Abstract
Here we examine overlap between tactile and visual motion BOLD responses within the human MT+ complex. Although several studies have reported tactile responses overlapping with hMT+, many used group average analyses, leaving it unclear whether these responses were restricted to sub-regions of hMT+. Moreover, previous studies either employed a tactile task or passive stimulation, leaving it unclear whether or not tactile responses in hMT+ are simply the consequence of visual imagery. Here we carried out a replication of one of the classic papers finding tactile responses in hMT+ (Hagen et al. 2002). We mapped MT and MST in individual subjects using visual field localizers. We then examined responses to tactile motion on the arm, either presented passively or in the presence of a visual task performed at fixation designed to minimize visualization of the concurrent tactile stimulation. To our surprise, without a visual task, we found only weak tactile motion responses in MT (6% of voxels showing tactile responses) and MST (2% of voxels). With an unrelated visual task designed to withdraw attention from the tactile modality, responses in MST reduced to almost nothing (<1% regions). Consistent with previous results, we did observe tactile responses in STS regions superior and anterior to hMT+. Despite the lack of individual overlap, group averaged responses produced strong spurious overlap between tactile and visual motion responses within hMT+ that resembled those observed in previous studies. The weak nature of tactile responses in hMT+ (and their abolition by withdrawal of attention) suggests that hMT+ may not serve as a supramodal motion processing module.
Keywords: multisensory, cross-modal, multimodal, fMRI, attention
Introduction
Although human motion-sensitive middle temporal cortex (hMT+ complex) is traditionally thought of as visual cortex, in recent years the human neuroimaging literature has presented several studies suggesting tactile responses (Hagen et al. 2002; Blake et al. 2004; Beauchamp et al. 2007; Ricciardi et al. 2007; Summers et al. 2009; Matteau et al. 2010; Sani et al. 2010) selective for the direction of tactile motion (van Kemenade et al. 2014) in hMT+ within sighted subjects, as well as disruption of tactile processing with rTMS inhibition over the expected site of hMT+ (Ricciardi et al. 2011), even for passive tactile stimulation. This has led to the suggestion that regions within hMT+ may be supramodal, processing motion regardless of the sensory modality in which it is presented (Pascual-Leone and Hamilton 2001; Ricciardi et al. 2014).
Here we carried out a replication of one of the most influential papers showing tactile responses with hMT+, that of Hagen et al. (Hagen et al. 2002). This paper is often cited as evidence that in most normally sighted individuals hMT+ responds to passive tactile stimulation. Within this replication, we were particularly interested in examining three aspects of these tactile responses: (1) the extent of overlap between tactile responses and individually localized hMT+, (2) the location of responses within hMT+ with respect to MT, and (3) in an extension of the original design we examined whether responses to tactile stimulation near hMT+ would survive withdrawal of attention.
We were interested in examining how clearly tactile motion responses overlapped with hMT+ because many (though not all, see Discussion) of the studies cited above relied on stereotactic coordinates (Matteau et al. 2010; Wacker et al. 2011), or group averaged data localizers (Ricciardi et al. 2007; Summers et al. 2009; Sani et al. 2010) to identify the location of hMT+. However the expected location of hMT+ varies widely across individuals (Watson et al. 1993; Dumoulin et al. 2000; Huk et al. 2002). Thus in some studies tactile motion responses from either MST (Beauchamp et al. 2007), satellite regions of hMT+ or nearby polysensory areas (Beauchamp et al. 2008) might easily have contribute to group averaged responses attributed to MT proper.
We were interested in the location of tactile responses with respect to subregions of the hMT+ complex and the superior temporal sulcus because temporal cortex contains a number of regions sensitive to visual motion including MT, ventral and dorsal MST, and more anteriorly, regions in the lower superior (LST) and the fundus of the superior temporal (FST) sulcus. These motion areas are thought to differ in their patterns of functional selectivity and role. A large literature suggests that neurons in MT are restricted to the contralateral visual field and respond to relatively simple as well as more complex motions. In contrast, neurons in MST tend to represent more complex optic flow patterns, and respond to ipsilateral as well as contralateral stimuli. There are also a number of more anterior regions that respond to object centered (FST/LST) and action-related (FST/LST/STPm) motions (Tanaka et al. 1993; Nelissen et al. 2006). These areas, like MST, respond to ipsilateral as well as contralateral stimulation (Tanaka et al. 1986). By using a visual motion stimulus that contained translational moving dots 10° from fixation either in the left or right visual field (based on Huk et al. 2002) our goal was to isolate MT from other regions of the hMT+ complex, and thereby examine whether tactile motion responses would be found in MT itself.
Finally, we were interested in whether tactile responses in hMT+ or the superior temporal sulcus would survive the withdrawal of visual attention from the tactile stimulus. There is now a converging body of literature showing that implicit motion within non-moving stimuli such as static pictures of moving objects (David and Senior 2000; Kourtzi and Kanwisher 2000; Senior et al. 2000) or sentences about moving stimuli (Saygin et al. 2010) are sufficient to elicit BOLD responses in hMT+. Given these findings, it seems plausible that tactile responses within hMT+ elicited by tactile stimuli might similarly be the result of ‘implicit’ motion rather than directly being driven by tactile stimulation. To examine this, we compared tactile motion responses in hMT+ and the superior temporal sulcus when subjects were either blindfolded, or carried out an attentionally demanding fixation task that consisted of a modified version of the memory game ‘Simon©’.
The Hagen et al. study used 9 subjects and found tactile responses within hMT+ in the majority of subjects. Because our goal was to very accurately localize hMT+ within each individual, we modified the Hagen et al. design slightly and chose protocol that focused on a small (5) number of individuals with high experimental power within each individual. We therefore collected out four sessions of data (2 sessions identifying the location of hMT+/MT/MST, and 2 sessions collecting tactile responses) on 5 individuals.
Methods
Participants
Participants were 5 young right handed individuals with normal vision (3 males; 27 ± 3.2 years old). All participants reported normal hearing and no history of psychiatric illness. Written and informed consent was obtained from all participants prior to the experiment, following procedures approved by the University of Washington.
