Abstract
Ecological constraints on independent breeding are recognized as major drivers of cooperative breeding across diverse lineages. How the prevalence and degree of cooperative breeding relates to ecological variation remains unresolved. Using a large dataset on cooperative nesting in Polistes wasps we demonstrate that different aspects of cooperative breeding are likely to be driven by different aspects of climate. Whether or not a species forms cooperative groups is associated with greater short-term temperature fluctuations. In contrast, the number of cooperative foundresses increases in more benign environments with warmer, wetter conditions. The same dataset reveals that intraspecific responses to climate variation do not mirror genus-wide trends and instead are highly heterogeneous among species. Collectively these data suggest that the ecological drivers that lead to the origin or loss of cooperation are different from those that influence the extent of its expression within populations.
Keywords: social evolution, bet hedging, ecological constraints, helping behavior, climate predictability, social insects, group size, reproductive skew, thermoregulation
INTRODUCTION
Although the importance of relatedness in shaping patterns of cooperation has recently been debated (Nowak et al. 2010; Liao et al. 2015), there is broad theoretical and empirical consensus that ecological constraints on independent breeding can favor cooperation (Brockmann 1997; Hatchwell & Komdeur 2000; Nowak et al. 2010; Jetz & Rubenstein 2011; Purcell 2011). Comparative and field studies have documented diverse ecological constraints on independent breeding including habitat saturation (Komdeur 1992), harsh foraging conditions (Faulkes et al. 1997), predation (Strassmann et al. 1988), and parasitism (Feeney et al. 2013). Despite several early comparative studies comparing cooperation and environmental factors (Reeve 1991; Faulkes et al. 1997; Arnold & Owens 1999), the nature of the environmental constraints that favor cooperative breeding and the extent of their influence across lineages remain largely unresolved.
There has been renewed interest in recent years in using phylogenetic comparative methods and global climate datasets to identify aspects of environmental variation that are associated with cooperative breeding (e.g. Jetz & Rubenstein 2011; Gonzalez et al. 2013). However, two major critiques of comparative studies of cooperative breeding have emerged. First, comparative analyses of cooperative breeding tend to rely on binary classifications of social systems, while ignoring the variation in the intensity of cooperation among taxa (Ligon & Burt 2004; Cockburn 2013). Second, macroevolutionary patterns in some groups contradict findings from many population-level studies, complicating the interpretation of results (Cockburn & Russell 2011; Cockburn 2013). At the heart of both critiques is our ability to distinguish which environmental factors are associated with the presence versus extent of cooperative breeding. Categorical classification systems may reveal which environmental factors are associated with the presence of cooperative strategies, but only data comparing the degree of cooperation across taxa can provide insights into what factors shape the extent of cooperation among lineages.
There are at least two alternative views on the role that environmental factors play on the occurrence and intensity of cooperative breeding. One view argues for cooperation being considered as a continuum (Sherman et al. 1995; Avilés & Harwood 2012). Under such a scenario, elevated values of a particular environmental feature might be associated with cooperation and the most cooperative species are expected to occupy ranges with the most extreme environmental values. Changes in cooperation levels in this scenario are also expected to be associated with the tightening or relaxation of environmental constraints. Under such a model, cooperative breeding is expected to evolve as a continuum, such that a shift from singular breeding to breeding in groups of two should not be fundamentally different from a shift going from two to three and even further increases thereafter (Sherman et al. 1995). If cooperation is a continuum, we may also expect the environmental features that shape macroevolutionary patterns to explain intraspecific patterns of variation in cooperative breeding as well (Cockburn 2013).
Alternatively, several authors have argued that non-cooperative and cooperative breeding systems represent shifts between qualitatively distinct social regimes (Brown 1987; Wcislo & Tierney 2009), and that the factors influencing whether or not a species cooperates are likely to differ from those that shape the proportion of the population that pursue a helping strategy (West et al. 2007). This hypothesis predicts that the presence of cooperation should be favored on one side of an environmental threshold and that the rates of helping among cooperative species are not necessarily driven by the same environmental factors.
Whether variation in cooperative breeding among species is best explained by a continuous or a threshold model has important implications for understanding the evolution of cooperation. The major challenge in distinguishing between these alternatives has been a lack of quantitative estimates of variation in rates of cooperation across species as the few data available on social systems tend to be coarse-grained, and potentially arbitrarily categorized (Cockburn 2013). High-resolution datasets that provide quantitative estimates of the extent of cooperation across species are sorely needed.
To test the relationship between climate and cooperative breeding in Polistes paper wasps, a model genus in sociobiology (Jandt et al. 2014), we constructed a dataset of nesting behavior for over 30,000 wasps from 51 species (Table S1). Paper wasp colonies are initiated by adult females, known as foundresses or queens (Reeve 1991). In temperate habitats, colony foundation occurs in the spring, after adult wasps emerge from winter diapause. Colony foundation is more asynchronous in the tropics (Reeve 1991). While Polistes wasps are eusocial (i.e., there are queens and workers), there is marked variation across species in the extent to which new nests are founded by solitary foundresses (non-cooperative) or associations of multiple foundresses (cooperative) (Fig 1). Thus, species appear to differ in the extent to which foundresses seek to join established nests, accept potential cooperators or some combination of those two. Within cooperative associations, foundresses engage in dominance contests with the most dominant foundress assuming the role of the primary egg layer while lower ranking individuals engage in more foraging and less reproduction (Jandt et al. 2014). Thus, Polistes foundress associations present a classic example of cooperative breeding with skewed reproduction among nest members (Reeve et al. 2000; Seppa et al. 2002; Leadbeater et al. 2011).
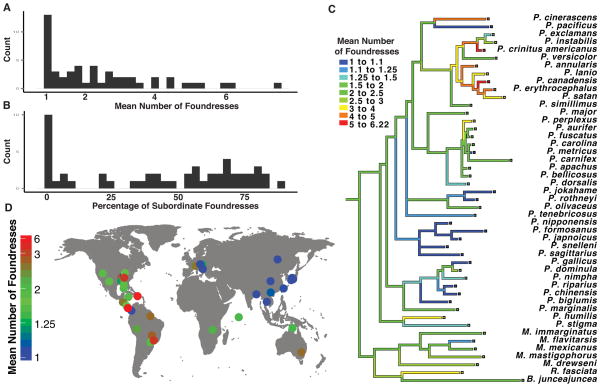
FIGURE 1.

