Abstract
All forms of cell signaling occur in discreet cellular microdomains in which the ER is the main participant and include microdomains formed by the ER with lysosomes, endosomes, the nucleus, mitochondria and the plasma membrane. In the microdomains the two opposing organelles transfer and exchange constituents including lipids and ions. As is the case for other forms of signaling pathways, many components of the receptor-evoked Ca2+ signal are clustered at the ER/PM microdomain, including the Orai1-STIM1 complex. This review discusses recent advances in understanding the molecular components that tether the ER and plasma membrane to form the ER/PM microdomains in which PI(4,5)P2 is enriched, and how dynamic targeting of the Orai1-STIM1 complex to PI(4,5)P2-poor and PI(4,5)P2-rich microdomains controls the activity of Orai1 and its regulation by Ca2+ that is mediated by SARAF.
Introduction
All forms of cell signaling occur in discreet cellular microdomains, depending on the cellular function they regulate. Accordingly, Ca2+ signaling proteins are assembled into complexes within such microdomains. This can be seen very nicely in polarized cells, such as secretory epithelial cells, where expression of both Ca2+ and cAMP signaling proteins is highly polarized. These include G proteins-coupled receptors [1-3], the plasma membrane Ca2+ pump (PMCA) and the Endo/Sarcoplasmic reticulum Ca2+ pump (SERCA) [4, 5], all IP3 receptors [6, 7], the Ca2+ influx channels TRPC1 [8], TRPC3 [9] and Orai1 [8], the ER Ca2+ sensor STIM1 [8] and several adenylyl cyclase isoforms [10]. These proteins are expressed at high levels in the apical pole, the site of many specialized activities of polarized cells. Disruption of such polarized signaling is associated with disease states (Reviewed by Petersen OH, this Special Issue of Cell Calcium).
Organization of signaling complexes in microdomains increases signaling fidelity and strength and allows cross-talk and synergism between signaling modalities. A good example is the well-established cross-talk and synergism between the cAMP and Ca2+ signaling pathways. Synergism in activation of exocytosis [11] and fluid secretion [12] in epithelia, exocytosis by endocrine glands [13] and other cellular functions have been known for many years. However, the molecular mechanism of synergism and cross-talk has only been understood recently with increased understanding of the organization of signaling complexes in microdomains. For example, the response of IP3 receptors (IP3Rs) to IP3 is modulated by cAMP/PKA-mediated phosphorylation of the IP3Rs on specific serine/threonine residues [14, 15]. Ca2+-dependent adenylyl cyclases (ACs) are regulated by specific components of the Ca2+ signal and both of which are localized at specific ER/PM microdomains. AC8 is associated with the N terminus of Orai1 in an endoplasmic reticulum/plasma membrane (ER/PM) microdomain [16] that may also express TRPC1 [17]. Ca2+ entering the cells specifically through Orai1 and TRPC1 activate AC8 [16, 17] (See also review by Cooper DM in this Special Issue of Cell Calcium). Plasma membrane ACs are also regulated by STIM1 [18]. Clustering and translocation of STIM1 to the plasma membrane in response to store depletion increases cAMP by activation of plasma membrane ACs, independent of Orai1, Ca2+ influx and an increase in cytoplasmic Ca2+ [18]. This may involve formation or expansion of ER/PM microdomains (see below discussion of STIM1 and ER/PM microdomains by Hogan PG in this Special Issue of Cell Calcium).
Synergism between the Ca2+ and cAMP pathways is mediated by IRBIT (IP3Rs binding protein released with IP3) in the apical pole microdomain of polarized cells [19]. In resting cells IRBIT binds to the IP3 binding pocket of the IP3Rs to inhibit Ca2+ signaling [20], with IP3Rs acting to buffer and restrict availability of IRBIT for target proteins. A relatively large increase in IP3 is required to release IRBIT from the unphosphorylated IP3Rs. However, under physiological stimulus intensity, activation of the cAMP/PKA pathway phosphorylates the IP3Rs at specific serine residues [14, 15]. This increases the affinity of the IP3Rs for IP3 and facilitates release of IRBIT from the IP3Rs upon stimulation of Gq-coupled receptors at the apical pole [19]. IRBIT then activates transporters at the luminal membrane, such as the Cl− channel CFTR [21, 22] and the Cl−/HCO3− exchanger slc26a6 [19], resulting in synergistic activation of fluid and HCO3− secretion by secretory ducts [23].
Many of the microdomains in which signaling complexes are assembled and communicate are formed by the ER with several organelles. This topic has been reviewed extensively in recent years (for example [24, 25]) and in other parts of this Special Issue of Cell Calcium and will be discussed briefly below.
The ER-formed microdomains
In addition to functioning as a hub for signaling pathways, cellular microdomains serve to transmit and exchange signals and molecules between organelles. Microdomains are formed in regions of close apposition of cellular membranes when the membranes of two organelles are within a distance of 10-30 nm and are tethered by structural proteins that span the distance between the two apposed membranes. The ER forms microdomains with multiple organelles, including the plasma membrane [24], the mitochondria [26, 27], lysosomes [28] and the nucleus [25]. Several of the molecular components that form the tethers have been identified in the last few years, although some of these may have additional functions like lipid metabolism and transfer [29, 30] or modulation of channel activity [31]. Another important component of the microdomains is enrichment with specific lipids, most commonly cholesterol in the plasma membrane and phosphatidylinositols in other sites beside the plasma membrane [32].