MRI scanning
Scanning was performed at the DISC Center at the University of Washington with a 3T Philips Achieva system equipped with 32 channel SENSE head coil. Three-dimensional (3D) anatomical images were acquired at 1×1×1mm resolution using a T1-weighted MPRAGE (magnetization-prepared rapid gradient echo) sequence. Blood oxygenation-level dependent (BOLD) functional scans were acquired with following common parameters: 2.75 × 2.75 × 3 mm voxels; flip angle = 76°; field of view = 220 × 220. A continuous block design was used for all functional scans: a repetition time (TR) of 2s was used to acquire 30 transverse slices (TE 30ms).
Tactile stimulation
Tactile motion, no visual task
Following Hagen et al. (2002), we used a tactile motion stimulus consisting of a brush stroked proximal-to-distal along the right or the left volar forearm (Figure 1A). A goat hair oval mop brush (3/4 inch) was manually applied with a velocity of 6-8 cm/s over 30-40 cm of skin. Each stroke took about 5s, with successive strokes separated by a delay of 1s. Participants were blindfolded and were instructed to pay attention to the brush. Each tactile block lasted 24s, during which a total of four strokes were applied. Tactile blocks were separated by a 12s resting block, during which participants were presented with no strokes. Through headphones, the experimenter received beep instructions indicating the beginning and ending of stroking and resting periods. Right and left arms were stimulated in separate scans. Every participant performed four scans, two for each arm. Each scan lasted 6 min, and included 10 24s tactile blocks and 10 12s resting blocks.
Figure 1.
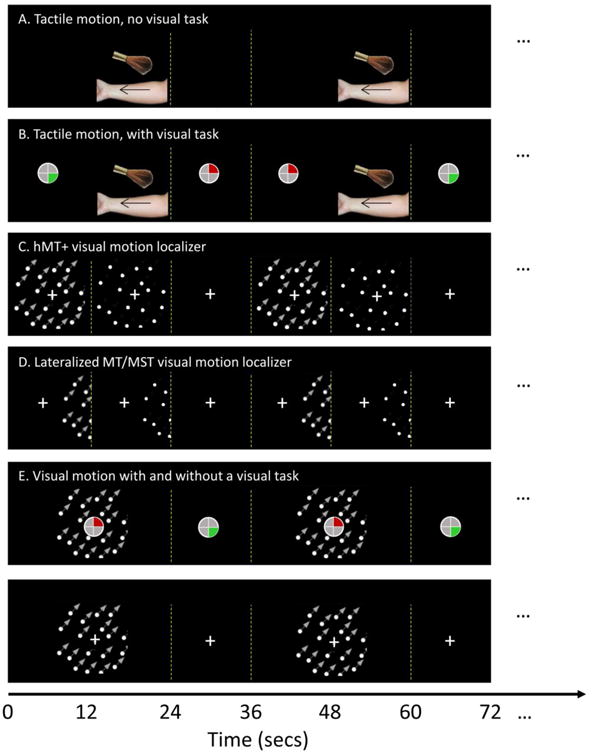
Schematic of the tactile and visual paradigm for the following conditions: (A) Tactile stimulation without a visual task. Left and right arms were stimulated on separate scans. (B) Tactile motion with a visual task, (C) hMT+ visual motion localizer, (D) Lateralized MT/MST visual motion localizer. Stimuli were presented to left and right visual field on separate scans. (E) Visual motion with and without a visual task.
Tactile motion, with visual task
This condition was identical to that described above except that subjects were instructed to perform a visual task at fixation with their unstimulated hand (Figure 1B). We used a modified version of the memory game ‘Simon©’. This task was designed to not contain motion, and to be temporally uncorrelated with the tactile task. Four quadrants of a 0.5 degree circle were defined by the four arms of the fixation cross. We only used the two quadrants on the right side of the circle and two corresponding colors (upper quadrant red, lower quadrant green). Subjects memorized a sequence of color flashes in which each color was flashed for 0.25 s with an interval of 0.33s in between colors. Subjects then reproduced it with 2-key response box, with each response button corresponding to one quadrant/color. If subjects correctly reproduced the sequence, the number of flashes in the sequence increased by 1 after a pause of 0.25s. If subjects made a mistake in reproducing the sequence, the last shown color was flashed four times for a total duration of 1s. Then the sequence length was reset to 1. Subjects played the game at their own pace throughout each scan with their unstimulated hand so no aspect of the Simon game (either the timing of the presentation of the flashes or the timing of subjects' responses) was systematically related to the timing of tactile stimulation.
Every participant performed four scans, two for each arm. Each scan lasted 6 min, and included 10 24s tactile blocks and 10 12s resting blocks.
Visual stimulation conditions
All visual stimuli were generated using Matlab and PsychToolboox (Brainard 1997; Pelli 1997). Visual stimuli were back-projected onto a screen mounted in the bore of the magnet and viewed through a mirror attached to the MR head coil. The display area covered ∼27 × 22 deg at a viewing distance of 68 cm.
For all the conditions described below dots were white on a black background. To prevent the tracking of individual dots, dots had limited life time (200 ms). In the moving condition all the dots moved coherently in one of 8 directions (spaced evenly between 0° and 360°) with a speed of 8° per second. The direction of motion changed once per second (the same direction was prevented from appearing twice in a row). In static conditions, dots were presented without motion, and the positions of the dots were reset once per second. In fixation conditions, participants were presented with only the fixation cross but no dots.
Full field hMT+ visual motion localizer
The hMT+ visual motion localizer stimulus (Figure 1C) consisted of blocks of moving, static and a fixation condition containing no dots. Dots were presented within a circular aperture (radius 8°) with a central fixation cross surrounded by a gap (radius 1.5°, to minimize motion induced eye-movements) in the dot field. Each dot subtended 0.3° (dot density 1 per degree). Participants were asked to fixate throughout the scan and performed no task. Each block lasted 10 s, during which one of the three visual stimulation conditions (motion, static, and fixation) was presented. The three conditions were cycled in a fixed order (motion, static, and fixation). Every participant performed two scans. Each scan lasts approximately 5 min, and included 30 10s blocks.
Lateralized MT/MST visual motion localizer
The lateralized visual stimuli (Figure 1D) used to delineate MT from MST consisted of blocks of moving and static dots presented either to the left or to the right of a fixation cross (Huk et al., 2002; Beauchamp et al., 2007). Each dot subtended 0.15° (dot density 2 per degree). Participants were asked to fixate throughout the scan and performed no task. Dots were restricted to a peripheral circular aperture (radius 7°) with its closest edge 10° from fixation. To compensate for our limited horizontal visual angle, the fixation was presented 3 deg off center, and roughly half of the circular aperture was presented (see Figure 1D). This design was chosen with the two goals of limiting stimulation of receptive fields spanning the midline and using a stimulus that extended to as far peripheral as possible.