Polistine wasp nests are initiated by single foundress or groups of foundresses. The open-structure of the nest makes determination of group size and colony stage straightforward. Species shown left to right are P. fuscatus, P. annularis and P. bahamensis (Photos by M.J. Sheehan).
In this paper we set out to answer three fundamental questions regarding the relationship between climate and cooperative nesting in Polistes. First, what aspects of climate are associated with the presence or absence of cooperative nesting? Second, are these same climatic features associated with the extent of cooperation among species? Third, to what extent do climatically driven patterns of variation in cooperative nesting within species match patterns of variation among species within a genus?
METHODS
Data on cooperative behavior
We collected information on cooperative behavior from published data on wasp nesting, our own unpublished field records, and data from online natural history databases including Bugguide (http://bugguide.net) the Atlas of Australia (http://www.ala.org.au) and iNaturalist (www.inaturalist.org) of foundress associations in wasps. The uncovered nests of Polistes wasps allow for the easy determination of the stage of colony progression, even from photographs. As a general rule, published accounts of wasp nests (and our own field data) report counts made during the early morning or late evening, when all foundresses are present and quantifying the number of wasps on a nest is straightforward. However, because the number of foundresses observed at a nest can fluctuate over the course of colony development and throughout the day, the numbers reported here should be seen as estimates rather than the ‘true’ numbers of foundresses per species (West Eberhard 1969). Because we were interested in the number of foundresses that associate in the formation of colonies, we included observations of colonies during the pre-emergence phase of the colony cycle during which only foundresses are present (West Eberhard 1969; Reeve 1991). If observations of the pre-emergence phase nests were not available we used records of the number of foundresses present on nests as determined by dissection of ovarian development or the number of foundresses that contributed to the brood via genetic analysis. Indirect measures of foundress number (i.e., photographs and ovarian counts) constituted only 1.17% (101/8613) of the nests observed in Polistes in our dataset (Supplemental text).
Analyzing the present dataset requires a balance between including data on more species and stringent filtering for data quality. We strike a balance between inclusivity and data quality by conducting two separate analyses. In the first analysis we use all the data available and report aggregate values for each species (hereafter referred to as the ‘aggregate’ analysis). The aggregate dataset has the benefit of including as many species as possible, though estimates of the size of cooperative nesting groups are based on small numbers of foundresses in some cases (Table S1). In the second analysis, we stringently filtered the data by only considering well-sampled localities for each species (hereafter referred to as the ‘locality’ analysis). The locality analysis includes fewer species and phylogenetic contrasts, but the continuous estimates are robust as they are based on the behavior of many foundresses from the same location (Supplemental Text).
In the aggregate dataset, we made use of all available data for each species to estimate rates of cooperation, aggregating nest observation data from all sources (Table S1). We use the aggregate dataset (Table S1) for three analyses: (1) the distribution of rates of cooperation across species, (2) ancestral state reconstructions, (3) comparative analyses of the relationship between cooperation and climate. In addition to continuous estimates of the average size of cooperative foundress associations, we also categorized species as either ‘cooperative’ or ‘non-cooperative’ based on categorizations used in the literature. We note that the continuous estimates of cooperation are in agreement with traditional descriptive categories.
In the locality dataset (Table S2), we made an attempt to define localities as narrowly as possible, to the level of municipality, using the verbal descriptions or specific place names of sampling in each study. Although some variation in climate can be expected within large metropolitan areas or municipalities, it is unlikely that characterizing the climate variables of such localities from a single georeference will bias our results because variation in precipitation and temperature at a local scale is minimal in comparison to the regional differences observed between distant localities from the same or different species. We were conservative in our locality dataset and only considered localities where the nesting behavior of at least 20 foundresses from a given species had been observed (N = 129 localities across 28 species, range 1–22 localities per species, Table S2).
For both the aggregate and locality datasets, we calculated the mean number of foundresses as well as the percentage of foundresses in a subordinate role, measures that have been previously used to compare rates of cooperative nesting in Polistes (Hughes et al. 1993). We chose to measure cooperative nesting behavior as mean number of foundresses and percent subordinates because it was possible to calculate these statistics for the largest number of records in our dataset. We considered the number of foundresses observed in excess of the number of nests as subordinate foundresses because, by extension, such foundresses could not have nested solitarily and because each nest has a single most dominant female. For example, if 150 foundresses were observed on a total of 100 nests, then the 50 excess foundresses were considered subordinate, meaning that 33% of the foundresses observed in the population were subordinate. These measures are related to each other, though not in a linear manner (Fig S1). In particular, calculating percentage of foundresses in a subordinate role places greater emphasis on variation between means of 1 and 2 foundresses (i.e., 0–50%) than between higher rates of cooperation, e.g. an increase from a mean of 2–3 foundresses corresponds to 50–66.67%. Overall, we believe that these measures reasonably capture variation in the extent of cooperation across species as they distinguish between species with nests of different foundress-association sizes.
It is important to emphasize that our measures deal with size of cooperative foundress associations and are not measures of how reproduction is apportioned within groups. In general, multiple foundress associations in Polistes wasps show evidence of reproductive skew among foundress, though the extent of skew is highly variable even within populations (Reeve et al. 2000; Seppa et al. 2002). While dominant foundresses typically enjoy a disproportionate share of reproduction within multiple foundress associations, they are not the sole breeders; subordinate reproduction is commonly reported in Polistes (Reeve et al. 2000; Seppa et al. 2002). Regardless of the amount of skew, multiple foundress associations are cooperative in the sense that foundresses provide care to offspring that are not their own (West Eberhard 1969; Reeve 1991; Jandt et al. 2014).
Phylogenetic reconstruction
Phylogenetic reconstruction was performed on 71 taxa, 17 out-group species in the genera Apoica, Mischocyttarus, Polybia, Protopolybia and Ropalidia, and 54 Polistes taxa, using sequences from two mitochondrial loci. All sequence data was taken from GenBank (Table S3). A 563 base pair portion of the 16S ribosomal RNA gene was used for all taxa except ten, P. apachus, P. carnifex, P. biglumis, P. olivaceus, P. erythrocephalus, P. satan, P. instabilis, P. versicolor, M. immarginatus, M. mexicanus, and R. fasciata, for which only 350 or fewer bases were available. Additionally, a 1234 base pair portion of the cytochrome oxidase subunit I (COI) gene was used. For COI sequences 56 taxa had at least 75% shared sequence length included, however, only 376–658 bases were available for 19 of the taxa. Sequences for each gene were aligned separately using ClustalW (Thompson et al., 1994) and manually adjusted for accuracy. These alignments were then concatenated and used for Bayesian analyses in MRBAYES v3.1.2 (Huelsenbeck and Ronquist, 2001). Two runs of four parallel Markov chain Monte Carlo chains under the GTR + I + Γ model were performed for 800,000 generations, sampling every 1000 generations, at which point the standard deviation of split frequencies was effectively zero. From each analysis a 50% majority rule consensus tree was produced from 1000 samples with a 25% burn-in of trees. Multiple polytomies with low support were recovered in the analysis. However, the overall topology of the tree is very similar to that resolved previously using morphological data (Pickett & Carpenter 2010), suggesting that the low support values stem from a need for more informative sequence data rather than inaccurate tree reconstruction. A full version of the phylogeny is shown in Fig S2.