A group of proteins participating in the formation of most microdomains are the VAMP-associated proteins (VAPs) [33]. The major VAP isoforms are VAP-A and VAP-B. The VAPs are type II integral membrane proteins that are anchored in the ER and bind proteins containing the phenylalanines in an acid tract (FFAT) motifs [34]. Proteins of particular interest containing FFAT motifs that bind to the VAPs are the Oxysterol-binding protein (OSBP)-related proteins (ORPs). The ORPs have in addition to Oxysterol binding site a PH domain that binds phosphatidylinositols [35]. Accordingly, the ORPs bind and transfer lipids, including cholesterol [36] and phosphatidylinositol-4-phosphate (PI4P) [37].
Another group of proteins in the microdomains are the septins [38]. The septins are GTP-binding proteins that heteropolymerize into filaments and rings, which interact with actin filaments and with microtubules [39, 40]. The septins are present in a variety of microdomains including the ER/PM microdomain and the ER/nuclear envelope microdomain [41], the cell division site [42], base of cilia [43] and dendrites [44]. All septins have a central core domain, which contains a polybasic region that can bind phospholipids, a GTP binding domain and a septin unique element [45]. Binding to phospholipids regulates septin filament assembly and, in turn, septins appear to regulate phospholipids at the plasma membrane [46-48]. A major role of the septins is to function as a diffusion barrier to prevent mixing proteins between domains and probably regulate the size and perhaps the dimension of the microdomains [41].
Many of the proteins that function as tethers contain a conserved membrane-binding domain, called synaptotagmin-like-mitochondrial-lipid protein (SMP) domain, that targets the proteins to membrane contact sites [49]. The SMP domain is conserved and has the same function in all species, with the yeast and mammalian homologues targeted to the same site [49].
The ER-formed microdomains are tethered with proteins specific to the organelle that is in close contact with the ER. Other reviews in this Special Issue discuss the role of the various tether proteins in forming the ER/Mitochondria microdomain, the SR-Plasmalema microdomain in muscle and the ER/lysosomes/endosomes microdomain and will not be discussed further here. Below we discuss the ER/PM microdomain in relation to the function of the Orai1/STIM1 complex.
The Molecular components of the ER/PM microdomain
An ER(SR)/PM microdomain has been first noted in muscle by Porter and Palade [50], which are essential for excitation-contraction coupling. Subsequent studies revealed that in skeletal muscle the SR/PM microdomains are formed by interaction of the type 1 ryanodine receptor is the SR and the voltage-activated Ca2+ channels in the plasma membrane [51]. In cardiac muscle type 2 ryanodine receptor is in the SR [52]. Assembly and stabilization of the microdomain is dependent on the tethering protein Junctophilin (reviewed in this Special Issue by Takeshima et al). ER/PM microdomains have been observed in the immunological synapse [53], neurons [54] and in many cells at the site of the native and expressed STIM1 [55]. However, the proteins that tether the ER and plasma membrane and their potential functions have only been found recently and studied.
The proteins that tether the ER and plasma membrane (PM) to form the ER/PM microdomain have been examined more extensively in yeast than in mammalian cells, and even now only some of them are known [24, 25, 29]. The first protein to be identified is Ist2 (increased sodium tolerance 2), which was identified as a protein that is asymmetrically distributed to the bud and its polarized localization required border formation by septin [56]. A subsequent important finding was that Ist2 is targeted to the peripheral ER in yeast, with its C terminus polybasic domain tethering the ER to the plasma membrane [57-59]. Moreover, the Ist2 C terminus is sufficient to target several ER and plasma membrane proteins to the ER/PM microdomain [57, 60]. Ist2 is predicted to have eight transmembrane domains and is homologous to the mammalian TMEM16 family proteins [24] that are also known as Anoctamines [61, 62]. However, recent crystallization of TMEM16F indicates that the family has ten transmembrane domains [63], raising the possibility that Ist2 also has ten transmembrane domains. TMEM16A (ANO1) [61, 64, 65] and TMEM16B (ANO2) [66] function as a Ca2+-activated Cl− channel and TMEM16F (ANO6) functions as phospholipids scramblase [67] and anion and cation channel [68, 69]. The role and isoform of the TMEM16 (ANO) proteins in tethering the ER/PM is unknown at this time and so is the function of Ist2 besides tethering the ER to the plasma membrane.
A second important group of proteins in the ER/PM microdomain are the Tricalbins (Tcbs) in yeast [49, 70, 71] and their mammalian homologues the Extended-Synaptotagmins (E-Syts) [72, 73]. The Tcbs/E-Syts are integral ER membrane proteins with a membrane anchor hydrophobic patch [73], a conserved SMP domain [49] and multiple Ca2+ and PI(4,5)P2 binding C2 domains [49, 73]. The Tcbs are localized in the peripheral ER at the ER/PM junction [49]. Similarly, all three E-Syts localize to the ER/PM junction and expression of all E-Syts markedly increases the number of the ER/PM microdomains, with E-Syt2 and E-Syt3 being particularly efficient in generating ER/PM microdomains [73]. Interestingly, targeting the E-Syts to the ER/PM microdomain requires PI(4,5)P2,. It is dependent and is mediated by Ca2+ binding to the fifth C2 domain [73].
The last proteins known to be present in and required for formation of the ER/PM microdomains are the VAP proteins discussed above [33]. In yeasts the VAPs bind the Oxysterol binding protein related proteins (ORPs) through an FFAT motif and recruit it to the ER/PM microdomains [74]. The ORPs have a PH domain that anchors them by binding PI4P in the plasma membrane [75]. The ORPs can also interact with the PI4P phosphatase Sac1 and recruit it to the ER/PM microdomain to control PI metabolism at this site [76, 77].