Each block lasted 10 s, during which one of the three visual stimulation conditions (motion, static, and fixation) was presented. The three conditions were cycled in a fixed order (motion, static, and fixation). Each scan lasted approximately 5 min, and included 30 10s blocks. Right and left visual fields were stimulated in separate scans. Every participant performed four scans, two for each visual fields. One subject performed two additional scans, resulting a total of three scans for each hemifield.
Visual motion, with and without a visual task
Finally, for one subject we examined the effect of the Simon© task on visual motion responses (Figure 1E). The same visual dots stimulus as was used for the hMT+ localizer was presented for 24 s periods of motion separated by a 12s fixation block. Note only motion and fixation conditions were included here, with the intention to match the conditions used in tactile stimulation (see above). Each scan lasted 6 min, and included 10 24s motion blocks and 10 12s resting blocks. The participant performed four scans, two that included a passive fixation spot and two that included the Simon task.
Data Analysis
Data were analyzed using Brain Voyager QX (Version 2.3, Brain Innovation, Maastricht, the Netherlands) and MATLAB (Mathworks, MA). Prior to statistical analysis, functional data underwent preprocessing steps that included 3D motion correction (trilinear/sinc interpolation), slice scan time correction (cubic spine), linear trend removal, and high pass filtering to remove nonlinear low frequency drifts using a standard GLM approach implemented with BrainVoyager that uses a Fourier basis set consisting of 2 cycles of sines/cosines as predictors for lower frequencies (BrainVoyager Users Guide: Temporal High Pass Filtering). No spatial smoothing was applied to functional data.
For each individual participant, pre-processed functional data were co-registered to their corresponding anatomical data. The initial alignment was based on header information from functional and anatomical sessions and fine-tuning alignment was gradient based (rigid body affine transformation). Anatomical and functional data were then transformed and up-sampled into Talairach space (Talairach and Tournoux 1988) at 1×1×1mm resolution (trilinear interpolation).
ROI selection
Responses to the visual motion localizer stimulus and the lateralized visual motion localizer and the criteria used to define hMT+, MT, and MST are included within Supplementary Materials. Briefly, hMT+ was defined functionally based on the full field hMT+ visual motion localizer as a contiguous region near the posterior part of the inferior temporal sulcus that activated significantly (q(FDR)<0.05) for moving vs. static dots. MT and MST ROIs were defined as subregions of hMT+ using criteria very similar to those of Huk et al. (2002).
Beta weights were then estimated for all experimental conditions within these ROIs in Brain Voyager using a fixed effects standard generalized linear model with baseline z normalization. Beta weights are condition-associated coefficients that quantify the potential contribution of each condition in explaining the voxel time course (BrainVoyager Users Guide: The General Linear Model). Further custom analyses were carried out using custom software written in MATLAB (Mathworks, MA).
Results
Individual surface maps
Our first goal was to examine the location of tactile responses with respect to hMT+ and its subregions MT and MST (see Supplementary Figure 1). Figure 2 shows responses on the cortical surface to tactile motion. In the no visual task condition all subjects except S2 showed significant responses to the tactile motion stimuli in a location close to the expected location to hMT+. However individual surface maps reveal only limited overlap between visual and tactile motion: S1, (who had exceptionally robust responses to the visual motion stimuli), showed large amounts of overlap between tactile and visual responses for both hemispheres. S4 showed a small amount of overlap for both hemispheres. S2, S3 and S5 showed minimal or no overlap between tactile and visual motion responses. The tactile responses close to hMT+ generally (except for S1) persisted in the presence of a visual distraction task, through with reduced extent. Only in S4 in the left hemisphere was there any overlap between tactile responses and hMT+ in the absence of a visual task.
Figure 2.
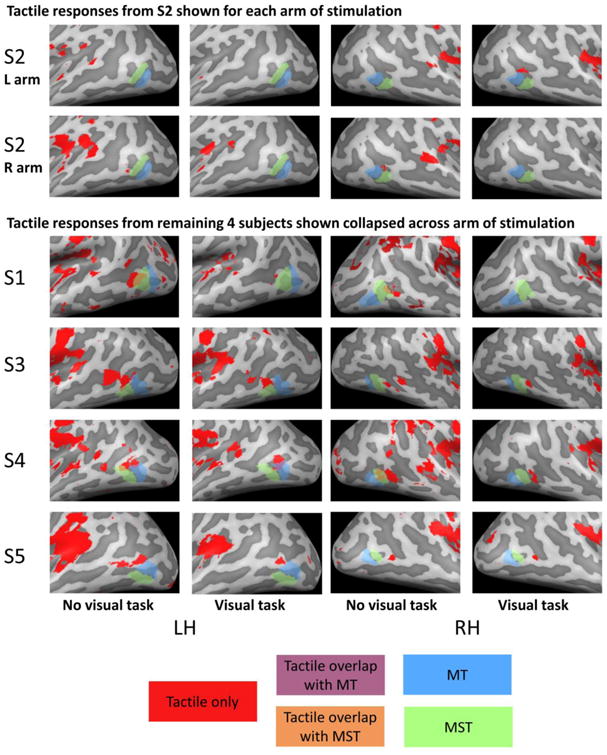
Cortical surface maps for tactile responses for the no visual task and visual task conditions. Upper panels show responses from a single subject for left and right arm separately. Lower panels show responses in the remaining 4 subjects, with responses collapsed across left and right arms such that voxels were considered responsive to tactile stimulation if they responded to tactile stimulation of either the left or the right arm. Significant responses to tactile stimulation of either the left or right arm vs. rest (q(FDR) < 0.05) is shown in red, MT is shown in blue, MST is shown in green. Overlap between tactile responses and MT and MST are shown in purple and orange respectively.