Ancestral State and Area Reconstruction
We reconstructed the evolutionary history of cooperative nesting in Polistes using the parsimony reconstruction model of continuous data in Mesquite v 2.75 (Maddison & Maddison 2001) using the mean number of foundresses and percent of subordinate foundresses as a continuous measures respectively. Additionally, we considered the evolution of cooperative breeding as a categorical variable, using the likelihood reconstruction model for categorical data in Mesquite v 2.75. We ran the analyses using the previously constructed Bayesian tree pruned to 47 species for which we had data on social systems: 40 species of Polistes and 5 species of Mischocyttarus, 1 species of Belonogaster and 1 species of Ropalidia as outgroups.
We reconstructed the evolution of geographic ranges in Polistes and its relatives using the maximum likelihood dispersal-extinction-cladogenesis model as implemented in “Lagrange” (Ree & Smith 2008). We employed a temporally unconstrained model in which dispersal probabilities between regions were assumed to be symmetric.
The following six biogeographic regions were used in the analysis: a) Neotropics (South America, Central America and the Caribbean), b) the Nearctic (North America); c) the Western Palearctic (Europe, Central Asia, Middle East, and North Africa); d) the Eastern Palearctic (temperate East Asia); e) Indo-Malaya and Oceania (South Asia, Peninsular and Insular Southeast Asia, New Guinea, and Australia); and f) the Afrotropics (Sub-Saharan Africa). See the supplemental methods for further justification of the choice of regions. We calculated a geographic reconstruction pertaining to one fully dichotomous phylogeny randomly resolved from the multichotomous tree using the R package ‘picante’ (Fig S3, Kembel et al. 2010).
Climate analysis
We used a total of 4103 georeferenced observations of species from museum specimens (GBIF: http://www.gbif.org), field observations (Bugguide: http://bugguide.net; Atlas of Australia: http://www.ala.org.au) and localities described in the published literature on each species (median number of records = 58, range: 4 to 1108). For each record of each species we extracted 13 variables capturing the mean, variance and predictability of temperature, precipitation and primary productivity variables from Bioclim and the CRU-TS 3.1 Climate Database (Mitchell & Jones 2005, see Table S4 for further information on the variables considered). Predictability of climate variables was measured as Colwell’s P (Colwell 1974), which takes into account both the contingency and constancy of climate patterns between years. The aggregate species mean for each of the variables was calculated and a principal component analysis performed on the 40 Polistes species used in the comparative analyses. The first two principal components explained approximately 75% of the variation in the aggregate dataset and can be interpreted as corresponding to variation in environmental harshness (PC1, 57% of the variance) and short-term temperature fluctuation (PC2, 17% of the variance) (Supplemental text, Fig S4a). For PC1, higher values are associated with lower mean temperatures and rainfall with lower values associated with warmer, wetter conditions. High values of PC2 are associated with low differences between the high and low temperatures within a month and lower values have higher amplitude short-term temperature fluctuations. We ran an additional PCA analysis with the climate data limited to georeference points used in our locality dataset (Table S2). The results of this analysis are similar to those found for the aggregate dataset climate PCA (Fig S4b): the first two principal components explain approximately 66% of the variation and correspond roughly to the same environmental features as those captured in the aggregate species PCA. That is, environmental harshness or PC1 captured 47% of the variance, and short-term temperature fluctuation or PC2 captured 20% of the variance. Indeed, the loadings on PC1 for both the species-level and population-level datasets are nearly identical (linear regression, r2 = 0.95, B = 1.01, P < 0.0001) and the loadings on PC2 are very similar (linear regression, r2 = 0.71, B = 0.79, P = 0.0003), reflecting the fact that both of these analyses ultimately have similarly balanced global coverage. Loading of the variables on different PC axes can be found in Table S5. Although the PC axes in both cases are not exact replicas, the high level of similarity allows for reasonably direct comparisons between the inclusive aggregate and more stringently filtered locality datasets (Fig S5).
Four temperate species - P. dominula, P. exclamans, P. fuscatus, and P. metricus - were sampled at a sufficiently large and geographically disparate set of localities to allow us to investigate intraspecific relationships between climate and cooperative nesting. Using subsets of the locality dataset for each species, we modeled the mean number of foundresses observed in a given locality as a function of environmental factors.
Comparative Analyses
We examined the relationships between the principal components of climate variation and our different measures of cooperation. First, we examined both PC axes as predictors of cooperation coded as a categorical variable (cooperative v. non-cooperative). Next, we considered both PC axes as predictors of continuous measures of cooperative nesting (i.e., mean number of foundresses and the percent of subordinate foundresses). We next examined the effects of both climate axes on continuous variation in cooperation in a reduced dataset including only the cooperative species. Species were categorized as either cooperative or non-cooperative based on traditional categorizations and descriptions of the species in the literature. We examined the relationship between climate and cooperative breeding with Bayesian phylogenetic mixed models using the package MCMCglmm in R (Hadfield 2010) with flat non-informative priors, 600000 iterations, a burnin of 200000 and a thinning interval of 100 iterations used in all analyses. Visual inspection of the MCMC chain demonstrated convergence in all cases. Additionally, we analyzed our data using phylogenetic generalized least squares analyses using the R package Caper (Orme et al. 2012) to ascertain their robustness to different modeling procedures. For each analysis we pruned the overall phylogenetic tree (Fig S2) to taxa for which we had data. In the locality analysis, species observed at multiple localities were represented by a polytomy with multiple tips.
FIGURE 2.