Considering the many functions of the ER/PM microdomains and the functional specificity of three E-Syts with respect to regulation of the STIM1-Orai1 gating (see below), it is likely that additional proteins are present and function in the microdomain and are still to be discovered. A common feature of these proteins might be binding to polar lipids in the plasma membrane. Indeed the E-Syts, Ist2, the ORPs and Sac1 all bind PI(4,5)P2 and/or PI4P, which serve to anchor them to the plasma membrane. This also allows regulation of proteins in the ER/PM microdomains or their function by PI4P and PI(4,5)P2.
The Orai1-STIM1 complex in the PI(4,5)P2 microdomain
To illustrate the functional importance of localization to the ER/PM microdomain below we discuss how localization of the Orai1-STIM1 complex in the ER/PM microdomain affects regulation of channel function. The Orai1-STIM1 complex mediates an essential component of the receptor-evoked Ca2+ signal. The receptor-evoked Ca2+ signal is initiated by PLC-mediated generation of IP3 [78] and generation of other second messengers, like cADPR and NAADP [79], to release Ca2+ from the ER and the endolysosomal system, respectively. Ca2+ release from the ER causes co-clustering of STIM1 and the pore forming Ca2+-selective Orai1 channel at the ER/PM microdomain, resulting in activation of Ca2+ influx across the plasma membrane. Ca2+ influx sustain the Ca2+ signal during cell stimulation and replenishes the Ca2+ lost from the ER during both physiological Ca2+ oscillations and maximal activation of Ca2+ release and efflux [80].
Over activation of Ca2+ influx causes Ca2+ toxicity, which is mediated by excesive activation of TRPC [81] and Orai channels [82]. Orai channels have a dominant role in Ca2+ toxicity since Orai1 is essential for Ca2+ influx and regulates the activity of the TRPC channels [83, 84]. Hence, cells strictly regulate the activity of the Orai1 channel. The most potent and immediate form of regulator of Orai1 is by Ca2+ itself. Two modes of regulation of Orai1 by an increase in cytoplasmic Ca2+ have been described and extensively studied; fast Ca2+-dependent inactivation (FCDI), which occurs within milliseconds after channel activation, and slow Ca2+-dependent inactivation (SCDI) that requires several minutes to be completed (reviewed in [85, 86]). A stretch of negatively charged residues in STIM1 appears to participate in FCDI of Orai1 [87-89], and a protein interacting with STIM1 named SARAF was shown to be essential for SCDI of Orai1 [90]. SARAF interacts with the STIM1 SOAR domain [91], which is regulated by a STIM1 domain downstream of SOAR that we named C terminal inhibitory domain (CTID) [92]. More recently, we discovered that interaction of SARAF with STIM1 requires the presence of the STIM1-Orai1 complex at the ER/PM microdomain, which was used to reveal important features of the ER/PM microdomains and its role in regulating Orai1 function [31]. Interestingly, SARAF regulated both modes of Ca2+-mediated inhibition of Orai1, the FCDI and SCDI [31]. How SARAF regulates FCDI remains to be studied.
Since the discovery of STIM1 [93, 94] and Orai1 [95-97] it was clear that the Orai1-STIM1 complex is clustered in ER/PM microdomain and that STIM1 mediates the clustering and targets the complex to the ER/PM microdomain [55, 98]. The proteins that form the microdomain and whether targeting into the microdomain has a regulatory function besides determining the Ca2+ influx sites has just begun to be revealed. A significant recent finding was that septin 4 (and likely septin 5) forms a PI(4,5)P2 microdomain around the Orai1-STIM1 complex [46]. The role of PI(4,5)P2 in regulation of Orai1 was unclear since PI(4,5)P2 does not regulate the activity of store-operated Ca2+ influx or of Orai1 current [31, 99], although a role for plasma membrane PI4P in targeting of proteins that interact with acidic lipids, like STIM1, has been suggested [99, 100]. Another recent finding showed that altering the ER/PM microdomain by knockout of E-Syt1 prolonged the receptor-evoked Ca2+ signal upon repeated stimulation [101], although another study concluded that knockout of E-Syt1 and E-Syt2 alone or together did not affect polarized localization of IP3Rs and the receptor-stimulated Ca2+ signal in hepatocytes [102].
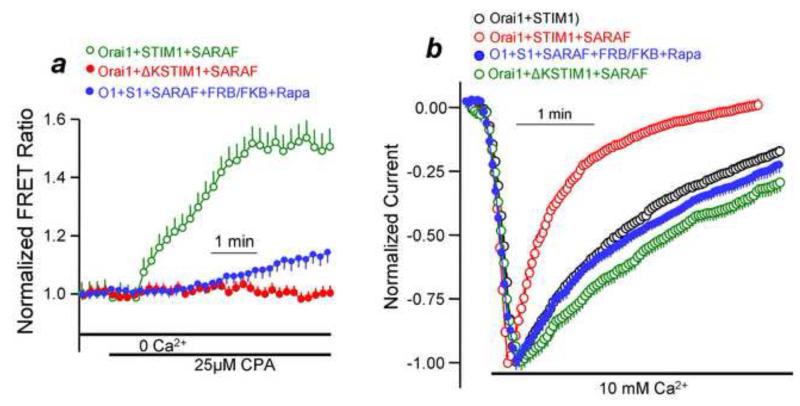
Recently, we reexamined the role of the ER/PM microdomain in the regulation of the Orai1-STIM1 complex and in the role of SARAF [31]. We used inhibition by SARAF as a readout of the STIM1 conformation and localization of the Orai1-STIM1 complex in plasma membrane microdomains. In the resting state SARAF weakly interacts with STIM1 in the ER and only to a limited extent. In response to Ca2+ store depletion and formation of the Orai1-STIM1 complex SARAF is recruited to the complex and specifically interacts with STIM1 [31]. Requirement of SARAF and interaction with STIM1 is delayed and its characterization revealed that it requires the presence of the Orai1-STIM1 complex in a PI(4,5)P2-rich microdomain. This is illustrated in Figure 1. Measurement of FRET between STIM1 and SARAF in the presence of Orai1 to assay the interaction between them shows that the interaction required PI(4,5)P2 and the PI(4,5)P2 binding polybasic domain of STIM1 (Fig. 1a). Similarly, the SARAF-mediated SCDI of Orai1 current was eliminated by depletion of PI(4,5)P2 and by deletion of the STIM1 polybasic domain (Fig. 1b). Importantly, when the Orai1-STIM1 complex was in a PI(4,5)P2-poor microdomain it was still fully active. It just lost the regulation by SARAF.