Beta weights
Although the surface maps shown in Figure 2 reveal little overlap between regions selective for tactile and visual motion, it remains possible that tactile motion responses were present within visual ROIs, but did not reach threshold. The leftward panels of Figure 3 shows beta weights for tactile motion with and without a distractor visual task, within each of ROIs defined by the visual motion. For comparison, responses to contralateral visual stimulation are also shown. The corresponding time courses in percent signal change are shown in Figure 4.
Figure 3.
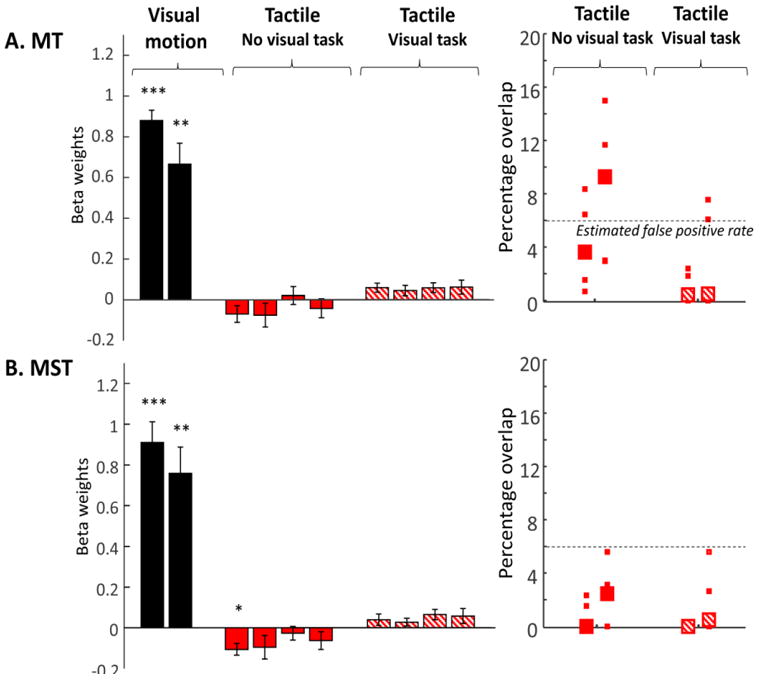
Leftward panels show Beta weights for tactile responses within MT and MST. For comparison, responses to the contralateral visual motion stimulus are shown for each ROI (black bars). Responses in the absence of a distractor visual task are shown in red, and responses in the presence of a distractor visual task are shown with red hatched bars. Data are shown separately for left and right hemispheres, and left and right arms of stimulation. Each bar represents data averaged across subjects and single standard error bars are shown. Asterisks represent whether responses were significantly different from zero. * p<0.05, * p<0.01, * p<0.001. Rightward panels show the percentage of voxels within MT and MST that responded to tactile motion with and without a visual task. Again, voxels were considered to respond to tactile motion if they showed significant responses to tactile stimulation of either the left or the right arm vs. rest. The dotted line represents the number of voxels that might be expected to respond to tactile stimulation simply as a consequence of false positives.
Figure 4.
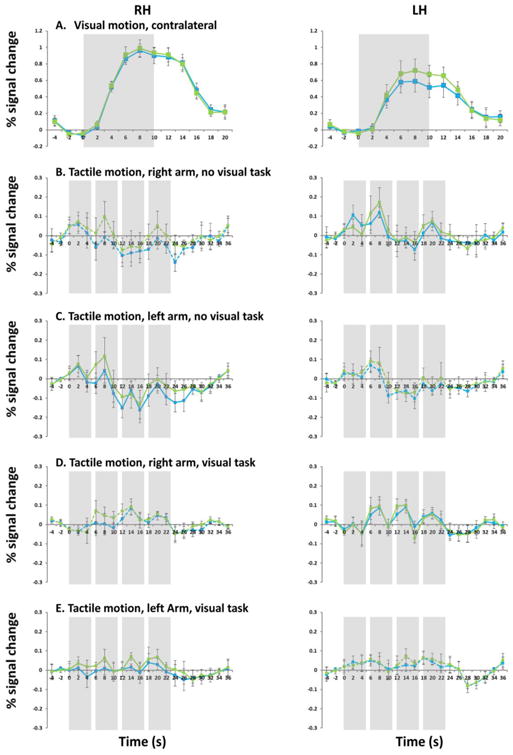
fMRI time course in percent signal change within MT and MST for contralateral visual motion stimulus (A), tactile motion stimulus without a visual task (B: right arm; C: left arm), tactile motion stimulus with a visual task (D. right arm; E: left arm). Note that scale on the y-axis was changed for all tactile conditions. Leftward panels show time course data in right MT and MST, and rightward panels show time course data in left MT and MST. MT is shown in blue and MST is shown in green. Solid lines show contralateral stimulation, and dashed lines show ipsilateral stimulation. Shading represents the period of visual/tactile stimulation (10s block for visual stimulation, 24s block for tactile stimulation consisting of 4 5-s brushes).
To our surprise given the previous literature, when subjects were blindfolded and there was no visual task there was no suggestion of sub-threshold positive tactile responses in either MT or MST. Indeed, tactile stimulation in the absence of a visual task resulted in a slight suppression of BOLD responses within visually defined MST. In the presence of a visual task tactile responses resulted in positive responses in MT and MST, though comparison with visually evoked motion responses indicates that although these positive responses were robust (as evidenced by relatively high p values and medium to large effect sizes) they were small in magnitude. We found extremely similar pattern of results using ROIs defined using a variety of thresholds of q(FDR)<0.1, q(FDR)<0.01 and Bonferroni corrected 0.05 (data not shown). Similar results were also obtained using an STS visual motion ROI defined as showing significant responses to the hMT+ full field localizer but being non-contiguous with hMT+ (see Supplementary Figure 2 leftward panel), suggesting that our failure to find tactile responses in hMT+ was not due to an overly stringent definition of hMT+.
A three way ANOVA carried out on Beta weight responses in each ROI to tactile stimulation, with Task vs. No Task, arm of tactile stimulation (RA vs. LA), and hemisphere (RH vs. LH) as factors. For MT and MST there was a significantly significant main effect of task (MT: F(1, 32)=12.36, p<0.01; MST: F(1, 32)=22.17, p<0.001). No statistically significant main or interaction effects for arm of stimulation or hemisphere were found for either ROI.