(A) The mean number of foundreses shows a leptokurtotic distribution across Polistes species. (B) Rates of cooperation measured as the percent of subordinate foundresses are bimodally distributed among Polistes paper wasp species. (C) Phylogeny of Polistes wasps with rates of cooperative nesting mapped onto the tree. Rates of cooperation have been evolutionary labile, with multiple independent losses of cooperative breeding. (D) Range centers for species examined in our cooperation data set. The dots each represent the average latitude and longitude for each species examined, with the color denoting the level of cooperation observed in that species. Non-cooperative species are clustered in eastern Asia while the most cooperative species are found in the Neotropics.
RESULTS
Distribution and evolutionary history of cooperative breeding
The average number of foundresses observed on pre-emergence nests in Polistes species ranges from 1 to 7.5, showing a positive skewed distribution (Fig 2A, skewness = 1.19). The percent of subordinate foundresses varies from 0 to 87% across species and shows a strongly bimodal distribution. (Fig 2B, Hartigan’s dip test, D = 0.098, P = 0.0004, N = 51 species). Removing poorly sampled species (< 15 foundresses observed) does not alter this result (Hartigan’s dip test, D = 0.10, P = 0.0003, N = 40 species). Notably, the species on either side of the break in the distribution (greater or less than 10% subordinates) have historically been categorized as non-cooperative and cooperative, suggesting that these categorical descriptors may capture a biologically relevant break in patterns of cooperative nesting.
Ancestral state reconstruction indicates that cooperative nest founding has been evolutionarily labile. There is broad agreement across reconstructions using mean number of foundresses, percent subordinate foundresses, and categorical measures (Fig 2C, S6). Cooperative breeding has been lost multiple independent times in Polistes, with increased rates of cooperation seen in some lineages (Figs 2C). At least three independent losses of cooperative nesting in Polistes involve species or clades that have independently invaded eastern Asia (Fig 2C–D, S3). Additionally, species with the highest rates of cooperation are found in the Neotropics (Fig 2D). The clustering of non-cooperative and highly cooperative species in different geographic regions suggests that climatic factors may have played a role in the evolution of cooperative nesting behavior in this genus.
Climatic correlates of cooperative breeding across species
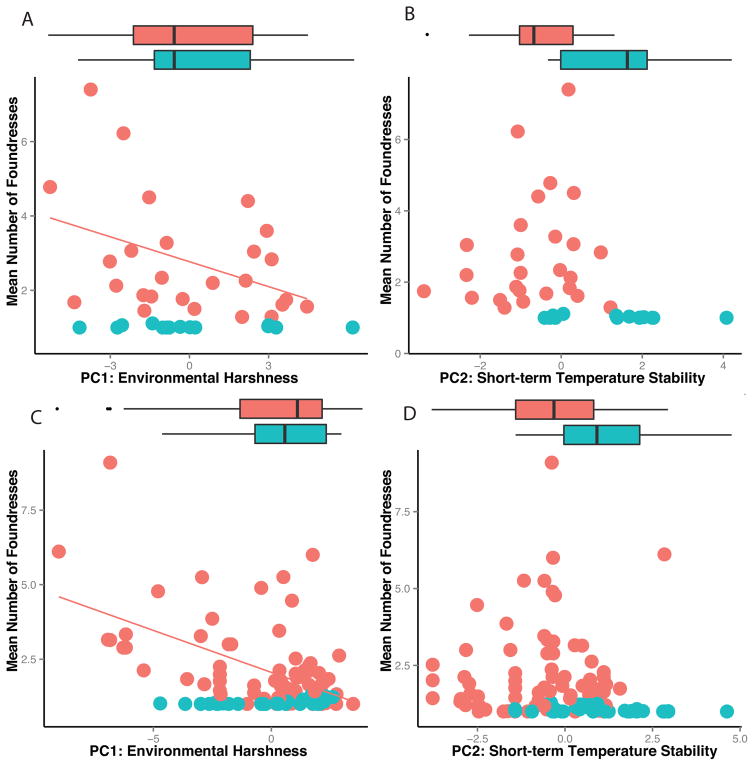
Different aspects of climate variation are correlated with the formation versus the size of cooperative foundress associations in Polistes. Table 1 shows the output for models of categorical and continuous measures of cooperation with the first two PC axes, environmental harshness and short-term temperature fluctuation as main effects. Categorical models using both the aggregate and locality datasets show that non-cooperative species occur in regions with greater short-term temperature stability (Fig 3). Conversely, cooperative nesting is associated with higher amplitude fluctuations in temperature. Continuous models show that the size of cooperative nesting associations is inversely related to environmental harshness, with higher rates of cooperative nesting occurring in more benign regions, i.e. the tropics (Fig 3). Notably, environmental harshness is a better predictor of rates of cooperation when non-cooperative species are excluded from both the aggregate and locality datasets (Table 1). The overall pattern of results is equivalent whether we measure cooperation as the mean number of foundresses (Table 1) or the percentage of subordinate foundresses (Supplemental text, Table S6).
Table 1.
Comparative results
| Cooperation measure | Climate PC axis | posterior mean | Bayesian | pMCMC | PGLS | ||
|---|---|---|---|---|---|---|---|
| 95% CI Bounds | t | P | r2 | ||||
| All aggregate data (N = 40 species) | |||||||
| Categorical | Env. Harshness | −8.68 | −56.92 – 38.17 | 0.72 | −0.28 | 0.78 | 0.37 |
| Temp. Stability | 175.66 | 36.69 – 318.94 | 0.0013 | −5.00 | <0.0001 | ||
| Mean | Env. Harshness | −0.112 | −0.30 – 0.065 | 0.21 | −1.19 | 0.24 | 0.02 |
| Temp. Stability | −0.191 | −0.52 – 0.15 | 0.26 | −1.11 | 0.28 | ||
| Cooperative species only aggregate data (N = 26 species) | |||||||
| Mean | Env. Harshness | −0.186 | −0.435 – 0.065 | 0.12 | −1.89 | 0.07 | 0.09 |
| Temp. Stability | 0.090 | −0.51 – 0.72 | 0.77 | 0.41 | 0.69 | ||
| All locality data (N = 129 localities, 28 species) | |||||||
| Categorical | Env. Harshness | −7.76 | −62.85 – 49.87 | 0.81 | 1.37 | 0.17 | 0.05 |
| Temp. Stability | 58.06 | −2.10 – 136.13 | 0.041 | −2.68 | 0.008 | ||
| Mean | Env. Harshness | −0.177 | −0.293 – −0.062 | 0.003 | −2.97 | 0.004 | 0.05 |
| Temp. Stability | −0.032 | −0.191 – 0.130 | 0.69 | −0.36 | 0.72 | ||
| Cooperative species only locality data (N = 86 localities, 19 species) | |||||||
| Mean | Env. Harshness | −0.262 | −0.383 – −0.124 | 0.001 | −5.91 | <0.001 | 0.28 |
| Temp. Stability | 0.046 | −0.172 – 0.256 | 0.66 | 0.81 | 0.41 | ||
FIGURE 3.