Fig. 1. Interaction of SARAF with STIM1 and the SARAF-mediated SCDI requires the STIM1 polybasic domain and plasma membrane PI(4,5)P2.
Panel (a): FRET was measured between STIM1 (green) or STIM1(ΔK) (red) and SARAF and in the absence of PI(4,5)P2 (blue). Panel (b): Orai1 current was measured in the presence of STIM1 (black) alone, STIM1+SARAF (red), STIM1(ΔK) (green) and in the presence of STIM1+SARAF and absence of PI(4,5)P2 (blue). The results are reproduced from [31].
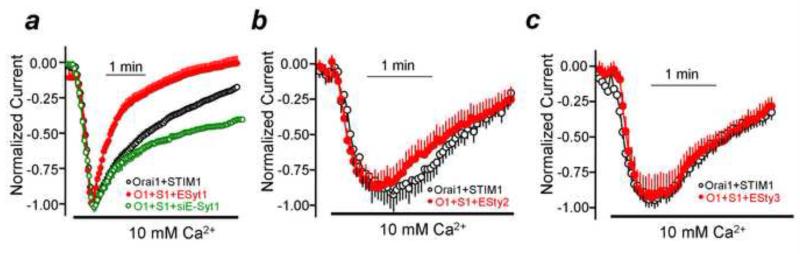
The PI(4,5)P2-rich microdomain in which SARAF interacts with STIM1 is demarcated and tethered by septin 4 and E-Syt1. Septin 4 was shown to form a PI(4,5)P2-rich domain around a Orai1-STIM1 complex [46], whereas E-Syt1 in addition to tethering the ER and plasma membrane may also transport PI(4,5)P2 to the domain, as suggested by the resolved structure of the lipid accommodating barrel shape of E-Syt2 lipid interacting domain [103]. Hence, depletion of septin 4 and E-Syt1 eliminated the STIM1-SARAF FRET and SARAF-mediated SCDI [31]. The role of all E-Syts was examined further by their knockdown and by their overexpression. Remarkably, both the knockdown [31] and the over-expression (Figure 2) showed that the PI(4,5)P2 rich microdomain at which the Orai1-STIM1 complex is regulated by SARAF is specifically tethered by E-Syt1 with no role for E-Syt2 and E-Syt3. This was quite unexpected considering that all E-Syts tether the ER to the plasma membrane and that E-Syt2 and E-Syt3 appear to do be more efficient than E-Syt1 [73].
Fig. 2. Only E-syt1 formed tethers regulate the Orai1-STIM1 complex.
Panel (a): Orai1 current was measured in the presence of STIM1 (black), STIM1+E-Syt1 (red) and in cells treated with siE-Syt1 (green). Panels (b, c): Orai1 current was measured in the presence of STIM1 (black), STIM1+E-Syt2 (red in b) or E-Syt3 (red in c). Note that only E-Syt1 increased SCDI and knockdown of E-Syt1 reduced SCDI. The results in panel (a) are reproduced from [31].
Together, the findings suggest diversity and specificity in the properties of the ER/PM microdomains. Thus, all E-Syts interact with PI(4,5)P2 [73] and many processes and proteins have been shown to be regulated by phosphatidylinositols [32]. The ER/PM microdomains also mediate exchange of lipids and other constituents between organelles [25, 29], are the site for initiation of endocytosis [104] and the localization of the various Ras proteins [105]. It should be of interest to determine which of the E-Syts participate in each of these activities that take place at the ER/PM microdomains. However, it is important to note that a single E-Syt is not sufficient to form a defined an ER/PM microdomain. In fact, disruption of the ER/PM microdomain in yeast required deletion of six proteins: the three tricalbins, Ist2, VAP-A and VAP-B [77]. This can also explain the minor phenotype of the E-Syt2/E-Syt3 double knockout mice [106] and the luck of effect of knockdown of E-Syt1/E-Syt2 on localization of IP3Rs in hepatocytes [102].