Because we found a main effect of task, but no effect of either arm of stimulation or hemisphere we also examined whether responses were significantly different from zero after collating across both stimulation arm and hemisphere. When there was no visual distractor task Beta weights were significantly lower than zero in MST (No task, MST: Mean=-0.073, std=0.092, t(19)=-3.451, p<0.01, d= -0.7857). When there was a visual distractor task Beta weights were significantly larger than zero in both ROIs (Visual task, MT: Mean=0.055, std=0.055, t(19)= 4.439, p<0.001, d=0.9930; MST: Mean=0.047, std=0.06, t(19)= 3.525, p<0.01, d=0.7880).
To help interpret these results we ran a single subject on a visual motion stimulus with and without the Simon task. As expected, the response to visual motion in hMT+ was consistently smaller in the presence of the Simon task (data not shown).
Overlap between visual and tactile responses
One possibility was that the lack of positive response to tactile motion within areas sensitive to visual motion might possibly be due to only small subregions showing significantly positive responses to tactile motion. To examine this we calculated the percentage overlap between visual and tactile motion responses. This was simply calculated as the percentage of voxels within each ROI that showed significant responses to tactile stimulation on either arm. Data shown in the rightward panels of Figure 3 are based on a significance level of q(FDR)<0.05 for both visual and tactile motion. We found an extremely similar pattern of results using alternative thresholds of q(FDR)<0.1, q(FDR)<0.01 and Bonferroni corrected 0.05 (data not shown). Similar results were also obtained for an STS visual motion ROI defined as showing significant responses to the hMT+ full field localizer but being non-contiguous with hMT+ (see Supplementary Figure 2 rightward panel), suggesting that our failure to find overlap between tactile responses and visual motion responses in hMT+ was not due to an overly stringent definition of hMT+.
It should be noted that it is difficult to estimate what overlap between neighboring but non-overlapping tactile and visual motion responses might be expected simply due to false positives, shared vasculature and the smooth spatial structure of the BOLD signal. Simulations (based on Genovese et al. 2002; Chumbley and Friston 2009) suggest that a false positive rate of 6% is a reasonable estimate given that we included any voxels that responded to either left or right arm stimulation. Except for MT in the no task condition, most subjects showed levels of overlap less than 6%.
Estimates of spatial blur from (Aquino et al. 2012) suggest adjoining regions can produce statistically significant spurious overlap over a surface distance of 5mm. Except for S1, all the cases of overlap found in Figure 3 that fell above the 6% false positive threshold, fell within this 5mm boundary, making it impossible to exclude the possibility that the apparent overlap in MT in the no task condition found for some subjects was simply due to fMRI spatial blur. Thus, we find little evidence of overlap between visual and tactile responses in most individual subjects.
Group average responses
Our next concern was that our findings of extremely limited overlap with hMT+ might be an artifact of our particular task, which involved brushing the arm with a brush. Although this choice of task was deliberately chosen as a replication of a previous study (Hagen et al. 2002) it was possible that other tasks might elicit tactile responses that overlapped more substantially with hMT+.
To examine this, we analyzed our data using group averaging methods, as has been used in several previous studies. Figure 5 Panels A and B show group averaged data from our study (thresholded at q(FDR)<0.05) based on tactile motion responses reported in Figure 2. Even though, as described above, these data fail to show convincing overlap between visual and tactile motion in individual subjects, there is clear (spurious) overlap when the data is group averaged. In this no task condition, using the individual subject approach of Figure 2, an average of 6.4% of voxels within hMT+ showed significant responses to tactile stimulation on either arm (averaged across subjects and hemispheres). However when data is analyzed using the group averaging techniques (as in Figure 5) 15.2% percent of voxels in hMT+ are spuriously identified as responding to tactile stimulation at a significance level of q(FDR) < 0.05. Although the presence of a visual task reduced the extent of group tactile motion responses near hMT+, spurious overlap between visual and tactile motion remained (12.9%).
Figure 5.
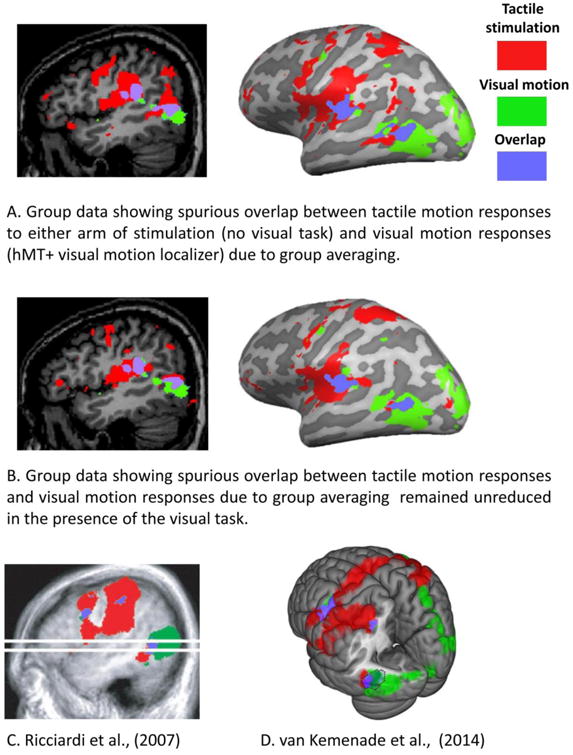
The potential for spurious overlap between visual and tactile activation due to group averaging. Activation patterns are recolored so as to be consistent across all figures. Red shows tactile responses, green shows visual motion responses, and purple shows regions of overlap. (A, B) Group average tactile motion responses from the no visual task condition (C) and the visual task condition (D) reported in Figure 2, showing regions that responded to stimulation of either arm. Data are thresholded at q(FDR)<0.05. (C) Group average data reprinted from (Ricciardi et al. 2007). Tactile stimulation was via Braille-like dot patterns on a plastic surface undergoing translational and rotational tactile flow. Data are thresholded at z>+/- 3.5. (D) Group average data reprinted from (van Kemenade et al. 2014). Subjects performed a tactile motion direction discrimination task on moving Braille-like dot stimuli applied bilaterally to the fingertip. Data are thresholded at FWE p <0.05. The dotted line shows the estimated location of hMT+.