Similar patterns of results are found for analyses using aggregate (A–B) and locality datasets (C–D). In the aggregate dataset, the climate and cooperation data are based on the aggregate of all available data for each species. For the locality dataset, climate and cooperation data are specific to particular localities. In both datasets, environmental harshness PC is negatively associated with the rate of cooperative nesting among cooperative species but does not separate cooperative from non-cooperative species (A, C). Greater short term temperature stability is associated with non-cooperative nesting species in both the aggregate and locality datasets (B,D). The scatterplots show the continuous variation in raw data for each analysis with trend lines denoting a significant phylogentically corrected relationship. Boxplots show the distribution of climate variables for each category of species. Cooperative species are denoted with red and non-cooperative with blue.
Categorical analyses are a better fit to the aggregate dataset whereas continuous analyses are a better fit to the locality dataset. A model that considers short-term temperature fluctuation as the sole predictor of categorical cooperation data is a substantially better fit in the aggregate (PGLS, F2,38 = 25.63, r2 = 0.39, P < 0.0001) compared to the locality dataset (PGLS, F2,127 = 6.96, r2 = 0.04, P = 0.009). For the continuous data, a model that considers solely environmental harshness as a predictor of extent of cooperation in cooperative species within the locality dataset fits better (PGLS, F2,84 = 34.55, r2 = 0.28, P < 0.0001) than its equivalent model with the cooperative-only aggregate dataset (PGLS, F2,24 = 4.57, r2 = 0.13, P = 0.021).
Climatic correlates of cooperation within species
Global axes of climate variation that explain patterns of cooperative nesting across the genus are relatively poor predictors of variation in cooperative nesting within individual species. In the genus-wide analysis of the locality dataset environmental harshness correlates with variation in the extent of cooperative nesting (Fig 3C). However, short-term temperature stability tends to better explain variation in size of cooperative nesting associations within the four species examined here (Fig 4A–B, Table 2). In both P. dominula and P. exclamans, short-term temperature stability tends to be negatively associated with the mean number of foundresses. In contrast, in P. fuscatus short-term temperature stability tends to be positively associated with cooperative nesting. Neither of the genus-wide climate PCs explained variation in cooperative nesting in P. metricus. The same pattern of results is found when analyzing variation in the percent subordinate foundresses for P. exclamans, P. fuscatus and P. metricus (Table 2). For P. dominula, however, the percentage of subordinate foundresses is associated with environmental harshness rather than short-term temperature stability. This difference arises because mean foundress number and percent subordinate foundresses differentially emphasize variation among populations showing high or low rates of cooperative nesting respectively (Fig S1).
FIGURE 4.
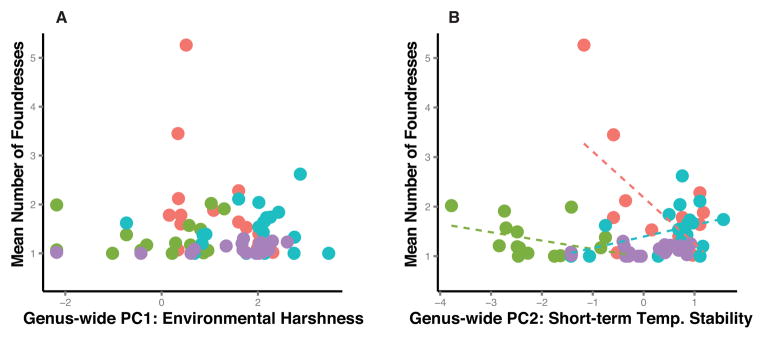
(A) Environmental harshness that correlates with interspecific variation in the extent of cooperation across the genus, but does not explain intraspecific variation in rates of cooperation in any of the four species examined: P. dominula (red), P. fuscatus (blue), P. metricus (purple), and P. exclamans (green). (B) Short term temperature stability, which is associated with the presence of cooperation at the macroevolutionary scale, tends to explain variation in the extent of cooperative nesting among populations in three of the species. The trends in P. dominula and P. exclamans are in line with the genus-wide patterns though P. fuscatus shows an opposite response to fluctuating temperatures.
Table 2.
Interspecific analyses
| Genus-wide Population-level Climate PCs | |||||||
|---|---|---|---|---|---|---|---|
| Mean Foundresses | Percent Subordinate | ||||||
| Species | F or t | r2 | P | F or t | r2 | P | |
| P. dominula | Whole model | 6.07 | 0.36 | 0.010 | 6.24 | 0.37 | 0.010 |
| Env. Harshness | −0.62 | 0.54 | 2.3 | 0.036 | |||
| Fluctuating Temp. | −2.08 | 0.054 | −0.39 | 0.71 | |||
| P. exclamans | Whole model | 2.21 | 0.13 | 0.15 | 1.86 | 0.097 | 0.19 |
| Env. Harshness | −1.07 | 0.3 | 0.87 | 0.398 | |||
| Fluctuating Temp. | −2.10 | 0.055 | −1.90 | 0.078 | |||
| P. fuscatus | Whole model | 1.9 | 0.09 | 0.18 | 3.85 | 0.23 | 0.042 |
| Env. Harshness | −0.36 | 0.73 | 1.14 | 0.27 | |||
| Fluctuating Temp. | 1.89 | 0.076 | 2.77 | 0.013 | |||
| P. metricus | Whole model | 1.65 | 0.06 | 0.22 | 1.88 | 0.08 | 0.18 |
| Env. Harshness | 0.91 | 0.37 | −0.86 | 0.40 | |||
| Fluctuating Temp. | 0.39 | 0.698 | 0.54 | 0.60 | |||
Genus-wide PC axes are derived from a global dataset of localities, and although they are relevant axes of climate variation at a global scale, they may not accurately reflect patterns of climatic variation within the range of single species. For example, the environmental factors that explain broad patterns of variation in cooperation between temperate and tropical zones or between rainforests and deserts may be highly uninformative when it comes to the variation in cooperation observed within a species that is only present in temperate deciduous forests. Thus, we conducted a second-set of intraspecific analyses where we calculated climate PCs specific to the population datasets for each species. As expected from the limited distributions of the focal species, species-specific climate PCs differ considerably from genus wide PCs (Fig S7). Analyses of patterns of variation in cooperative nesting relative to species-specific climate PCs reveals considerable heterogeneity among species (Table S7) – in P dominula cooperation is positively associated with warmer, predictable temperature regimes (Fig S8A, F1,17 = 10.78, r2 = 0.35, P = 0.004); in P. fuscatus cooperation is higher with more predictable precipitation patterns (Fig S8B, F1,18 = 6.60, r2 = 0.23, P = 0.019); in P. metricus cooperation is highest with less predictable precipitation patterns (Fig S8C, F1,20 = 7.27, r2 = 0.23, P = 0.014); in P. exclamans neither of the first two principal components explain variation in cooperation rates (Fig S8D).