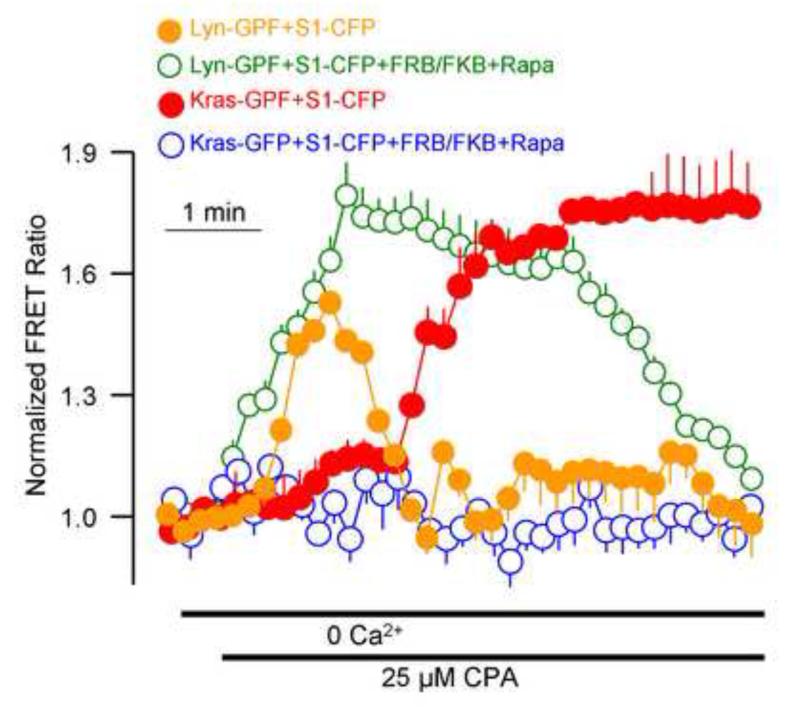
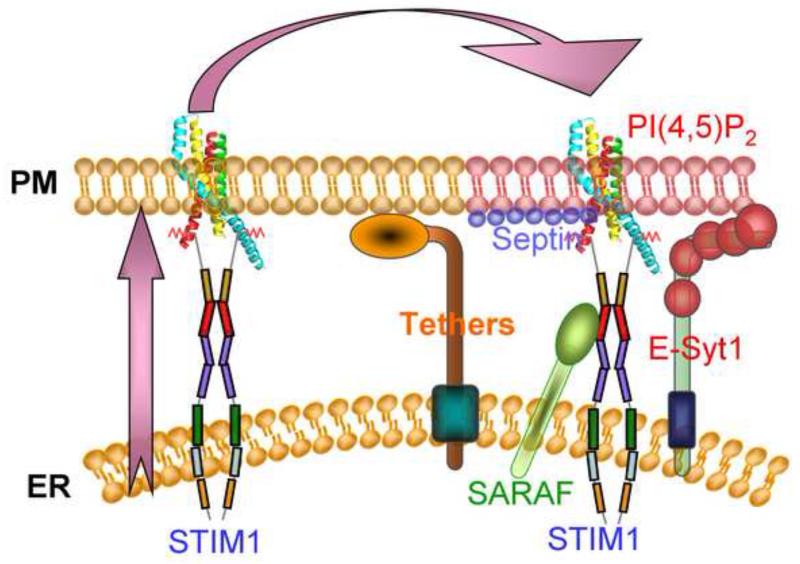
An interesting aspect of the ER/PM microdomains is their dynamic nature. This is clearly demonstrated in the formation of the Orai1-STIM1 complex in response to store depletion. Another aspect of the dynamic nature of the microdomains is that they can be poor or rich in particular lipids such as PI(4,5)P2. It turned that the PI(4,5)P2-poor and PI(4,5)P2-rich domains can be selectively accessed by the targeting motifs of the Ras and Lyn proteins, where the Kras motif targets proteins to the PI(4,5)P2-rich domain and the Hras and Lys motifs target proteins to a PI(4,5)P2-poor domain. Using these tools we were able to provide evidence to suggest that after its formation the Orai1-STIM1 complex is targeted first to a PI(4,5)P2-poor domain and is then translocated to a PI(4,5)P2-rich domain. It is also possible that the PI(4,5)P2-rich domain is formed around the Orai1-STIM1 complex after its formation in the PI(4,5)P2-poor domain. Once the Orai1-STIM1 complex is in a PI(4,5)P2-rich domain SARAF accesses STIM1 to mediated gating of Orai1 by SCDI. This is illustrated in Fig. 3, which shows that upon store depletion clustered STIM1 is transiently present in a PI(4,5)P2-poor domain and only after a 30-60 seconds delay the complex is seen in a PI(4,5)P2-rich domain. The overall findings lead to the model in Figure 4 illustrating assembly and targeting of the Orai1-STIM1 complex to a PI(4,5)P2-poor domain that is free of septin 4 and E-Syt1. In this location the channel is fully active and is not inhibited by Ca2+ to allow maximal Ca2+ influx. Once cytoplasmic Ca2+ raises to a desired concentration, the Orai1-STIM1 complex is translocated to a PI(4,5)P2-rich domain (or a PI(4,5)P2-rich domain is formed around it) and now SARAF interacts with STIM1 to initiate SCDI that markedly reduces Ca2+ influx to match the Ca2+ loss by PMCA and guard against Ca2+ toxicity.
Fig. 3. Dynamic translocation of STIM1 between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains in response to Ca2+ store depletion.
The time course of the translocation of STIM1 to the PI(4,5)P2-poor and PI(4,5)P2-rich domains was evaluated by following FRET between CFP-STIM1 and Lys-GFP and Kras-YFP, respectively, and in the presence or absence of PI(4,5)P2 depletion. Note that store depletion results in a rapid by transient translocation to the Lyn domain (orange) that became more sustained upon PI(4,5)P2 depletion (green), while the translocation to the Kras domain is delayed (red) and is inhibited by deletion of PI(4,5)P2 (blue). The results are reproduced from [31].
Fig. 4. Gating of Orai1 by translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains.