The location and extent of tactile responses found within hMT+ when using a group averaged approach also look remarkably similar to the results of previous studies, as is illustrated by the two examples of Panels C and D (as well as others (Beauchamp et al. 2007). Panel C shows group average data from Ricciardi et al. (2007) in a study in which subjects passively experienced Braille-like tactile flow patterns on the finger. Panel D shows group average data of van Kemenade et al. (2014). In this study subjects had to identify the direction of motion of Braille-like tactile stimulation to the fingertip. In all these datasets the center of group averaged tactile motion is anterior and superior to visually localized MT and MST.
Thus, while we cannot exclude the possibility that our failure to find tactile responses overlapping with visually responsive areas was unique to our particular stimulation paradigm, our results do show that it is easy to generate the spurious appearance of cross-modal responses in hMT+ on the basis of group averaged responses even in the absence of convincing genuine overlap within individuals.
Discussion
As described in the Introduction, it has been argued that regions within hMT+ may be supramodal, with the role of processing motion regardless of the sensory modality in which it is presented (Pascual-Leone and Hamilton 2001; Ricciardi et al. 2014). Our goal in this study was to further examine this hypothesis by replicating a previous study examining tactile responses within hMT+ and neighboring regions. In particular, we were interested in the location of tactile responses with respect to MT and MST, and whether tactile responses would be found after withdrawal of attention from the tactile stimulus.
To our surprise, when individual data were examined, our findings did not replicate those of the original study: only one of the five subjects showed evidence for overlap between tactile and visual motion responses, and that overlap only occurred in the absence of a visual distractor task. Group averaging did result in significant spurious overlap, suggesting that group average results comparing overlap between conditions should be interpreted with caution for cortical regions that show significant variability in location.
Previous studies examining tactile responses in hMT+
A range of findings have been reported regarding responses to tactile activation in hMT+ that range from strong tactile activation to weak suppression. One possible reason for this wide range of findings is that a continuum of methods to define hMT+ have been used in the literature: stereotactic co-ordinates (Matteau et al. 2010; Wacker et al. 2011), group averaged response to visual motion localizers (Ricciardi et al. 2007; Summers et al. 2009), and finally using individual responses to visual motion to define hMT+ for each subject (Hagen et al. 2002; Blake et al. 2004; Beauchamp et al. 2007; van Kemenade et al. 2014). The choice of method used to define hMT+ is critical because although it has a relatively consistent position in relation to the sulcal patterns, its stereotaxic location is highly variable (Dumoulin et al. 2000). Indeed the Jülich probabilistic atlas for hMT+ is never higher than 50% (Wilms et al. 2005; Eickhoff et al. 2007; Malikovic et al. 2007). As shown in Figure 5 this variability can produce considerable apparent overlap between tactile and visual motion responses using a group averaging approach, even in the absence of overlap within individual subjects.
The two studies that identified hMT+ using a stereotaxic definition of hMT+ found positive responses to tactile stimulation within left stereotaxic hMT+ (Wacker et al. 2011) and bilateral positive responses within regions described as being within left and right hMT (Matteau et al. 2010). However in both studies the peaks of the identified region actually fell outside two standard deviations of the expected variance in the location of individually defined hMT+ (based on Dumoulin et al. 2000), though the location of peak activity being outside hMT+ doesn't exclude the possibility that activity extended to within the expected location of hMT+.
The studies that have used group averaged responses to visual motion stimuli to define hMT+ have tended to find positive responses to tactile motion that have ranged from small but significant (Summers et al. 2009) to a combination of strong positive and negative responses to tactile motion (Ricciardi et al. 2007; reanalyzed Sani et al. 2010). In the case of Summers et al. (2009) the peak coordinates of tactile activity in the right hemisphere fell within hMT+, but the region identified as left hMT+ fell outside the expected location of hMT+. In the case of Ricciardi et al. (2007) the peak coordinates of the region that showed positive activation to tactile stimulation fell outside the expected location of hMT+, whereas clusters in each hemisphere that showed suppression to tactile stimulation fell within the expected location of hMT+ in both hemispheres (Sani et al. 2010).
Several studies that have defined hMT+ individually using visual motion localizers, and found positive modulation of hMT+ by tactile stimulation within individually defined ROIs. However these responses have tended to be smaller than those found in the studies described above (Hagen et al. 2002; Blake et al. 2004; Beauchamp et al. 2007; van Kemenade et al. 2014). Our finding of a weak suppressive effect of tactile stimulation in the no task condition has also previously been observed (Ricciardi et al. 2007; Lewis et al. 2010). (Interestingly, a variety of studies show suppression of hMT+ when subjects attend to an auditory motion stimulus Lewis et al. 2000; Strnad et al. 2013; Jiang et al. 2014).
A primary goal of this study was to very carefully define hMT+, including its subdivisions into MT/MST. To do this we carried out two sessions devoted specifically to visual motion stimuli. This is more than any previous study, all of which have defined hMT+ based on less than 20 minutes of fMRI data (Blake et al. 2004; Beauchamp et al. 2007; van Kemenade et al. 2014) or 2-4 PET scans (Hagen et al. 2002). We believe that this likely led to a more accurate localization of hMT+ as evidenced by the close correspondence between regions defined as hMT+ across the two types of motion localizers (see Supplementary Figure 1) and across a range of thresholds (q(FDR)<0.1, q(FDR)<0.01 and Bonferroni corrected 0.05).
We also collected two full fMRI sessions of tactile data for each subject. Again this was a considerable amount of data compared to most previous studies. It can be seen in Figure 2 show that the pattern of activation across the two sessions of tactile data are very similar, though the extent of activation is highly reduced in the presence of a visual task. Thus we believe we were successful in obtaining the high quality data needed to accurately determine which regions are activated by tactile and visual motion respectively.
hMT+ fails to respond to auditory motion
One might expect a supramodal hMT+ to also respond to auditory motion. However a variety of studies have explicitly looked for, but failed to find, evidence of auditory motion responses in hMT+ (Lewis et al. 2000; Saenz et al. 2008; Bedny et al. 2010; Lewis et al. 2010; Alink et al. 2012; Jiang et al. 2014). Indeed, in an analysis closely analogous to that of Figure 5 it has been previously been shown by Saenz et al. (Saenz et al. 2008) that spurious auditory motion responses in hMT were elicited as a result of using group averaging methods to define hMT+. However, inspection of that same data using individual hMT+ ROIs (based on individual visual functional localizers) demonstrated that the vast majority of individually defined hMT+ ROIs did not respond to auditory motion.