DISCUSSION
Our results demonstrate that the axes of global environmental variation associated with shifts between cooperative and singular nesting are different from those that explain variation in the size of cooperative nesting associations across species. Put simply, the environmental pressures associated with increasing from one to two foundresses do not explain the increase from two to three foundresses. We find the same pattern of results using both a comprehensive, though noisy dataset of aggregate measures for all species and a stringently filtered dataset based solely on well-sampled localities, demonstrating that our findings are robust. Our results therefore suggest that being willing or able to form any cooperative nesting association is a fundamental step in social evolution. Notably, the bimodal distribution of the rates of cooperation across species is consistent with a model where non-cooperative and cooperative breeding represent two distinct states. In other realms of ecological research, bimodal distributions have been interpreted to be driven by regime shifts in other systems as well (Scheffer et al. 2014) or to be indicative of bistability of ecosystems (Staver et al. 2011). Specifically, the bimodal distribution in rates of cooperative nesting observed in Polistes wasps appear to be the result of opposing selection pressures favoring either cooperative or non-cooperative strategies at either side of an environmental threshold.
The loss of cooperative nesting is associated with reduced temperature fluctuations over short time scales. Comparative studies of cooperative breeding in vertebrates have focused on the role of year-to-year environmental predictability in shaping cooperative behavior (Faulkes et al. 1997; Jetz & Rubenstein 2011; Gonzalez et al. 2013). Compared to relatively long-lived cooperatively breeding vertebrates, paper wasps have a short lifespan with annual colony cycles (Reeve 1991; Brockmann 1997). Thus it is perhaps less surprising that variation during the course of a wasp’s life rather than between generations is more salient in this case.
A number of investigators have examined the influence of microhabitat temperature on nest site choice and colony productivity in Polistes (Cervo & Turillazzi 1985; Jeanne & Morgan 1992; Nadeau & Stamp 2003). However, little work has explicitly examined the influence of the amplitude of temperature fluctuations. We suggest two non-mutually exclusive routes through which short-term temperature fluctuations may influence cooperation in paper wasps. First, large diurnal and day-to-day temperature fluctuations can have negative implications for growth and development in insects (Colinet et al. 2015). Unlike many bees and ants, paper wasps have small, exposed nests, which offer little buffer from environmental fluctuations (Jones & Oldroyd 2006). This is especially true at the founding stage when nests are small (Hozumi & Yamane 2001). Higher amplitudes of temperature fluctuation may represent more stressful conditions for both larval development and adult physiology given the limited thermoregulatory capacity of Polistes wasps (Weiner et al. 2010). More stressful nesting conditions, in turn, may favor cooperation. Second, fluctuating temperatures may also affect wasps by reducing the amount of time available for foraging. Wasps tend to be inactive at lower temperatures and some species have narrow temperature ranges for optimal flight (Weiner et al. 2012). At higher temperatures, adults forgo nutrient foraging and invest in nest-directed thermoregulatory behaviors including fanning the nest and collecting water to drench the nest for evaporative cooling (Rau 1931). Cooperation may be advantageous when there are larger amplitude temperature fluctuations as groups of foundresses may be able to more effectively take advantage of windows suitable for foraging. The current findings call for work integrating studies of thermal physiology and cooperative nesting in Polistes wasps to elucidate the mechanisms driving the pattern uncovered in this study.
The largest cooperative groups are not found among species with the most extreme temperature fluctuations, but rather those occupying benign climates with warm and wet conditions. At face-value this finding appears to challenge much of the work emphasizing the role of ecological constraints on independent breeding in favoring cooperative breeding (Faulkes et al. 1997; Hatchwell & Komdeur 2000). Benign environmental conditions, however, have also been argued to potentially lead to increased rates of cooperation due to habitat saturation (Selander 1956; Arnold & Owens 1999; Gonzalez et al. 2013). There is some evidence of higher rates of cooperative nesting in denser Polistes populations (Brockmann 1997) though there is no evidence that Neotropical species with the highest rates of cooperation nest at higher density or are closer to their carrying capacity than Polistes in other parts of the world. Alternatively, it is possible that wasps in regions with benign abiotic conditions are faced with harsher biotic interactions. In particular, rates of ant predation on wasp larvae have been experimentally shown to be higher in the Neotropics compared to temperate North America (Jeanne 1979), and are thought to have been a major evolutionary force shaping nest site selection in tropical Polistine wasps (Corbara et al. 2009). Currently, data on any moderating effects of foundress number on mitigating ant attacks is lacking. More broadly, larger foundress associations have been shown to be more resilient against vertebrate predation as well as defending against parasitoids (Strassmann 1981; Strassmann et al. 1988). The Neotropics also has elevated levels of species diversity in Polistes and related genera (Corbara et al. 2009), raising the possibility that competition may be greater in the paper wasp niche in the Neotropics compared to temperate regions. Relatively little is known about the comparative population demography, predation and parasitism pressures across Polistes though future work in this area holds important promise for understanding patterns of cooperation across species.