The ER/PM microdomain is formed by E-Syt1-mediated tethering of the ER and plasma membrane and by other yet unknown proteins that most likely include the VAPs and TMAM16 isoform. This domain is rich in PI(4,5)P2 that may be calculated by E-Syt1 and controlled/stabilized by septin 4. Adjust to the PI(4,5)P2-rich microdomain exist a domain that is poor with PI(4,5)P2 . The two domains can be included within the same Orai1-STIM1 puncta. When the stores are depleted of Ca2+ STIM1 opens and form clusters within the ER and then translocates first to the PI(4,5)P2-poor microdomain to cluster and activate Orai1. At this domain the STIM1 polybasic domain is shielded and does not interact with plasma membrane PI(4,5)P2. After initiation of Ca2+ influx by Orai1 the Orai1-STIM1 complex gradually translocates to the PI(4,5)P2-rich microdomain and the complex is stabilize in this domain by interaction of STIM1 polybasic domain with PI(4,5)P2. In this domain STIM1 resumes a conformation that binds SARAF. Binding of SARAF to STIM1 SOAR domain initiates both FCDI and SCDI to reduce Orai1 activity, limiting Ca2+ influx to guard against Ca2+-dependent toxicity.
Regulation by PI(4,5)P2 has been demonstrated for many channels and transporters (reviewed in [32, 107]). For most cases it was assumed and in few cases it was shown that the transporters are regulated by direct interaction with the phosphoinositides that bind to a positively charged stretch of amino acids. The findings with the Orai1-STIM1 complex of the role of E-Syt1-formed PI(4,5)P2-rich domain in channel regulation would suggest that another form of regulation is not by synthesis and breakdown of PI(4,5)P2, but rather by translocation of the regulated channels and transporters between PI(4,5)P2 poor and rich microdomains. Now that we know some of the players like E-Syts, septins and other ER/PM microdomain proteins to be found, it should be informative to determine the role of translocation between PI(4,5)P2 domains in the regulation of the various channels and transporters by PI(4,5)P2 and other phosphoinositides.
Highlights.
-
1)
ER/PM microdomains are formed by tethering proteins such as the E-syts VAPs and the yeast homologues of Ist2
-
2)
The ER/PM microdomains are divided to PI(4,5)P2-poor and PI(4,5)P2-rich domains
-
3)
Dynamic translocation between the PI(4,5)P2-poor and PI(4,5)P2-rich domains is a new form of regulation of proteins in the ER/PM microdomains.
-
4)
Translocation of the Orai1-STIM1 complex from the PI(4,5)P2-poor to the PI(4,5)P2-rich domains determines STIM1 conformation and Orai1 gating by Ca2+.
Acknowledgments
This work was supported by NIH/NIDCR intramural grant DE000735 and by the National Foundation of Korea Grant funded by the Korean Government (NRF-2013S1A2A2035370).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG, Muallem S. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. The Journal of biological chemistry. 2001;276:44146–44156. doi: 10.1074/jbc.M105203200. [DOI] [PubMed] [Google Scholar]
- [2].Gee HY, Kim YW, Jo MJ, Namkung W, Kim JY, Park HW, Kim KS, Kim H, Baba A, Yang J, Kim E, Kim KH, Lee MG. Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology. 2009;137:607–617. 617, e601–604. doi: 10.1053/j.gastro.2009.01.065. [DOI] [PubMed] [Google Scholar]
- [3].Hodges RR, Zoukhri D, Lightman JP, Dartt DA. Identification and cellular localization of the components of the VIP signaling pathway in the lacrimal gland. Advances in experimental medicine and biology. 1998;438:169–176. doi: 10.1007/978-1-4615-5359-5_24. [DOI] [PubMed] [Google Scholar]
- [4].Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. The Journal of biological chemistry. 1997;272:15771–15776. doi: 10.1074/jbc.272.25.15771. [DOI] [PubMed] [Google Scholar]
- [5].Yang YM, Lee J, Jo H, Park S, Chang I, Muallem S, Shin DM. Homer2 protein regulates plasma membrane Ca(2)(+)-ATPase-mediated Ca(2)(+) signaling in mouse parotid gland acinar cells. The Journal of biological chemistry. 2014;289:24971–24979. doi: 10.1074/jbc.M114.577221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. The Journal of biological chemistry. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- [7].Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJ. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. The Journal of biological chemistry. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]
- [8].Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. The Journal of biological chemistry. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- [10].Nlend MC, Schmid A, Sutto Z, Ransford GA, Conner GE, Fregien N, Salathe M. Calcium-mediated, purinergic stimulation and polarized localization of calcium-sensitive adenylyl cyclase isoforms in human airway epithelia. FEBS letters. 2007;581:3241–3246. doi: 10.1016/j.febslet.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gardner JD, Jackson MJ, Batzri S, Jensen RT. Potential mechanisms of interaction among secretagogues. Gastroenterology. 1978;74:348–354. [PubMed] [Google Scholar]
- [12].Lee RJ, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Cohen NA. Vasoactive intestinal peptide regulates sinonasal mucociliary clearance and synergizes with histamine in stimulating sinonasal fluid secretion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:5094–5103. doi: 10.1096/fj.13-234476. [DOI] [PubMed] [Google Scholar]
- [13].MacDonald MJ. Synergistic potent insulin release by combinations of weak secretagogues in pancreatic islets and INS-1 cells. The Journal of biological chemistry. 2007;282:6043–6052. doi: 10.1074/jbc.M606652200. [DOI] [PubMed] [Google Scholar]
- [14].Betzenhauser MJ, Fike JL, Wagner LE, 2nd, Yule DI. Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937. The Journal of biological chemistry. 2009;284:25116–25125. doi: 10.1074/jbc.M109.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yule DI, Betzenhauser MJ, Joseph SK. Linking structure to function: Recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell calcium. 2010;47:469–479. doi: 10.1016/j.ceca.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Science signaling. 2012;5:ra29. doi: 10.1126/scisignal.2002299. [DOI] [PubMed] [Google Scholar]