Only two studies have found auditory responses in hMT+, and both may have failed to accurately isolate hMT+. Poirier et al. (2005) reported hMT+ BOLD responses to auditory motion stimuli in blindfolded sighted subjects using a definition of hMT+ based on group averaging in stereotaxic coordinates. However, within individuals only 2 of the 8 reported co-ordinates of activated clusters fell within 2 standard deviations of the expected location of hMT+ (also see Watson et al. 1993; Dumoulin et al. 2000). Using multivoxel pattern analysis, Strnad et al. (2013) recently showed that while the overall BOLD response to auditory motion was negative (in contrast to the Poirier et al. study described above, but similar to many auditory studies that used individual localizers), a region defined as hMT+ did contain classification information about different auditory motion conditions. However, hMT+ was defined as all voxels within a relatively generous 10 mm radius from MNI group peak coordinates and classification was carried out using only 50 out of ∼1000 voxels in the ROI. Thus this analysis is likely to be highly susceptible to the inclusion of voxels from areas adjoining hMT+.
The effect of attention
Within individual subjects, in the absence of the visual distractor task responses were slightly suppressed, though this effect was only significant in MST. In the presence of a visual distractor task, tactile stimulation elicited small but significantly positive responses in MT and MST.
What is the substrate of these modulations of hMT+ by tactile stimulation? Our finding of slight suppression of hMT+ in the absence of a visual task, and small positive responses within hMT+ with the addition of a visual task excludes a number of possibilities including: (1) a general arousal effect, (2) visual imagery, or (3) a direct response to tactile motion stimuli within hMT+. All of these explanations would predict a larger response within hMT+ in the absence of a visual task.
One possibility is that tactile responses are the result of featural and/or cross-modal attention. In one subject we confirmed that the presence of the Simon task served to reduce BOLD responses to the visual motion stimulus. Attending to the Simon task in isolation may reduce hMT+ BOLD responses more effectively then the Simon task in the presence of the tactile brush. There are many reasons that might be the case: for example, the tactile motion stimulus contains the shared feature of motion, the Simon task was extremely demanding so the addition of tactile stimulation may have ‘released’ some attention, or withdrawing visual spatial attention might be more effective at reducing hMT+ BOLD responses then withdrawing cross-modal attention to a tactile stimulus (Ciaramitaro et al. 2007). Thus, the modulation of hMT+ by the presence or absence of tactile stimulation may reflect the effects of cross-modal attention rather than signifying tactile responses within hMT+ per se. This explanation is also consistent with the fact that no primate electrophysiology paper to date has reported tactile responses in MT/V5: Presumably cross-modal attentional effects of tactile stimulation would be less likely to be casually observed than increases in spiking as a direct result of tactile stimulation.
Limitations of our findings
Given our small number of subjects, our findings cannot be taken as evidence that no individuals show responses to passive tactile stimulation in hMT+ (indeed one of our five subjects did show such responses, in the absence of a visual distractor task). However our results do suggest that these responses do not occur in the majority of individuals. We also only examined a single tactile task. Although we deliberately chose arm brushing because our goal was to replicate a previous influential positive finding in the literature it is nonetheless possible that tactile stimulation of other body parts might be more effective in eliciting responses in hMT+.
Finally, our goal was to examine whether hMT+ is ‘supramodal’ - processing motion regardless of the sensory modality in which it is presented (Ricciardi et al. 2014). Consequently we chose to replicate a paper that used a passive protocol for tactile stimulation that was related to the prediction for supramodal responses. Supramodal responses should be driven by the sensory stimulus itself, and therefore should be observable during passive stimulation and survive (albeit attenuated) withdrawal of attention to a visual stimulus. It remains perfectly likely that asking subjects to actively perform a tactile direction discrimination task (or some equivalent) would lead to enhanced hMT+ responses to tactile motion. Similarly, our results do not address whether hMT+ shows multisensory interactions: whether the response to a visual stimulus in hMT+ is influenced by a presence of a congruent or incongruent tactile stimulus, as suggested by Blake et al. (Blake et al. 2004).
Summary
Here we present data from a replication of Hagen et al. (2002). In contrast to that study, we did not find that passive tactile stimulation consistently activated hMT+. We also present analyses showing that failing to accurately localize hMT+ can easily lead to artifactual responses to tactile motion within hMT+, which may have contributed to positive findings in previous studies. Although the presence of tactile stimulation did produce a small modulation of responses in hMT+, the pattern of responses with respect to the withdrawal of attention were the opposite of what would be predicted if these small modulations were due to direct tactile motion responses in hMT+, as compared to cross-modal featural attention. Thus, the evidence for supramodal responses in hMT+ may be less conclusive than has sometimes been assumed.
Supplementary Material
Highlights.