The axis of climate variation that explains genus-wide patterns of variation in the size of cooperative nesting associations do not explain intraspecific patterns of cooperative nesting in the four temperate species examined. This result is especially noteworthy for two reasons. Previous authors have criticized phylogenetic comparative studies of cooperative breeding because they did not match species-level patterns (Cockburn 2013), even though studies at different scales have used different climate data. In the present study, the climate and cooperation data used to assess variation in each species was simply a subset of the locality data used in the genus-wide analysis. Arguably, this result provides the clearest evidence to date that different processes shape variation in the rates of cooperation within and between species. Indeed, analyses of intraspecific variation in rates of cooperation demonstrate that variation in cooperative nesting is often associated with environmental variation, though the relevant gradients differ across species (Fig. 4). The major axes of climate variation at the global scale are rarely replicated within the range of an individual species, so it is not surprising that important features of climate variation may differ at local and global scales (Fig S6). Notably, the species we examined shared partly overlapping ranges and still showed heterogeneous responses to climate variation suggesting that species’ cooperative nesting responses to climate variation are evolutionarily labile within Polistes (Fig 4). The heterogeneity in the relationships between cooperation and environmental conditions among species urges caution in extrapolating findings on the climatic drivers of cooperation from a single population or species to broader geographic and spatial scales. Thus, criticisms that the results of comparative studies examining the relationship between cooperation and the environment do not concur with intraspecific studies (Cockburn & Russell 2011; Cockburn 2013) should be re-evaluated in light of the fact that predictors of the formation of cooperative groups and size of those groups need not be the same (West et al. 2007, this study).
Conclusion
Detailed records of wasp nesting behavior have allowed us to examine the relationships between cooperative nesting and climate using different metrics across phylogenetic and spatial scales. These analyses reveal that different aspects of climatic variation are associated with the presence and extent of cooperation both within and across species. Interestingly, estimates of the average climate for each species are a better predictor of the presence of cooperation than climate variables from the limited subset of populations where species have been observed and vice versa for continuous measures of cooperation. This result suggests that the propensity to engage in cooperative nesting is a trait that evolves at the species-level in paper wasp while the extent of its expression (as measured by foundress group size) is potentially more plastic and dependent on local conditions. Taken together, our data provide support for variation in cooperative breeding as both an ecologically labile continuum and distinct evolutionary strategies. The disconnect between inter- and intra-specific patterns of cooperative nesting in responses to climate begs for further research documenting patterns of cooperation across species’ ranges, opening up a new line of questioning to understand the demographic, ecological and evolutionary processes that give rise to heterogeneity in climate responses across species.
Supplementary Material
Acknowledgments
We would like to thank E. Lacey, D. Elias and the Behavior Group at UC Berkeley for comments on earlier stages of this work. We acknowledge the following funding support, Edwin S. George Reserve Scholarship and NIH-NRSA postdoctoral fellowship F32 GM101863 to MJS, Initiative for Biological Complexity fellowship to CAB, AFRI-USDA-NIFA Postdoctoral Fellowship 11351209 to TAH, Smithsonian Tupper Fellowship to BES, NSF-IOS, Behavioral Systems Award # IOS- 1146410 and NSF-IOS, Evolution of Developmental Systems Award # IOS-1051808 to ALT, and NSF-IOS, Behavioral Systems Award # IOS-1146139 to EAT.
Footnotes
Author contributions:
MJS conceived and designed the study; MJS constructed the database of cooperative nesting; TAH performed phylogenetic analysis; MJS, JMJ, SW, ALT and EAT contributed field data on wasp nesting behavior; MJS, CAB and BES analyzed the nesting data in relation to climate; MJS wrote the paper with input from other authors.
Contributor Information
Michael J Sheehan, Email: ms3246@cornell.edu.
Carlos A Botero, Email: cbotero@wustl.edu.
Tory A Hendry, Email: th572@cornell.edu.
Brian E Sedio, Email: briansedio@gmail.com.
Jennifer M. Jandt, Email: jandt@iastate.edu.
Susan Weiner, Email: sweiner02@roosevelt.edu.
Amy L Toth, Email: amytoth@iastate.edu.
Elizabeth A Tibbetts, Email: tibbetts@umich.edu.
References
- 1.Arnold KE, Owens IPF. Cooperative breeding in birds: the role of ecology. Behav Ecol. 1999;10:465–471. [Google Scholar]
- 2.Avilés L, Harwood G. A Quantitative Index of Sociality and Its Application to Group-Living Spiders and Other Social Organisms. Ethology. 2012;118:1219–1229. doi: 10.1111/eth.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockmann HJ. The Evolution of Social Behavior in Insects and Arachnids. Cambridge University Press; Cambridge, UK: 1997. Cooperative breeding in wasps and vertebrates: The role of ecological constraints; pp. 347–372. [Google Scholar]
- 4.Brown JL. Helping and communal breeding in birds. Princeton University Press; Princeton: 1987. [Google Scholar]
- 5.Cervo R, Turillazzi S. Associative foundation and nesting sites in Polistes nimpha. Naturwissenschaften. 1985;72:48–49. [Google Scholar]
- 6.Cockburn A. 12 Cooperative Breeding in Birds: Toward a Richer Conceptual. Coop Its Evol. 2013:223. [Google Scholar]
- 7.Cockburn A, Russell AF. Cooperative Breeding: A Question of Climate? Curr Biol. 2011;21:R195–R197. doi: 10.1016/j.cub.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Colinet H, Sinclair BJ, Vernon P, Renault D. Insects in Fluctuating Thermal Environments. Annu Rev Entomol. 2015;60:123–140. doi: 10.1146/annurev-ento-010814-021017. [DOI] [PubMed] [Google Scholar]
- 9.Colwell RK. Predictability, constancy, and contingency of periodic phenomena. Ecology. 1974:1148–1153. [Google Scholar]