- [17].Willoughby D, Ong HL, De Souza LB, Wachten S, Ambudkar IS, Cooper DM. TRPC1 contributes to the Ca2+-dependent regulation of adenylate cyclases. The Biochemical journal. 2014;464:73–84. doi: 10.1042/BJ20140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nature cell biology. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- [19].Park S, Shcheynikov N, Hong JH, Zheng C, Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, Seki G, Yule D, Mikoshiba K, Muallem S. Irbit mediates synergy between ca(2+) and cAMP signaling pathways during epithelial transport in mice. Gastroenterology. 2013;145:232–241. doi: 10.1053/j.gastro.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Molecular cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- [21].Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. The Journal of clinical investigation. 2011;121:956–965. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3- secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. The Journal of clinical investigation. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiological reviews. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stefan CJ, Manford AG, Emr SD. ER-PM connections: sites of information transfer and inter-organelle communication. Current opinion in cell biology. 2013;25:434–442. doi: 10.1016/j.ceb.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prinz WA. Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. The Journal of cell biology. 2014;205:759–769. doi: 10.1083/jcb.201401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca(2)(+) and reactive oxygen species signaling. Journal of cell science. 2013;126:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Naon D, Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochimica et biophysica acta. 2014;1843:2184–2194. doi: 10.1016/j.bbamcr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- [28].Penny CJ, Kilpatrick BS, Han JM, Sneyd J, Patel S. A computational model of lysosome-ER Ca2+ microdomains. Journal of cell science. 2014;127:2934–2943. doi: 10.1242/jcs.149047. [DOI] [PubMed] [Google Scholar]
- [29].Lahiri S, Toulmay A, Prinz WA. Membrane contact sites, gateways for lipid homeostasis. Current opinion in cell biology. 2015;33C:82–87. doi: 10.1016/j.ceb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lahiri S, Chao JT, Tavassoli S, Wong AK, Choudhary V, Young BP, Loewen CJ, Prinz WA. A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria. PLoS biology. 2014;12:e1001969. doi: 10.1371/journal.pbio.1001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maleth J, Choi S, Muallem S, Ahuja M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nature communications. 2014;5:5843. doi: 10.1038/ncomms6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiological reviews. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lev S, Ben Halevy D, Peretti D, Dahan N. The VAP protein family: from cellular functions to motor neuron disease. Trends in cell biology. 2008;18:282–290. doi: 10.1016/j.tcb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- [34].Loewen CJ, Roy A, Levine TP. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. The EMBO journal. 2003;22:2025–2035. doi: 10.1093/emboj/cdg201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Olkkonen VM, Li S. Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Progress in lipid research. 2013;52:529–538. doi: 10.1016/j.plipres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- [36].Wang PY, Weng J, Anderson RG. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science. 2005;307:1472–1476. doi: 10.1126/science.1107710. [DOI] [PubMed] [Google Scholar]
- [37].Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- [38].Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- [39].Sellin ME, Holmfeldt P, Stenmark S, Gullberg M. Microtubules support a disk-like septin arrangement at the plasma membrane of mammalian cells. Molecular biology of the cell. 2011;22:4588–4601. doi: 10.1091/mbc.E11-09-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Developmental cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- [41].Chao JT, Wong AK, Tavassoli S, Young BP, Chruscicki A, Fang NN, Howe LJ, Mayor T, Foster LJ, Loewen CJ. Polarization of the endoplasmic reticulum by ER-septin tethering. Cell. 2014;158:620–632. doi: 10.1016/j.cell.2014.06.033. [DOI] [PubMed] [Google Scholar]
- [42].Kinoshita M, Noda M. Roles of septins in the mammalian cytokinesis machinery. Cell structure and function. 2001;26:667–670. doi: 10.1247/csf.26.667. [DOI] [PubMed] [Google Scholar]
- [43].Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Current biology : CB. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC evolutionary biology. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature. 2013;499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bertin A, McMurray MA, Thai L, Garcia G, 3rd, Votin V, Grob P, Allyn T, Thorner J, Nogales E. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. Journal of molecular biology. 2010;404:711–731. doi: 10.1016/j.jmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tanaka-Takiguchi Y, Kinoshita M, Takiguchi K. Septin-mediated uniform bracing of phospholipid membranes. Current biology : CB. 2009;19:140–145. doi: 10.1016/j.cub.2008.12.030. [DOI] [PubMed] [Google Scholar]
- [49].Toulmay A, Prinz WA. A conserved membrane-binding domain targets proteins to organelle contact sites. Journal of cell science. 2012;125:49–58. doi: 10.1242/jcs.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Porter KR, Palade GE. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. The Journal of biophysical and biochemical cytology. 1957;3:269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dirksen RT. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Frontiers in bioscience : a journal and virtual library. 2002;7:d659–670. doi: 10.2741/A802. [DOI] [PubMed] [Google Scholar]
- [52].Shaw RM, Colecraft HM. L-type calcium channel targeting and local signalling in cardiac myocytes. Cardiovascular research. 2013;98:177–186. doi: 10.1093/cvr/cvt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Quintana A, Hoth M. Mitochondrial dynamics and their impact on T cell function. Cell calcium. 2012;52:57–63. doi: 10.1016/j.ceca.2012.02.005. [DOI] [PubMed] [Google Scholar]
- [54].Hayashi M, Raimondi A, O’Toole E, Paradise S, Collesi C, Cremona O, Ferguson SM, De Camilli P. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Orci L, Ravazzola M, Le Coadic M, Shen WW, Demaurex N, Cosson P. From the Cover: STIM1-induced precortical and cortical subdomains of the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19358–19362. doi: 10.1073/pnas.0911280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- [57].Juschke C, Wachter A, Schwappach B, Seedorf M. SEC18/NSF-independent, protein-sorting pathway from the yeast cortical ER to the plasma membrane. The Journal of cell biology. 2005;169:613–622. doi: 10.1083/jcb.200503033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Franz A, Maass K, Seedorf M. A complex peptide-sorting signal, but no mRNA signal, is required for the Sec-independent transport of Ist2 from the yeast ER to the plasma membrane. FEBS letters. 2007;581:401–405. doi: 10.1016/j.febslet.2006.12.048. [DOI] [PubMed] [Google Scholar]
