M, Steinbuchel A, et al. CD4+ Th1-cells predominate in low-grade B-cell lymphoma of gastric mucosa-associated lymphoid tissue (MALT type) Scand J Gastroenterol. 2001;36:1198–203. doi: 10.1080/00365520152584842. [DOI] [PubMed] [Google Scholar]
- 54.Hauer AC, Finn TM, MacDonald TT, Spencer J, Isaacson PG. Analysis of TH1 and TH2 cytokine production in low grade B cell gastric MALT-type lymphomas stimulated in vitro with Helicobacter pylori. J Clin Pathol. 1997;50:957–9. doi: 10.1136/jcp.50.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rusakiewicz S, Nocturne G, Lazure T, Semeraro M, Flament C, Caillat-Zucman S, et al. NCR3/NKp30 contributes to pathogenesis in primary Sjogren's syndrome. Science translational medicine. 2013;5:195ra96. doi: 10.1126/scitranslmed.3005727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Yamamoto K, Nishiumi S, Nakamura M, Matsui H, Takahashi S, et al. Interferon-gamma-producing B cells induce the formation of gastric lymphoid follicles after Helicobacter suis infection. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.66. [DOI] [PubMed] [Google Scholar]
- 57.Kubo-Murai M, Hazeki K, Nigorikawa K, Omoto T, Inoue N, Hazeki O. IRAK-4-dependent degradation of IRAK-1 is a negative feedback signal for TLR-mediated NF-kappaB activation. J Biochem. 2008;143:295–302. doi: 10.1093/jb/mvm234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Caption for Suppl. Fig.1 (S1): No statistically significant difference in IFN II/IFN I score between SS non lymphoma and SS lymphoma patients was detected.
Caption for Suppl. Fig.2 (S2): Peripheral blood type I and II Interferon (IFN) scores according to the presence (+) or absence (-) of SS-related clinical and serological parameters. A-C. SS patients with salivary gland enlargement (SGE) showed significantly increased type I IFN score (mean±SD: 23.4±18.7 versus 10.1±12.0, p-value: 0.02), as well as anti-Ro/SSA positive patients (mean±SD: 16.9±16.4 versus 5.0±7.2.2, p-value <0.05), patients with elevated γ globulins (mean±SD: 16.9±14.2 versus 8.4±13.8, p-value: 0.04) and patients with lymphopenia (mean±SD: 25.6±17.1 versus 12.1±14.2, p-value: 0.04). B-D. Significantly higher type II IFN score was found only among patients with elevated γ globulins (mean±SD: 4.0±4.3 versus 1.7±4.3, p-value: 0.04). * p<0.05, RE: Relative expression.
Caption for Suppl. Fig.3 (S3): Minor Salivary Gland (MSG) tissue type I and II IFN scores according to the presence (+) or absence (-) of SS-related clinical and serological parameters. A-C. Higher type I IFN score was observed in patients with arthralgias (mean±SD: 6.4±5.3 versus 1.7±2.8, p-value: 0.03). B-D. Higher type II IFN score was only found in patients with dry eye symptoms (mean±SD: 66.8±42.6 versus 30.2±15.8, p-value: 0.03). * p<0.05, RE: Relative expression.
Caption for Suppl. Fig.4 (S4): IFNα and IFNγ mRNA expression in MSG tissues according to the presence (+) or absence (-) of SS-related clinical and serological parameters. A-C. Higher IFNα transcript levels were found in patients with arthralgias (mean±SD: 3.8±8.2 versus 0.3± 0.6, p-value: 0.003). B-D. Significantly increased IFNγ transcript levels were detected in patients with Raynaud's phenomenon (mean±SD: 40.4±27.4 versus 11.5±11.0, p-value <0.05), purpura (mean±SD: 30.9±22.9 versus 14.3±20.4, p-value <0.05) and low C4 levels (mean±SD: 22.8±19.4 versus 13.9±25.5, p-value: 0.04). * p<0.05, ** p<0.01, RE: Relative expression.
Supplementary Table 1: Specific genes and primers sequence for the gene expression analysis.