- 10.Corbara B, Carpenter JM, Céréghino R, Leponce M, Gibernau M, Dejean A. Diversity and nest site selection of social wasps along Guianese forest edges: assessing the influence of arboreal ants. C R Biol. 2009;332:470–479. doi: 10.1016/j.crvi.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Faulkes CG, Bennett NC, Bruford MW, Obrien HP, Aguilar GH, Jarvis JUM. Ecological constraints drive social evolution in the African mole-rats. Proc R Soc Lond Ser B-Biol Sci. 1997;264:1619–1627. doi: 10.1098/rspb.1997.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feeney WE, Medina I, Somveille M, Heinsohn R, Hall ML, Mulder RA, et al. Brood Parasitism and the Evolution of Cooperative Breeding in Birds. science. 2013;342:1506–1508. doi: 10.1126/science.1240039. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez J-CT, Sheldon BC, Tobias JA. Environmental stability and the evolution of cooperative breeding in hornbills. Proc R Soc B Biol Sci. 2013:280. doi: 10.1098/rspb.2013.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw. 2010;33:1–22. [Google Scholar]
- 15.Hatchwell BJ, Komdeur J. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim Behav. 2000;59:1079–1086. doi: 10.1006/anbe.2000.1394. [DOI] [PubMed] [Google Scholar]
- 16.Hozumi S, Yamane S. Incubation ability of the functional envelope in paper wasp nests (Hymenopteta, Vespidae, Polistes): I. Field measurements of nest temperature using paper models. J Ethol. 2001;19:39–46. [Google Scholar]
- 17.Hughes CR, Queller DC, Strassmann JE, Davis SK. Relatedness and Altruism in Polistes Wasps. Behav Ecol. 1993;4:128–137. [Google Scholar]
- 18.Jandt JM, Tibbetts EA, Toth AL. Polistes paper wasps: a model genus for the study of social dominance hierarchies. Insectes Sociaux. 2014;61:11–27. [Google Scholar]
- 19.Jeanne RL. A latitudinal gradient in rates of ant predation. Ecology. 1979:1211–1224. [Google Scholar]
- 20.Jeanne RL, Morgan RC. The influence of temperature on nest site choice and reprodcutive strategy in a temperate zone Polistes wasp. Ecol Entomol. 1992;17:135–141. [Google Scholar]
- 21.Jetz W, Rubenstein DR. Environmental Uncertainty and the Global Biogeography of Cooperative Breeding in Birds. Curr Biol. 2011;21:72–78. doi: 10.1016/j.cub.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 22.Jones JC, Oldroyd BP. Nest thermoregulation in social insects. Adv Insect Physiol. 2006;33:153–191. [Google Scholar]
- 23.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 24.Komdeur J. IMPORTANCE OF HABITAT SATURATION AND TERRITORY QUALITY FOR EVOLUTION OF COOPERATIVE BREEDING IN THE SEYCHELLES WARBLER. Nature. 1992;358:493–495. [Google Scholar]
- 25.Leadbeater E, Carruthers JM, Green JP, Rosser NS, Field J. Nest Inheritance Is the Missing Source of Direct Fitness in a Primitively Eusocial Insect. Science. 2011;333:874–876. doi: 10.1126/science.1205140. [DOI] [PubMed] [Google Scholar]
- 26.Liao X, Rong S, Queller DC. Relatedness, Conflict, and the Evolution of Eusociality. PLoS Biol. 2015;13:e1002098–e1002098. doi: 10.1371/journal.pbio.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligon JD, Burt DB. Ecology and Evolution of Cooperative Breeding in Birds. Cambridge University Press; Cambridge, UK: 2004. Evolutionary origins. [Google Scholar]
- 28.Maddison W, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2001. [Google Scholar]
- 29.Mitchell TD, Jones PD. An improved method of constructing a database of monthly climate observations and associated high-resolution grids. Int J Climatol. 2005;25:693–712. [Google Scholar]
- 30.Nadeau H, Stamp N. Effect of prey quantity and temperature on nest demography of social wasps. Ecol Entomol. 2003;28:328–339. [Google Scholar]
- 31.Nowak MA, Tarnita CE, Wilson EO. The evolution of eusociality. Nature. 2010;466:1057–1062. doi: 10.1038/nature09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, et al. R package version 0.5. 2012. Caper: Comparative Analyses of Phylogenetics and Evolution in R. [Google Scholar]
- 33.Pickett KM, Carpenter JM. Simultaneous Analysis and the Origin of Eusociality in the Vespidae (Insecta: Hymenoptera) Arthropod Syst Phylogeny. 2010;68:3–33. [Google Scholar]
- 34.Purcell J. Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol Rev. 2011;86:475–491. doi: 10.1111/j.1469-185X.2010.00156.x. [DOI] [PubMed] [Google Scholar]
- 35.Rau P. Polistes wasps and their use of water. Ecology. 1931;12:690–693. [Google Scholar]
- 36.Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst Biol. 2008;57:4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- 37.Reeve HK. Polistes. In: Ross KG, Matthews RW, editors. The Social Biology of Wasps. Cornell University Press; Ithaca, NY: 1991. pp. 99–149. [Google Scholar]
- 38.Reeve HK, Starks PT, Peters JM, Nonacs P. Genetic support for the evolutionary theory of reproductive transactions in social wasps. Proc R Soc Lond Ser B-Biol Sci. 2000;267:75–79. doi: 10.1098/rspb.2000.0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheffer M, Vergnon R, Cornelissen JHC, Hantson S, Holmgren M, van Nes EH, et al. Why trees and shrubs but rarely trubs? Trends Ecol Evol. 2014;29:433–434. doi: 10.1016/j.tree.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Selander RK. Speciation in wrens of the genus Campylorhynchus. University of California; Berkeley: 1956. [Google Scholar]
- 41.Seppa P, Queller DC, Strassmann JE. Reproduction in foundress associations of the social wasp, Polistes carolina: conventions, competition, and skew. Behav Ecol. 2002;13:531–542. [Google Scholar]
- 42.Sherman PW, Lacey EA, Reeve HK, Keller L. The eusociality continuum. Behav Ecol. 1995;6:102–108. [Google Scholar]
- 43.Staver AC, Archibald S, Levin SA. The global extent and determinants of savanna and forest as alternative biome states. Science. 2011;334:230–232. doi: 10.1126/science.1210465. [DOI] [PubMed] [Google Scholar]
- 44.Strassmann JE. Parasitoids, predators, and group size in the paper wasp, Polistes exclamans. Ecology. 1981:1225–1233. [Google Scholar]
- 45.Strassmann JE, Queller DC, Hughes CR. Predation and the evolution of sociality in the paper wasp Polistes bellicosus. Ecology. 1988:1497–1505. [Google Scholar]
- 46.Wcislo WT, Tierney SM. The evolution of communal behavior in bees and wasps: an alternative to eusociality. Organ Insect Soc Genome Sociocomplexity. 2009:148–169. [Google Scholar]
- 47.Weiner SA, Noble K, Upton CT, Flynn G, Woods WA, Jr, Starks PT. The cost of flight: a role in the Polistes dominulus invasion. Insectes Sociaux. 2012;59:81–86. [Google Scholar]
- 48.Weiner SA, Upton CT, Noble K, Woods WA, Jr, Starks PT. Thermoregulation in the primitively eusocial paper wasp, Polistes dominulus. Insectes Sociaux. 2010;57:157–162. [Google Scholar]
- 49.West Eberhard MJ. The Social Biology of Polistine Wasps. Museum of Zoology, University of Michigan; Ann Arbor: 1969. [Google Scholar]
- 50.West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17:R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.