- [59].Wolf W, Kilic A, Schrul B, Lorenz H, Schwappach B, Seedorf M. Yeast Ist2 recruits the endoplasmic reticulum to the plasma membrane and creates a ribosome-free membrane microcompartment. PloS one. 2012;7:e39703. doi: 10.1371/journal.pone.0039703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lavieu G, Orci L, Shi L, Geiling M, Ravazzola M, Wieland F, Cosson P, Rothman JE. Induction of cortical endoplasmic reticulum by dimerization of a coatomer-binding peptide anchored to endoplasmic reticulum membranes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6876–6881. doi: 10.1073/pnas.1002536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- [62].Jang Y, Oh U. Anoctamin 1 in secretory epithelia. Cell calcium. 2014;55:355–361. doi: 10.1016/j.ceca.2014.02.006. [DOI] [PubMed] [Google Scholar]
- [63].Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- [64].Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- [66].Pifferi S, Dibattista M, Menini A. TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Archiv : European journal of physiology. 2009;458:1023–1038. doi: 10.1007/s00424-009-0684-9. [DOI] [PubMed] [Google Scholar]
- [67].Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- [68].Grubb S, Poulsen KA, Juul CA, Kyed T, Klausen TK, Larsen EH, Hoffmann EK. TMEM16F (Anoctamin 6), an anion channel of delayed Ca(2+) activation. The Journal of general physiology. 2013;141:585–600. doi: 10.1085/jgp.201210861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yang H, Kim A, David T, Palmer D, Jin T, Tien J, Huang F, Cheng T, Coughlin SR, Jan YN, Jan LY. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell. 2012;151:111–122. doi: 10.1016/j.cell.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Schulz TA, Creutz CE. The tricalbin C2 domains: lipid-binding properties of a novel, synaptotagmin-like yeast protein family. Biochemistry. 2004;43:3987–3995. doi: 10.1021/bi036082w. [DOI] [PubMed] [Google Scholar]
- [71].Toulmay A, Prinz WA. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. The Journal of cell biology. 2013;202:35–44. doi: 10.1083/jcb.201301039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Min SW, Chang WP, Sudhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. The Journal of cell biology. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. The Journal of biological chemistry. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- [76].Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- [77].Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- [78].Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochimica et biophysica acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [79].Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. The Journal of biological chemistry. 2012;287:31633–31640. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS letters. 2010;584:2022–2027. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. 2115, e2101–2104. doi: 10.1053/j.gastro.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gerasimenko JV, Gryshchenko O, Ferdek PE, Stapleton E, Hebert TO, Bychkova S, Peng S, Begg M, Gerasimenko OV, Petersen OH. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13186–13191. doi: 10.1073/pnas.1300910110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native Store-operated Ca2+ Influx Requires the Channel Function of Orai1 and TRPC1. The Journal of biological chemistry. 2009;284:9733–9741. doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca(2)+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca(2)+ signals required for specific cell functions. PLoS biology. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiological reviews. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- [86].Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- [87].Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14687–14692. doi: 10.1073/pnas.0904664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15495–15500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Derler I, Fahrner M, Muik M, Lackner B, Schindl R, Groschner K, Romanin C. A Ca2(+ )release-activated Ca2(+) (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2(+)-dependent inactivation of ORAI1 channels. The Journal of biological chemistry. 2009;284:24933–24938. doi: 10.1074/jbc.C109.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–438. doi: 10.1016/j.cell.2012.01.055. [DOI] [PubMed] [Google Scholar]
- [91].Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nature cell biology. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jha A, Ahuja M, Maleth J, Moreno CM, Yuan JP, Kim MS, Muallem S. The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. The Journal of cell biology. 2013;202:71–79. doi: 10.1083/jcb.201301148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. The Journal of cell biology. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- [97].Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. The Journal of cell biology. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T. Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. The Journal of biological chemistry. 2009;284:21027–21035. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337:727–730. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep. 2013;5:813–825. doi: 10.1016/j.celrep.2013.09.038. [DOI] [PubMed] [Google Scholar]
- [102].Amaya MJ, Oliveira AG, Schroeder LK, Allgeyer ES, Bewersdorf J, Nathanson MH. Apical localization of inositol 1,4,5-trisphosphate receptors is independent of extended synaptotagmins in hepatocytes. PloS one. 2014;9:e114043. doi: 10.1371/journal.pone.0114043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature. 2014;510:552–555. doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Jean S, Tremblay MG, Herdman C, Guillou F, Moss T. The endocytic adapter E-Syt2 recruits the p21 GTPase activated kinase PAK1 to mediate actin dynamics and FGF signalling. Biology open. 2012;1:731–738. doi: 10.1242/bio.2012968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhou Y, Hancock JF. Ras nanoclusters: Versatile lipid-based signaling platforms. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbamcr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- [106].Herdman C, Tremblay MG, Mishra PK, Moss T. Loss of Extended Synaptotagmins ESyt2 and ESyt3 does not affect mouse development or viability, but in vitro cell migration and survival under stress are affected. Cell cycle. 2014;13:2616–2625. doi: 10.4161/15384101.2014.943573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC. Phosphoinositides regulate ion channels. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbalip.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]