We re-examine overlap between tactile and visual motion responses within hMT+
Little overlap between tactile and visual motion responses within hMT+
Group averaging results in spurious tactile motion responses within hMT+
Visual distractor task reduces tactile motion responses in MT and MST
hMT+ may not serve as a supramodal motion processing module
Acknowledgments
This work was supported by the National Institutes of Health (EY-014645 to Ione Fine). Fang Jiang was supported the Pathway to Independence Award (K99EY023268).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final ci form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alink A, Euler F, Kriegeskorte N, Singer W, Kohler A. Auditory motion direction encoding in auditory cortex and high-level visual cortex. Hum Brain Mapp. 2012;33:969–978. doi: 10.1002/hbm.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino KM, Schira MM, Robinson PA, Drysdale PM, Breakspear M. Hemodynamic traveling waves in human visual cortex. PLoS computational biology. 2012;8:e1002435. doi: 10.1371/journal.pcbi.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. Neuroimage. 2008;41:1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Kishan N, Ro T. Human MST but not MT responds to tactile stimulation. J Neurosci. 2007;27:8261–8267. doi: 10.1523/JNEUROSCI.0754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Konkle T, Pelphrey K, Saxe R, Pascual-Leone A. Sensitive period for a multimodal response in human visual motion area MT/MST. Curr Biol. 2010;20:1900–1906. doi: 10.1016/j.cub.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Sobel KV, James TW. Neural synergy between kinetic vision and touch. Psychol Sci. 2004;15:397–402. doi: 10.1111/j.0956-7976.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Ciaramitaro VM, Buracas GT, Boynton GM. Spatial and cross-modal attention alter responses to unattended sensory information in early visual and auditory human cortex. J Neurophysiol. 2007;98:2399–2413. doi: 10.1152/jn.00580.2007. [DOI] [PubMed] [Google Scholar]
- David AS, Senior C. Implicit motion and the brain. Trends Cogn Sci. 2000;4:293–295. doi: 10.1016/s1364-6613(00)01511-4. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hagen MC, Franzen O, McGlone F, Essick G, Dancer C, Pardo JV. Tactile motion activates the human middle temporal/V5 (MT/V5) complex. Eur J Neurosci. 2002;16:957–964. doi: 10.1046/j.1460-9568.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. J Neurosci. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Stecker GC, Fine I. Auditory motion processing after early blindness. J Vis. 2014;14 doi: 10.1167/14.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Activation in Human MT/MST by Static Images with Implied Motion. J Cognitive Neurosci. 2000;12:48–55. doi: 10.1162/08989290051137594. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Beauchamp MS, DeYoe EA. A comparison of visual and auditory motion processing in human cerebral cortex. Cereb Cortex. 2000;10:873–888. doi: 10.1093/cercor/10.9.873. [DOI] [PubMed] [Google Scholar]
- Lewis LB, Saenz M, Fine I. Mechanisms of cross-modal plasticity in early-blind subjects. J Neurophysiol. 2010;104:2995–3008. doi: 10.1152/jn.00983.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT1: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17:562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- Matteau I, Kupers R, Ricciardi E, Pietrini P, Ptito M. Beyond visual, aural and haptic movement perception: hMT+ is activated by electrotactile motion stimulation of the tongue in sighted and in congenitally blind individuals. Brain research bulletin. 2010;82:264–270. doi: 10.1016/j.brainresbull.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W, Orban GA. Charting the lower superior temporal region, a new motion-sensitive region in monkey superior temporal sulcus. J Neurosci. 2006;26:5929–5947. doi: 10.1523/JNEUROSCI.0824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Hamilton R. The metamodal organization of the brain. In: Casanova C, Ptito M, editors. Progress in Brain Research. 2001. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Poirier C, Collignon O, Devolder AG, Renier L, Vanlierde A, Tranduy D, Scheiber C. Specific activation of the V5 brain area by auditory motion processing: an fMRI study. Brain Res Cogn Brain Res. 2005;25:650–658. doi: 10.1016/j.cogbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Basso D, Sani L, Bonino D, Vecchi T, Pietrini P, Miniussi C. Functional inhibition of the human middle temporal cortex affects non-visual motion perception: a repetitive transcranial magnetic stimulation study during tactile speed discrimination. Exp Biol Med (Maywood) 2011;236:138–144. doi: 10.1258/ebm.2010.010230. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Bonino D, Pellegrini S, Pietrini P. Mind the blind brain to understand the sighted one! Is there a supramodal cortical functional architecture? Neuroscience & Biobehavioral Reviews. 2014;41:64–77. doi: 10.1016/j.neubiorev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Vanello N, Sani L, Gentili C, Scilingo EP, Landini L, Guazzelli M, Bicchi A, Haxby JV, Pietrini P. The effect of visual experience on the development of functional architecture in hMT+ Cereb Cortex. 2007;17:2933–2939. doi: 10.1093/cercor/bhm018. [DOI] [PubMed] [Google Scholar]
- Saenz M, Lewis LB, Huth AG, Fine I, Koch C. Visual Motion Area MT+/V5 Responds to Auditory Motion in Human Sight-Recovery Subjects. J Neurosci. 2008;28:5141–5148. doi: 10.1523/JNEUROSCI.0803-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani L, Ricciardi E, Gentili C, Vanello N, Haxby JV, Pietrini P. Effects of Visual Experience on the Human MT+ Functional Connectivity Networks: An fMRI Study of Motion Perception in Sighted and Congenitally Blind Individuals. Frontiers in systems neuroscience. 2010;4:159. doi: 10.3389/fnsys.2010.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin AP, McCullough S, Alac M, Emmorey K. Modulation of BOLD response in motion-sensitive lateral temporal cortex by real and fictive motion sentences. J Cogn Neurosci. 2010;22:2480–2490. doi: 10.1162/jocn.2009.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior C, Barnes J, Giampietro V, Simmons A, Bullmore ET, Brammer M, David AS. The functional neuroanatomy of implicit-motion perception or representational momentum. Curr Biol. 2000;10:16–22. doi: 10.1016/s0960-9822(99)00259-6. [DOI] [PubMed] [Google Scholar]
- Strnad L, Peelen MV, Bedny M, Caramazza A. Multivoxel pattern analysis reveals auditory motion information in MT+ of both congenitally blind and sighted individuals. PLoS One. 2013;8:e63198. doi: 10.1371/journal.pone.0063198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers IR, Francis ST, Bowtell RW, McGlone FP, Clemence M. A functional-magnetic-resonance-imaging investigation of cortical activation from moving vibrotactile stimuli on the fingertip. J Acoust Soc Am. 2009;125:1033–1039. doi: 10.1121/1.3056399. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tanaka K, Hikosaka K, Saito H, Yukie M, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Sugita Y, Moriya M, Saito H. Analysis of object motion in the ventral part of the medial superior temporal area of the macaque visual cortex. J Neurophysiol. 1993;69:128–142. doi: 10.1152/jn.1993.69.1.128. [DOI] [PubMed] [Google Scholar]
- van Kemenade BM, Seymour K, Wacker E, Spitzer B, Blankenburg F, Sterzer P. Tactile and visual motion direction processing in hMT+/V5. Neuroimage. 2014;84:420–427. doi: 10.1016/j.neuroimage.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Wacker E, Spitzer B, Lutzkendorf R, Bernarding J, Blankenburg F. Tactile motion and pattern processing assessed with high-field FMRI. PLoS One. 2011;6:e24860. doi: 10.1371/journal.pone.0024860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wilms M, Eickhoff SB, Specht K, Amunts K, Shah NJ, Malikovic A, Fink GR. Human V5/MT+: comparison of functional and cytoarchitectonic data. Anatomy and embryology. 2005;210:485–495. doi: 10.1007/s00429-005-0064-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


